Abstract
Psoriasin (S100A7) is a calcium-binding protein that has shown to be highly expressed in high-grade ductal carcinoma in situ (DCIS) and a subset of invasive breast cancers. However, its role in invasion and metastasis is not very well known. In this study, we have shown that S100A7 differentially regulates epidermal growth factor (EGF)-induced cell migration and invasion in ERα− MDA-MB-231 cells and ERα+ MCF-7 and T47D breast cancer cells. Further signaling studies revealed that S100A7 enhances EGF-induced EGFR phosphorylation and actin remodeling that seems to favor lamellipodia formation in ERα− cells. In addition, S100A7 overexpression enhanced NF-κB-mediated matrix metalloproteinase-9 (MMP-9) secretion in MDA-MB-231 cells indicating its role in enhanced invasiveness. However, S100A7 overexpression inhibited migration and invasion of MCF-7 cells by inactivating Rac-1 pathway and MMP-9 secretion. Moreover, S100A7 overexpressing MDA-MB-231 cells showed enhanced metastasis compared to vector control in in vivo nude mice as detected by bioluminescence imaging. Our tissue microarray data also revealed predominant expression of S100A7 in ERα− metastatic carcinoma, especially in lymph node regions. Overall these studies suggest that S100A7 may enhance metastasis in ERα− breast cancer cells by a novel mechanism through regulation of actin cytoskeleton and MMP-9 secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasin (S100A7) is a low molecular weight S100 gene family protein, originally isolated from skin psoriatic lesions [1]. The S100 gene family consisting of ~20 members is defined by calcium binding helix–loop–helix structural EF hand motif [2]. Apart from two calcium-binding sites, S100A7 has an additional zinc-binding site [3]. S100A7 expression has been reported in epithelial malignancies such as breast, lung, bladder, skin, esophageal, gastric, and head and neck [4–7]. In breast cancer, S100A7 expression is highly associated with high-grade ductal carcinoma in situ (DCIS) and invasive carcinoma compared to normal breast tissues [4, 8, 9]. Differential expression of S100A7 in breast cancer was initially observed in primary breast cancer compared to normal tissue [10]. Previous studies have shown that S100A7 is expressed in ~50 % of ERα− and only ~20 % of ERα+ cases of breast cancer [4, 8, 9, 11–13]. Interestingly, in terms of prognosis, both DCIS and invasive breast cancer forms showed consistent association of S100A7 with ERα− tumors [4]. Furthermore, S100A7 has been known to modulate tumor growth by activating several signaling pathways, including PI3K, NF-κB, AP-1, and Jab1 [13–15]. However, recent studies have reported the tumor suppressive effects of S100A7 in ERα+ breast cancer cells [16, 17].
Breast carcinoma is classified based on the expression of three receptors: EGFR, ER, and HER-2. EGFR expression is closely related to ER receptor status and has adverse association to overall patient survival with poor prognosis. One prominent feature of ERα− tumors, especially triple-negative basal-like subtype, is the expression of EGFR [18]. These basal-like tumors are associated with aggressive histological features, poor prognosis and are extremely difficult to treat. High EGFR expression is also associated with metastatic and invasive form of breast cancer [18]. EGFR activation provokes a plethora of signaling pathways that includes cell proliferation, adhesion and motility and promotes invasion and angiogenesis [19]. Recent studies in our laboratory and others have shown that S100A7 regulates epidermal growth factor (EGF)/EGFR-mediated signaling pathways [13–15]. Studies have also shown that S100A7 and EGFR are associated with ERα− tumors in a large unselected cohort of breast cancer patients [13].
Actin dynamics and remodeling have been identified as major determinant of metastasis and invasion that are the key basis of most cancer-related deaths. During cell motility, branched network of actin filaments are required to assemble beneath the plasma membrane to consistently progress the cell forward to form lamellipodia [20]. The recruitment of active Rac1, a small Rho GTPase at the leading edge, is itself sufficient for cell extension and further movement [21, 22]. Moreover, the influence of S100A7 in calcium-mediated signal transduction and cellular events through direct interactions with intermediate filaments also implies its role in modulation of the cytoskeleton [2].
Hence, present study investigated the influence of S100A7 on metastatic and invasive abilities of ERα− and ERα+ breast cancer cells upon EGF stimulation. Our studies showed that S100A7 differentially regulates migration and invasion in ERα− and ERα+ cells. S100A7 overexpression enhanced EGF-induced migration/invasion in ERα− cells, while its overexpression inhibited migration/invasion in ERα+ cells. We also showed that S100A7 enhances metastasis in vivo and its predominant expression was observed in ERα− lymph node metastatic group of breast patient samples. In addition, actin polymerization pathway seems to play an important role in establishing the differential effect of S100A7 in ERα− and ERα+ breast cancer cells.
