Abstract
The role of the tumour microenvironment and complex cellular interactions has attracted interest in responses to primary chemotherapy. Of particular interest are tumour-infiltrating T cells and tumour-infiltrating macrophages (TIMs). We evaluated TIMs and their key activation markers in patients with breast cancer undergoing primary chemotherapy related to response and survival. One hundred and ninety nine patients with large or locally advanced breast cancers received primary chemotherapy. Clinical data, histopathological responses to chemotherapy and survival were examined related to infiltrating cells in tumour microenvironments: cluster of differentiation (CD)3 (pan T cell); CD4 (helper T cells); CD8 (cytotoxic T cells); CD25 (activated T cells); CD68, suppressor of cytokine signalling (SOCS)1, SOCS3 (macrophages); and CD11c and CD205 (dendritic). In tumours demonstrating better responses to chemotherapy, there were significantly fewer CD4+ T-helper cells than a poorer response (p < 0.05). There were increased numbers of SOCS3 expressing macrophages (pro-inflammatory) in tumours with complete pathological responses compared with no response to chemotherapy (p < 0.05). There was no association between SOCS1 expressing macrophages (anti-inflammatory) and tumour response. Multivariate analysis revealed that factors indicating better survival were receiving anthracycline plus docetaxel (ExpB = 1.166; p = 0.006), better pathological chemotherapy response (ExpB = 0.309; p = 0.009) and a low macrophage SOCS1 expression (ExpB = 13.465; p = 0.044). This study highlights the heterogeneity of TIMs and provides further insight into complex interactions within tumours. The results emphasise the importance of characterising activation status of infiltrating macrophages and provides proof of principle for using macrophage SOCS protein expression as a survival predictor. The apparent impact of macrophage subsets on overall survival underlines the therapeutic potential of manipulating macrophage activation in cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary chemotherapy is used in the management of patients with large and locally advanced breast cancers with the intention of down-staging the primary tumour to allow breast conservation surgery [1–5]. Randomized trials have indicated primary chemotherapy and adjuvant chemotherapy are equally efficacious in terms of survival [5–7].
A complete clinical response occurs in up to 25 % of patients with at least a further 50 % having a partial response [3, 5]. When breast tissue is examined histologically after chemotherapy, a complete pathological response usually occurs in less than 20 % of patients’ tumours, although this increases to more than 30 % by adding docetaxel [3, 5, 6, 8]. In many tumours, there is a variable degree of tumour destruction, but the best predictor of survival is a complete histological response [2, 7].
It would be advantageous to predict which patients would benefit most from primary chemotherapy and who might gain the best survival advantage. Previous studies have examined the role of tumour biological characteristics and expression of molecular markers. For example, the absence of oestrogen receptors, HER2 expression, high tumour grade, high proliferation or tumour gene signatures [9–14] may point to the tumours most likely to respond. Oncoproteins, e.g. Bcl-2 and p53, topoisomerase II and TIMP-1 expression may also be important in determining response to chemotherapy [15–18]. Others have evaluated the role of 5 fluoro-deoxyglucose uptake by PET scanning with promising results [19]. However, at present, there is no routinely used marker in this respect.
Further understanding of the tumour microenvironment has attracted increasing interest in terms of responses to chemotherapy and the respective roles of tumour-infiltrating lymphocytes [20, 21] including cluster of differentiation (CD)8+ T cytotoxic cells [21, 22] and CD4+ T regulatory cells [23, 24], and tumour-infiltrating macrophages (TIMs) are the subjected of increasing scrutiny [25]. Some [26, 27], but not all [28], studies suggest that TIMs may be associated with a better prognosis in breast cancer and there are specific reports that the macrophage infiltrate is associated with angiogenesis and possibly an adverse prognosis in breast cancer [29, 30] and Hodgkin’s disease [31]. These cells produce growth factors modulating tumour growth; the other proteins produced cause invasion and neo-angiogenesis leading to metastases.