Materials and methods
Cells, stable transfections, and antibodies
The vector information and generation of stable clones of MDA-MB-231, MCF7, and T47D breast carcinoma cells with stable vector and S100A7 overexpression used in the present study are as described earlier [16, 23]. Knockdown of p65-NF-κB was performed using its siRNA transfection (50 nM; Dharmacon) for 72 h using lipofectamine 2000 as per manufacturer’s protocol. Antibodies used were mostly from Cell Signaling, GAPDH (Santa Cruz Biotechnology) and Phalloidin-568 (Invitrogen).
Migration and invasion assay
Wound healing assay, chemotaxis assay, and invasion assay were performed and calculated as described previously [15, 16, 23, 24].
Gelatin zymography
This method was used to compare MMP-2 and MMP-9 with gelatinase activity of MDA-MB-231 and MCF-7 cells upon S100A7 overexpression (24 h). Supernatants containing secreted form of MMPs were concentrated using centrifugal filter units (Millipore) and detected using Novex gelatin zymography. Renaturing, developing, and staining steps were followed to visualize active MMP bands according to the manufacturer’s instructions (Life technologies).
Western blotting
Western blot analysis was done as previously described [23, 24].
Rac1 activation assay
Activation of Rac1 was determined using the Rac/Cdc42 activation assay kit as per manufacturer’s protocol (Millipore). Briefly, cell lysates were incubated with 10 μg/mL p21-activated kinase 1 agarose beads for 60 min at 4 °C. Agarose beads were collected by centrifugation followed by heat denaturation of samples and Rac1 activation was evaluated by immunoblotting by anti-human Rac1 antibody.
G-actin/F-actin in vivo biochemical assay
This quantitative assay was performed to determine the relative effect of EGF on filamentous actin (G-actin) versus free globular actin (F-actin) content. Briefly, cells were suspended in F-actin stabilizing buffer and separated from G-actin by ultra-centrifugation as per manufacturer’s directions (Cytoskeleton Inc.). The difference in G-actin and F-actin content was examined by western blot using G-actin antibody.
Confocal microscopy
Briefly, treated cells were fixed with 4 % paraformaldehyde at room temperature. Cells were washed with 1× PBS, blocked with 5 % BSA in 1× PBS for 60 min and incubated with Phalloidin-568 overnight at 4 °C. Cells were washed with 1× PBS and mounted using vectashield mounting medium containing DAPI and examined under Olympus FV1000 Filter confocal microscope. Images were acquired with 40× objective and modified using FV10-ASW2.0 software.
Bioluminescent imaging (BLI) and analysis
Nude mice obtained from Charles River Laboratories, were maintained at Ohio State University animal facility under IACUC rules and regulations. Nude mice (n = 10) were injected intracardially with MDA-MB-231-luc-D3H2LN-S100A7-luciferase or vector control (1 × 105/100 μL) and were weekly assessed for tumor burden (IVIS System 200, Xenogen Corporation). Mice were anesthetized intraperitoneally with 0.15 mg/mL of d-luciferin (PBS) and bioluminescent images were collected between 2 and 5 min post-injection. The light intensity was detected by IVIS camera system, integrated, digitalized, and displayed for relative photon flux as calculated per mouse.
Tissue microarrays (TMA) and immunohistochemical analysis
TMA were obtained from Imgenex (San Diego, CA) and immunohistochemistry (IHC) analysis was performed on paraffin-embedded formalin fixed breast tissue specimens. TMAs were de-paraffinized according to manufacturer’s recommendation and immunostained with S100A7 antibody at 1:50 dilution (Imgenex). Vectastain Elite ABC reagents (Vector Laboratories) using avidin DH:biotinylated horseradish peroxidase H complex, 3,3′-diaminobenzidine (Polysciences), and Mayer’s hematoxylin (Fisher Scientific) were used for detection of the bound antibodies.
Statistical analysis
All the experiments were performed at least three to four times to confirm the results. The results were then expressed as mean ± SD of data obtained from these three or four experiments. The statistical significance was determined by the Student’s t test and value of p < 0.05 was considered significant as denoted by asterisks.
Results
S100A7 overexpression differentially activates EGFR in ERα− and ERα+ breast cancer cells
It has been shown that S100A7 downregulation inhibits EGFR-mediated signaling in ERα− cells [15]. Here, we have analyzed the effect of S100A7 overexpression on EGF-induced receptor activation in ERα− (MDA-MB-231) and ERα+ (MCF-7 and T47D) cells by EGFR phosphorylation. We observed an increase in EGFR phosphorylation in S100A7 overexpressing MDA-MB-231 cells upon EGF treatment (Fig. 1a). However, S100A7 overexpression reduced EGF-induced EGFR phosphorylation in MCF7 cells compared to vector (Fig. 1b). In another ERα+ cell line, T47D, we observed similar results of time-dependent inhibition of EGFR phosphorylation upon EGF stimulation (Fig. 1c). The quantitative analysis of all immunoblots showed consistent increase and decrease in EGFR phosphorylation of S100A7 overexpressing ERα− and ERα+ cells, respectively (Fig. 1d–f). Therefore, differential EGFR phosphorylation might play an important role in S100A7 overexpressing ERα− and ERα+ breast cancer cells.
EGF-induced differential phosphorylation of EGFR in ERα− and ERα+ breast cancer cells with S100A7 overexpression. EGFR phosphorylation status was analyzed in S100A7 overexpressing ERα− MDA-MB-231 cells (a) and ERα+ MCF-7 (b) and T47D cells (c) on EGF stimulation (100ng/mL) by western blotting. The relative EGFR phosphorylation levels in all cells are representative of three independent experiments, calculated with respect to GAPDH for MDA-MB-231, MCF-7, and T47D (d–f) at indicated time. The statistically significant p values were indicated as *<0.05 and **<0.005
S100A7 overexpression affects cell motility of ERα− and ERα+ cells
The motile ability of tumor cells determines their metastatic phenotype. In the present study, EGF-induced cell migration was performed to analyze the cell motility of ERα− and ERα+ cells upon S100A7 overexpression. The wound healing assay revealed the effect of S100A7 in directional cell migration of ERα− and ERα+ cells. The assay showed S100A7 to significantly increase EGF-mediated migratory abilities of S100A7 overexpressing MDA-MB-231 cells (Fig. 2a). We observed significant increase in wound closure of S100A7 overexpressing MDA-MB-231 cells compared to vector control. In contrast, S100A7 inhibited the directional cell migration of ERα+ MCF-7 cells by relatively slowing down their wound closure compared to vector cells (Fig. 2b). Moreover, cell migration assay using transwell chambers showed ~five fold increase in EGF-induced migration of S100A7 overexpressing MDA-MB-231 cells compared to its vector control (Fig. 2c). SCP6, a single cell progeny of MDA-MB-231 cells, which has previously been characterized as a low metastatic cell line, was also analyzed to evaluate the effect of S100A7 overexpression on cell migration (Supplementary Fig. 1a) [25]. Importantly, S100A7 was able to promote EGF-induced cell migration in SCP6 as well. However, S100A7 overexpression in MCF-7 cells inhibited EGF-induced cell migration by ~eight fold (Fig. 2d). Similar results were seen on S100A7 overexpression in ERα+ T47D cell line, which showed lesser inhibition in cell migration compared to MCF-7 cells (Supplementary Fig. 1b). Taken together, these data reveal the differential consequence of S100A7 on migratory response of breast cancer cells depending on their ERα status.
Effect of S100A7 on EGF-induced cell motility, migration, and invasion of ERα− and ERα+ cells. Wound healing assay images represent EGF-induced effect on cell motility of S100A7 overexpressing MDA-MB-231 and MCF-7 cells compared to their vector control (a, b). Their quantitative analysis represent one of the three independent experiments performed with p value denoted as *<0.05. Relative migratory potential of S100A7 overexpression in MDA-MB-231 (c) and MCF-7 cells (d) was compared to vector by transwell migration assay, represented as percent cell migration with P value as *<0.05 and **<0.005. EGF-induced effects were analyzed at 100ng/mL concentration for cell motility assays
Role of MMP-9 activation in invasiveness of S100A7 overexpressing ERα− and ERα+ cells
One of the hallmarks of tumor metastasis is its ability to degrade extracellular matrix to invade distant organs. Matrigel invasion assay was performed to analyze the EGF-induced invasion of S100A7 overexpressing ERα− and ERα+ cells. We found that S100A7 overexpression has significantly increased EGF-induced invasive ability of MDA-MB-231 cells compared to vector control by approximately twofold (Fig. 3a). However, there was a considerable decrease in invaded population of S100A7 overexpressing MCF7 cells upon EGF stimulation (Fig. 3b). Similar EGF-mediated inhibition of cell invasion was observed in S100A7 overexpressing T47D cells compared to control cells (Supplementary Fig. 2). The difference in invasion of vector and S100A7 overexpressing cells has been expressed as percentage, which was statistically significant. It is known that cancer cells require matrix metalloproteinases (MMPs) to invade the extracellular matrix underlying their basement membrane and stroma. Hence, we have analyzed the presence of S100A7 expression on activation status of MMP-2 and -9 that have implications in the process of tumor invasion [26]. We observed an increase in active form of MMP-9 secretion in S100A7 overexpressing MDA-MB-231 cells, while its activity was decreased in S100A7 overexpressing MCF7 cells (Fig. 3c, d). However, the MMP-2 activation was not affected in both vector and S100A7 overexpressing MDA-MB-231 and MCF-7 cells. Therefore, diverse MMP-9 activation in S100A7 overexpressing MDA-MB-231 and MCF7 cells suggests the significance of MMP-9 in S100A7 associated invasiveness.