Infiltrates in the microenvironment may be important in mediating responses to chemotherapy and radiotherapy. A T cell infiltrate was associated with a better response to radiotherapy in patients with cervical cancer and a better response to chemo-radiotherapy in oesophageal cancer [32, 33] and breast cancer [20, 22]. Patients with follicular lymphomas had better survival after chemotherapy when there were macrophages in the tumour [34], and in non-small cell lung cancer [35], increased numbers of macrophages and CD8+ T cells indicated a better prognosis. Patients with ovarian cancer had a prolonged survival after platinum chemotherapy if tumours contained increased T cell infiltrates [36].
In this study, we have evaluated the presence of TIMs and key markers of activation in patients with breast cancer undergoing primary chemotherapy in relationship to (i) the clinical and pathological response of the tumour to chemotherapy and (ii) the overall survival of these patients.
Methods
Patients
Consecutive 199 patients were included and were treated in the Aberdeen Breast Unit between 1997 and 2004. Patients had large breast cancers (>3 cm) or locally advanced breast cancers (defined as T3, T4 or any T stage, but with N2 nodal disease). All patients had invasive carcinoma of the breast without detectable metastatic disease. Complete followup details are available for all patients studied. The project had approval from the North of Scotland Research Ethics Committee (reference: 06/S0801/94) following the national process, and consent from patients was not required as this study was carried out anonymously.
Chemotherapy
This consecutive series of patients was treated with one of three regimens currently in use in the Aberdeen Breast Unit during this period of time. Sixty-two patients were from the Aberdeen Tax 301 study with core biopsy tumour material being available from these patients [4]. The chemotherapy for this study comprised CVAP, six to eight cycles given in three weekly intervals (cyclophosphamide [1,000 mg/m2], doxorubicin [50 mg/m2], vincristine [1.5 mg/m2] all given by intravenously followed by oral prednisolone [40 mg/day] for 5 days) (CVAP), or four cycles of CVAP followed by four cycles of docetaxel (100 mg/m2). A further 137 patients received either anthracycline with docetaxel or anthracycline with cyclophosphamide in the above dosages. The total number of patients receiving anthracycline together with docetaxel was 77 and the number receiving anthracycline-based chemotherapy without docetaxel was 122.
Surgery
Following chemotherapy, the responses of patients were assessed clinically (complete, partial, stasis or progression of disease) by standard UICC criteria. Patients underwent surgical resection of residual tumour with breast conservation or mastectomy. The surgical procedure depended on the residual tumour size (clinically less than 3 cm) and patient preference. In patients where there was no residual mass and undergoing breast conservation, the original tumour site was radiologically localised before surgery. Axillary surgery was performed for all patients (axillary sample was the standard practice).
Pathology of tissue removed at surgery
The residual tumour size was measured macroscopically and histological sections were taken. The histological response to chemotherapy was assessed by a five-point grading system [4, 37] where grade 1 was no response, grade 2—minor response, grade 3—moderate response, grade 4—marked response and grade 5—complete pathological response [4, 37]. This grading system has been shown to predict disease-free interval and overall survival in patients receiving primary chemotherapy for breast cancer [37].
Slides were assessed for intensity of staining—no (0), weak (1), moderate (2) or strong (3)—by two independent observers blinded to the clinicopathological data and any discrepant scores (>90 % agreement) were re-assessed at a multi-header microscope and consensus was achieved.
Preparation of core biopsies of breast cancers prior to primary chemotherapy
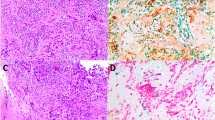
Pre-operative core biopsies were taken prior to primary chemotherapy and re-assessed by a breast pathologist (IDM). The areas of tumour to be sampled were identified and marked on the haematoxylin and eosin slide. Two 1 mm cores were taken from these areas of the wax-embedded tumour blocks (Beecher Instruments Microarrayer) and placed in a recipient paraffin block. The recipient array block was heated to 37 °C, and a glass slide was used to press down the cores to ensure they were at the same level. Microarray blocks were sectioned using a microtome (Leica RM2125) at a thickness of 5 μm, collected onto charged slides (Menzel Glaser Superfrost) and dried at 70 °C for 30 min.