Role of NF-κB in S100A7-mediated MMP-9 secretion in ERα− and ERα+ cells. The effect of EGF on relative invasiveness of MDA-MB-231 (a) and MCF-7 cells (b) on S100A7 overexpression was analyzed by matrigel invasion assay and represented as percent invasion with p value <0.05 as * and <0.005 as **. Gelatin zymography revealing the level of MMP-9 secretion in MDA-MB-231 cells (c) and MCF-7 cells (d) upon S100A7 overexpression without any effect on MMP-2 levels. Images represent one of the three independently performed experiments. Knockdown of p65-NF-κB subunit (e, f) markedly inhibited the MMP-9 secretion of MDA-MB-231 cells analyzed by gelatin zymography (g). Images represent one of the three experiments and their quantitative analyses were statistically significant with p value *<0.05 and **<0.005. Where, EGF used was 100ng/mL and Scr stands for scramble siRNA
Role of NF-κB in S100A7-mediated MMP-9 secretion in ERα− cells
It is known that NF-κB binding on MMP-9 gene regulates TNF-α-mediated MMP-9 secretion [27]. It has also been reported that S100A7 promotes pro-survival pathways in ERα− cells through Akt-mediated NF-κB activation [14]. Since, S100A7 regulates NF-κB activation in ERα− cells, we sought to see the effect of NF-κB knockdown on MMP-9 secretion in S100A7 overexpressing MDA-MB-231 cells. We observed a significant NF-κB downregulation in S100A7 overexpressing MDA-MB-231 cells (Fig. 3e, f). Active MMP-9 secretion was significantly reduced in NF-κB-knocked-down cells compared to scramble siRNA control indicating the direct role of NF-κB in S100A7-mediated MMP-9 secretion (Fig. 3g).
In vivo metastatic potential of S100A7 overexpressing MDA-MB-231 cells and clinicopathological S100A7 expression analysis in breast carcinomas
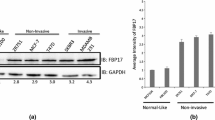
Since, S100A7 overexpression in breast cancer cell lines has been shown to enhance tumor growth [12], we investigated its significance in metastasis in vivo. In this study, we have used IVIS imaging system to analyze the metastatic potential of S100A7 overexpressing MDA-MB-231 with luciferase reporter gene (Fig. 4a). Higher metastatic progression with elevated radiation flux was observed in intracardially injected nude mice with S100A7 overexpressing MDA-MB-231 cells compared to vector control (Fig. 4b). This demonstrates that S100A7 plays an important role in promoting metastatic phenotype of ERα− breast cancer cells. Furthermore, we have analyzed the S100A7 expression in a cohort of breast tissue specimens using tissue microarray (n = 59). The TMA and IHC data has been summarized as in Supplementary Table 1. Our study revealed that S100A7 expression was highly prevalent in metastatic tumors, especially at lymph node region (Fig. 4c). Ninety percent of metastatic tumors showed good expression of S100A7, while normal breast tissues (n = 9) were devoid of S100A7 protein. Interestingly, S100A7 expressing lymph node metastatic group were mostly ERα negative. In addition, we observed predominant S100A7 expression in ERα− (~54 %) and PR− (~50 %) compared to ERα+ type (~36 %) and PR+ (~44 %) among 35 infiltrating ductal carcinoma carcinomas (Fig. 4d). Hence, the considerable role of S100A7 as a regulator of breast cancer metastasis seems to be directly linked to ERα status.
In vivo metastatic potential of S100A7 in ERα− cells and its association with ERα status. Representative BLI images show comparative metastases of control and S100A7 overexpressing MDA-MB231-luc-D3H2LN cells in nude mouse model (a), while the statistically significant high radiance flux was observed in the presence of S100A7 with p value <0.05 (b). Representative immunohistochemistry images show different levels of S100A7 staining pattern in lymph node region of metastatic tissue specimens compared to normal tissue, as a negative control (c). Based on clinico-pathologist analysis, the overall S100A7 expression levels were quantitatively represented in ERα− and ERα+ cells compared to normal breast tissues (d). Where 0, 1, 2, and 3 represent different S100A7 expression levels
Role of S100A7 overexpression on actin polymerization
Actin polymerization is a well-known process that drives cell migration with the most evident feature of lamellipodia formation at leading edges of motile cells. Our immunofluorescence studies showed increased actin accumulation at the leading edges of S100A7 overexpressing MDA-MB-231 cells compared to vector upon EGF stimulation (Fig. 5a). However, there was comparatively lesser actin accumulation at the leading edges of S100A7 overexpressing MCF7 cells compared to vector control. Instead, actin seems to be distributed as small actin-filament structures that became more prominent in vector compared to S100A7 overexpressing MCF-7 cells on EGF treatment (Fig. 5b). Actin accumulation at leading edges might be responsible for differential migratory response of S100A7 in ERα− and ERα+ breast cancer cells. Hence, a direct relationship between activity of actin polymerization and formation of migratory structures could be possible.
Effect of EGF on actin polymerization of S100A7 overexpressing ERα− and ERα+ cells. Immunofluorescence images showing EGF-induced consequences on actin-based cell protrusions of MDA-MB-231 (a) and MCF-7 cells (b) on S100A7 expression. Images represent one of three independent experiments with phalloidin-568 and DAPI staining as indicated at 100ng/mL of EGF treatment
S100A7 overexpression affect EGF-induced Rac1 activation and associated signaling
In order to investigate the role of actin polymerization pathway in S100A7-mediated differential effect in ERα− and ERα+ cells, we have analyzed the activity status of Rac1, which is a key molecule in actin polymerization. Rac1 pathway is downstream of LIMK1/2 and mediates its activation through intermediate kinases like PAK1 [28]. We observed that Rac1 activity was enhanced along with LIMK1/2 expression in S100A7 overexpressing MDA-MB-231 cells compared to vector control cells at all time points (Fig. 6a, c). However, Rac1 activation was inhibited in MCF-7 cells on S100A7 overexpression and even EGF stimulation did not affect its activity suggesting the importance of Rac1 pathway (Fig. 6e, f). We also observed the downregulated expression of LIMK1/2 upon EGF treatment in S100A7 overexpressing MCF7 cells compared to vector control cells (Fig. 6g, h). Consistently enhanced Rac1 activity and LIMK1/2 expression reveal Rac-1 pathway as a modulator of increased migration and metastasis in S100A7 overexpressing MDA-MB-231 cells. However, Rac1 appears to act as a negative regulator in cell migration of S100A7 overexpressing MCF-7 cells.
Rac-1 signaling in EGF-induced cell motility of S100A7 overexpressing ERα− and ERα+ cells. Actin regulators, Rac-1 and LIMK1/2 activation status were evaluated on EGF stimulation (100ng/mL) in S100A7 overexpressing MDA-MB-231 (a, c) and MCF-7 cells (e, g) by western blot. Their quantitative analysis corresponds to three independent repeats as indicated by asterisks * and ** with significant p value <0.05 and <0.005, respectively (b, d, f, h). Where, V stands for vector and S stands for S100A7
S100A7 overexpression affects cofilin
Cofilin is one of the downstream molecules of actin polymerization that directly has impact on cell motility through activation of actin-filament dynamics. Cofilin is de-phosphorylated upon Rac1 activation and leads to the polymerization of the F-actin filaments and lamellipodium formation [29]. Our results showed time-dependent decrease in phosphorylation of cofilin in S100A7 overexpressing MDA-MB-231 cells on EGF stimulation compared to vector control cells (Fig. 7a, b). Since p-cofilin is an inactive form of cofilin, its declined level leads to increased actin polymerization–depolymerization and enhanced lamellipodium formation as migratory structures in S100A7 overexpressing MDA-MB-231 cells seen in our immunofluorescence studies. This effect was not seen on cofilin phosphorylation in MCF7 cells upon S100A7 expression suggesting the effect of inactive Rac1 pathway and cofilin on slow mobility of S100A7 overexpressing ERα+ cells (Fig. 7c, d). Hence, actin-associated regulatory pathway appears to play an important role in migratory potential of S100A7 in ERα− and ERα+ breast cancer cells.