Immunohistochemistry
Antibodies used for characterisation of tumour infiltrate
CD3 (pan T cells); CD4 (helper T cells); CD8 (cytotoxic T cells); CD25 (activated T cells); CD68 (macrophages); suppressor of cytokine signalling (SOCS)1 and SOCS3 (anti- and pro-inflammatory macrophages, respectively); CD11c and CD205 (dendritic cells).
Procedure
Immunohistochemistry for each antibody was carried out using a Dako autostainer (Dako, Ely, UK) as previously described [38, 39]. Sections (5 μm) of tissue microarray were dewaxed, rehydrated and the antigen retrieval step performed when required. The antigen retrieval step consisted of microwaving the sections in 0.01 M citrate buffer at pH 6.0 for 20 min in an 800 W microwave, oven at full power. The sections were allowed to cool to room temperature.
Primary antibody appropriately diluted in antibody diluent (Dako) was applied for 60 min at room temperature, washed with buffer (Dako) followed by peroxidase blocking for 5 min (Dako), followed by a 2 min buffer wash. Pre-diluted peroxidase-polymer labelled goat anti-mouse/rabbit secondary antibody (Envision™, Dako) was applied for 30 min at room temperature, followed by washing with buffer to remove unbound antibody. Sites of peroxidase activity were demonstrated with diaminobenzidine (DAB) as the chromogen applied for three successive 5 min periods. Sections were washed in water, lightly counterstained with haematoxylin, dehydrated and mounted. Omitting the primary antibody from the immunohistochemical procedure and replacing it with non-immune rabbit or mouse immunoglobulin (Dako) acted as negative controls.
Double labelling of macrophages (CD68+) with either SOCS1 or SOCS3 was undertaken by sequential double staining (DakoG2 double stain Envision™ kit [K5361]). The readout for the first antibody (anti-SOCS1 or SOCS3) was DAB chromogen, and following this, staining step slides were blocked with a double staining blocking solution. The second antibody (anti-CD68) was added followed by alkaline phosphatase polymer labelled goat anti-mouse/rabbit secondary antibody (Envision™, Dako). Following incubation and a wash, liquid permanent red chromagen with added levamisole to block endogenous alkaline phosphatase was added for 7 min. Slides were counterstained as before.
Statistics
Statistical analyses were undertaken by means of SPSS for Windows (v17). Univariate and multivariate analyses correlated the presence or absence of cell types with clinical and pathological responses (grades 1–5) [34]. Clinical response was regarded as 'response' (complete or partial response) or 'no response' (stasis or progression). Analysis of variance for non-parametric data with post hoc testing was carried out (SPSS version 17). A p value less than 0.05 was considered to be statistically significant. Kaplan–Meier plots with log-rank testing determined the relationship of the different components of the tumour-infiltrating cells related to survival.
Results
Patients
A total of 199 patients were available for analysis, with demographics in Table 1. The patients’ ages ranged from 22 to 77 years (mean 51 years). 98 (49 %) patients were pre-menopausal and 101 (51 %) were post-menopausal.
Clinical responses to primary chemotherapy
Following completion of chemotherapy, 50 (25 %) patients had complete clinical responses, 99 (50 %) had partial responses, 32 (15 %) had stasis of disease and in the remaining 14 (7 %), there was disease progression. In four patients, there was no clinical documentation of the clinical response. The responses according to treatment are in Table 2, but there was no significant difference.
Pathological responses
In terms of the grade of histological response to primary chemotherapy (34), 53 patients (27 %) had a grade 1 response, 38 patients (19 %)—a grade 2 response, 34 patients (17 %)—a grade 3 response, 46 patients (22 %)—a grade 4 response and 28 patients (15 %)—a grade 5 response, signifying complete destruction. The responses according to whether patients had docetaxel are shown in Table 2, and although the trend was a better pathological response in patients receiving anthracycline and docetaxel, this was not significant. Although the study was not powered to detect differences in survival, Fig. 1 shows a survival advantage in patients receiving anthracycline and docetaxel.