Role of Cofilin signaling on actin remodeling of S100A7 overexpressing ERα− and ERα+ cells. Phosphorylation status of cofilin as a negative actin regulator was assessed to show effect of EGF on S100A7 overexpressing MDA-MB-231 (a) and MCF-7 cells (c). The relative phosphorylation levels appear to be significant with p value *<0.05 and **<0.005 (b, d). Effect of EGF-induced actin regulation in S100A7 overexpressing MDA-MB-231 (e) and MCF-7 (f) cells were quantitatively evaluated by in vivo actin turnover assay as a change in G-actin/F-actin ratio over the indicated time. Where EGF used was 100 ng/mL
S100A7 regulated EGF-induced actin turnover
So far, our studies suggest that S100A7-mediated cell motility is controlled by actin polymerization and associated downstream signaling. However, actin-filament subunits (F-actin) need to be recycled back to monomeric forms (G-actin) to maintain further polymerization and motility [30]. Hence, the influence of S100A7 associated actin polymerization on G-actin/F-actin content upon EGF stimulation was examined by an in vivo assay kit. The assay showed more G-actin content with decreased F-actin quantity in S100A7 overexpressing MDA-MB-231 cells on EGF stimulation (Fig. 7e). The decrease in F-actin and simultaneous increase in G-actin content might be responsible for enhancing ATP based actin recycling during actin polymerization, thus significantly enhancing actin turnover with time. Moreover, there was no effect of EGF on F-actin and G-actin content on S100A7 overexpression in MCF7 cells (Fig. 7f). The diverse influence of EGF on G-actin/F-actin content and actin turnover in S100A7 overexpressing ERα− and ERα+ cells significantly correlated with the activation of actin polymerization pathway.
Discussion
In this study, we report for the first time that S100A7 differentially regulates EGF-induced EGFR phosphorylation and migration of ERα− and ERα+ breast cancer cells. We observed an increase in EGFR phosphorylation in S100A7 overexpressing ERα−, whereas reduced EGFR phosphorylation was seen in ERα+ cells. The estrogen receptor pathway is known to crosstalk with EGFR pathway and since S100A7 negatively regulates ER, it could be possible that S100A7 may likely inhibit EGF activity in ERα+ cells [13, 16]. However, EGF-induced downregulation of EGFR phosphorylation in S100A7 overexpressing ERα+ cells could also be due to the downregulation of β-catenin/TCF4 pathway shown in previous studies [16, 31]. Therefore, it is reasonable to mention that S100A7-mediated differential EGF receptor activation appears to be regulated through different pathways in ERα− and ERα+ cells.
Increased invasive and migratory properties are important characteristics of metastatic breast cancer cells. Our previous studies on MVT-1 orthotopic syngeneic bi-transgenic mS100a7a15 mouse model showed enhanced metastasis through M2-macrophage recruitment [23]. EGF/EGFR-axis is known to regulate cell spreading, motility and invasion through extracellular matrix (ECM). In this study, we demonstrate EGF-induced increase in migration and invasion of S100A7 overexpressing MDA-MB-231 cells. In addition, our in vivo nude mouse model study revealed that S100A7 is associated with increased metastatic capacity of ERα− cells. Furthermore, our studies revealed that S100A7 enhanced NF-κB-mediated MMP-9 secretion in MDA-MB-231 cells. MMP-9 has been shown to play an important role in breast cancer invasion and metastasis [32]. Consistent with our studies, other S100 gene family proteins also promote tumor metastasis through MMP’s activation [33, 34]. Our patient sample data also suggest that S100A7 is widely expressed in metastatic carcinoma, especially in lymph node regions. Interestingly, all S100A7 expressing metastatic samples were ERα negative providing further evidence of S100A7 involvement in ERα− tumor metastasis. However, S100A7-mediated effects on cell migration and invasion were inhibited in ERα+ breast cancer cells with decreased MMP-9 activity.
Metastasis is a multi-step process which can be driven by several ways such as actin polymerization, cell adhesion, and acto-myosin contraction [35]. Hence, we have analyzed the influence of S100A7 in ERα− and ERα+ cells on actin polymerization pathway, which has been extensively studied in cancer metastasis. We revealed an increase in actin polymerization in MDA-MB-231 cells and decrease in MCF7 cells on S100A7 overexpression compared to vector control. Furthermore, we have shown that increased actin polymerization in S100A7 overexpressing ERα− cells is due to more lamellipodia formation at leading edges that is regulated by Rac1 pathway. Rac1 has been shown to control cofilin phosphorylation through the activity of class II PAKs that is regulated through LIM kinases and other downstream effectors of the Rho family of GTPases, Cdc42, Rac, and Rho [36]. Our results suggest that S100A7 overexpression in ERα− cells downregulates cofilin phosphorylation with increased LIMK1/2 expression. This can increase the number of barbed ends available during directional cell movement [28, 36]. Therefore, increased LIMK1/2 and cofilin de-phosphorylation mediates enhanced directional migration of S100A7 overexpressing ERα cells. However, downregulated LIMK1/2 expression and presence of inactive phosphorylated form of cofilin appears to inhibit local actin polymerization at leading edges and reduced EGF-induced migration in S100A7-overexpressing ERα+ cells. Interestingly, prominent cytoplasmic staining of actin filaments in S100A7 overexpressing MDA-MB-231 cells on EGF stimulation could be explained by increased actin turnover. Our in vivo G-actin/F-actin assay demonstrates that G-actin/F-actin ratio regulates actin turnover, which is maintained by S100A7-mediated activation of Rac1 pathway on EGF stimulation in MDA-MB-231 cells. However, ineffective actin turnover in MCF-7 cells could be due to inhibited actin regulatory Rac1 pathway.
In summary, our proposed model describes the EGF-induced differential role of S100A7-mediated actin remodeling and MMP-9 in ERα+ and ERα− breast cancer cells (Supplementary Fig. 3). S100A7 was expressed predominantly in lymph node ERα− metastatic tumors. Its overexpression enhanced in vivo metastasis of ERα− cells. Furthermore, our studies suggest that S100A7 regulates breast cancer metastasis through a novel pathway by modulating actin cytoskeleton and MMP-9 activation. Since metastasis is the leading cause of cancer-related deaths, S100A7 could be a novel therapeutic target in ERα− metastatic breast cancer.
Abbreviations
- S100A7:
-
Psoriasin
- ERα:
-
Estrogen receptor α
- EGF:
-
Epidermal growth factor
- DCIS:
-
Ductal carcinoma in situ
- IHC:
-
Immunohistochemistry
References
Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B et al (1991) Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol 97(4):701–712
Schafer BW, Heizmann CW (1996) The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci 21(4):134–140
Brodersen DE, Nyborg J, Kjeldgaard M (1999) Zinc-binding site of an S100 protein revealed. Two crystal structures of Ca2 + -bound human psoriasin (S100A7) in the Zn2+-loaded and Zn2 + -free states. Biochemistry 38(6):1695–1704. doi:10.1021/bi982483d
Al-Haddad S, Zhang Z, Leygue E, Snell L, Huang A, Niu Y, Hiller-Hitchcock T, Hole K, Murphy LC, Watson PH (1999) Psoriasin (S100A7) expression and invasive breast cancer. Am J Pathol 155(6):2057–2066
Ji J, Zhao L, Wang X, Zhou C, Ding F, Su L, Zhang C, Mao X, Wu M, Liu Z (2004) Differential expression of S100 gene family in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 130(8):480–486. doi:10.1007/s00432-004-0555-x
Wolf R, Ruzicka T, Yuspa SH (2010) Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids 41(4):789–796. doi:10.1007/s00726-010-0666-4
Tripathi SC, Matta A, Kaur J, Grigull J, Chauhan SS, Thakar A, Shukla NK, Duggal R, DattaGupta S, Ralhan R, Siu KW (2010) Nuclear S100A7 is associated with poor prognosis in head and neck cancer. PLoS One 5(8):e11939. doi:10.1371/journal.pone.0011939
Emberley ED, Alowami S, Snell L, Murphy LC, Watson PH (2004) S100A7 (psoriasin) expression is associated with aggressive features and alteration of Jab1 in ductal carcinoma in situ of the breast. Breast Cancer Res 6(4):R308–R315 10.1186/bcr791
Emberley ED, Niu Y, Njue C, Kliewer EV, Murphy LC, Watson PH (2003) Psoriasin (S100A7) expression is associated with poor outcome in estrogen receptor-negative invasive breast cancer. Clin Cancer Res 9(7):2627–2631
Moog-Lutz C, Bouillet P, Regnier CH, Tomasetto C, Mattei MG, Chenard MP, Anglard P, Rio MC, Basset P (1995) Comparative expression of the psoriasin (S100A7) and S100C genes in breast carcinoma and co-localization to human chromosome 1q21-q22. Int J Cancer 63(2):297–303
Emberley ED, Murphy LC, Watson PH (2004) S100A7 and the progression of breast cancer. Breast Cancer Res 6(4):153–159. doi:10.1186/bcr816
Emberley ED, Niu Y, Leygue E, Tomes L, Gietz RD, Murphy LC, Watson PH (2003) Psoriasin interacts with Jab1 and influences breast cancer progression. Cancer Res 63(8):1954–1961
West NR, Watson PH (2010) S100A7 (psoriasin) is induced by the proinflammatory cytokines oncostatin-M and interleukin-6 in human breast cancer. Oncogene 29(14):2083–2092. doi:10.1038/onc.2009.488
Emberley ED, Niu Y, Curtis L, Troup S, Mandal SK, Myers JN, Gibson SB, Murphy LC, Watson PH (2005) The S100A7-c-Jun activation domain binding protein 1 pathway enhances prosurvival pathways in breast cancer. Cancer Res 65(13):5696–5702. doi:10.1158/0008-5472.CAN-04-3927
Paruchuri V, Prasad A, McHugh K, Bhat HK, Polyak K, Ganju RK (2008) S100A7-downregulation inhibits epidermal growth factor-induced signaling in breast cancer cells and blocks osteoclast formation. PLoS One 3(3):e1741. doi:10.1371/journal.pone.0001741
Deol YS, Nasser MW, Yu L, Zou X, Ganju RK (2011) Tumor-suppressive effects of psoriasin (S100A7) are mediated through the beta-catenin/T cell factor 4 protein pathway in estrogen receptor-positive breast cancer cells. J Biol Chem 286(52):44845–44854. doi:10.1074/jbc.M111.225466
Yu SE, Jang YK (2012) The histone demethylase LSD1 is required for estrogen-dependent S100A7 gene expression in human breast cancer cells. Biochem Biophys Res Commun 427(2):336–342. doi:10.1016/j.bbrc.2012.09.057
Burness ML, Grushko TA, Olopade OI (2010) Epidermal growth factor receptor in triple-negative and basal-like breast cancer: promising clinical target or only a marker? Cancer J 16(1):23–32. doi:10.1097/PPO.0b013e3181d24fc1
Eccles SA (2011) The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol 55(7–9):685–696. doi:10.1387/ijdb.113396se
Ridley AJ (2011) Life at the leading edge. Cell 145(7):1012–1022. doi:10.1016/j.cell.2011.06.010
Donaldson JG, Porat-Shliom N, Cohen LA (2009) Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal 21(1):1–6. doi:10.1016/j.cellsig.2008.06.020
Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G (2009) Coordination of Rho GTPase activities during cell protrusion. Nature 461(7260):99–103. doi:10.1038/nature08242
Nasser MW, Qamri Z, Deol YS, Ravi J, Powell CA, Trikha P, Schwendener RA, Bai XF, Shilo K, Zou X, Leone G, Wolf R, Yuspa SH, Ganju RK (2012) S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res 72(3):604–615. doi:10.1158/0008-5472.CAN-11-0669
Qamri Z, Preet A, Nasser MW, Bass CE, Leone G, Barsky SH, Ganju RK (2009) Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther 8(11):3117–3129. doi:10.1158/1535-7163.MCT-09-0448
Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massague J (2005) Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 115(1):44–55. doi:10.1172/JCI22320
Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I, Bromberg JF (2004) Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA 101(29):10602–10607. doi:10.1073/pnas.0404100101
Lin CC, Tseng HW, Hsieh HL, Lee CW, Wu CY, Cheng CY, Yang CM (2008) Tumor necrosis factor-alpha induces MMP-9 expression via p42/p44 MAPK, JNK, and nuclear factor-kappaB in A549 cells. Toxicol Appl Pharmacol 229(3):386–398. doi:10.1016/j.taap.2008.01.032
Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM (2007) Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell 13(5):646–662. doi:10.1016/j.devcel.2007.08.011
Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393(6687):805–809. doi:10.1038/31729
Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112(4):453–465
Guturi KK, Mandal T, Chatterjee A, Sarkar M, Bhattacharya S, Chatterjee U, Ghosh MK (2012) Mechanism of beta-catenin-mediated transcriptional regulation of epidermal growth factor receptor expression in glycogen synthase kinase 3 beta-inactivated prostate cancer cells. J Biol Chem 287(22):18287–18296. doi:10.1074/jbc.M111.324798
Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N (2000) Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2(4):252–257
Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, Spiegelman VS, Setaluri V, Mukhtar H (2006) S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA 103(40):14825–14830. doi:10.1073/pnas.0606747103
Yong HY, Moon A (2007) Roles of calcium-binding proteins, S100A8 and S100A9, in invasive phenotype of human gastric cancer cells. Arch Pharm Res 30(1):75–81
Yamaguchi H, Condeelis J (2007) Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta 1773(5):642–652. doi:10.1016/j.bbamcr.2006.07.001
Wang W, Eddy R, Condeelis J (2007) The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer 7(6):429–440. doi:10.1038/nrc2148
Acknowledgments
This work was supported in part by grants from National Institutes of Health (CA109527 and CA153490) and Department of Defense to R.K. Ganju. Y.S. Deol was supported by Pelotonia Fellowship from Comprehensive Cancer Center, The Ohio State University. Authors thank Dr. Zahida Qamri, Dr. Nandu Thodi and Mohamed Adel for their help in in vivo BLI imaging. We also thank Viy McGaughy for IHC staining.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Amita Sneh and Yadwinder S. Deol contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sneh, A., Deol, Y.S., Ganju, A. et al. Differential role of psoriasin (S100A7) in estrogen receptor α positive and negative breast cancer cells occur through actin remodeling. Breast Cancer Res Treat 138, 727–739 (2013). https://doi.org/10.1007/s10549-013-2491-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2491-4