Characterisation of the tumour infiltrate and its relationship to tumour response to primary chemotherapy
The overall analysis of the characteristics of the infiltrate within the core biopsies taken prior to commencement of primary chemotherapy and related to the degree of tumour destruction after completion of primary chemotherapy is expressed graphically in Fig. 2a and b.
Tumour-Infiltrating T cells
Patients whose tumours had a better pathological response to chemotherapy had higher numbers of CD3+ cells and CD8+ cells. This was not statistically significant for individual grades (Fig. 2a) or for 'no response' (grade 1), 'incomplete response' (grades 2, 3 and 4) or for 'complete response' (grade 5) (Table 3).
In tumours demonstrating a better response to chemotherapy, there were significantly fewer CD4+ T-helper cells than in tumours with a poorer response to primary chemotherapy and this was statistically significant (p < 0.05) (Fig. 2a). There was no difference seen in CD25+ (activated) T cell numbers between pathological response groups (Fig. 2a).
Tumour-infiltrating macrophages
A detailed analysis of the relationship between the macrophage infiltration (as determined by cells with CD68 expression) and the characteristics of the tumour and response to chemotherapy was undertaken. The more aggressive the tumour was (as determined by pre-treatment histological grade), the greater the macrophage content.
Mean macrophage infiltrate number for histological tumour grade 1 was 21.5 ± 4.9 sem, tumour grade 2—30.25 ± 3.3 sem, tumour grade 3—42.12 ± 3.7 sem. There was a statistically significant difference of p < 0.05 between grades 1 and 3.
The correlation of macrophage count with pathological grade of response to chemotherapy is shown in Table 3. This shows an increased number of infiltrating CD68+ cells in both incomplete and complete pathological response groups and is statistically significant when compared to the no response group (p < 0.05).
The influence of macrophage activation was assessed by double staining for CD68 and antibodies directed against SOCS1 and SOCS3 because SOCS1 inhibits pro-inflammatory signalling pathways downstream of interferon (IFN)-γ and toll-like receptor 4, whilst SOCS3 inhibits signal transducer and activator of transcription 3 (STAT3) signalling responsible for macrophage anti-inflammatory effects and is highly expressed in classically activated cytotoxic M1 macrophages. There were increased numbers of SOCS3 expressing macrophages in patients whose tumours exhibited a complete pathological response (mean cell number 12.76 ± 2.18 sem) when compared with tumours where there had been no response to chemotherapy (mean cell number 5.13 ± 1.15 sem), p < 0.05.
There was no association between SOCS1 expressing macrophages and tumour response: no response 9.73 ± 1.98 sem, incomplete response 12.21 ± 1.6 sem and complete response 12.76 ± 2.18 sem. This is consistent with the hypothesis of a subset of TIMs with pro-inflammatory properties facilitating early response to chemotherapy.
Dendritic cells
Increased numbers of dendritic cells were seen in the complete response group, but were not significant (Table 3).
Characterisation of tumour infiltrate and relationship to survival in patients receiving primary chemotherapy
Tumour-infiltrating lymphocytes
The relationship of lymphocytic infiltrate with 5-year survival is shown in Fig. 3a–c comparing tumours with a cellular infiltrate in terms of cell numbers per core biopsy above and below the median.
There was a significant increase in 5-year survival in patients whose tumours had the lower levels of CD3+ cells when compared with higher levels (Fig. 2a). The results for CD4+ and CD8+ did not achieve significance (Fig. 3b, c).
Tumour-infiltrating macrophages
The relationship of macrophage infiltrate with 5-year survival is shown in Fig. 3d–f. This is shown as a comparison between patients with greater or less than the median number of cells per core in the pre-treatment diagnostic biopsy. A better long-term outcome was inversely correlated with numbers of infiltrating macrophages; better survival was seen in those with fewer macrophages (Fig. 3d; p = 0.026). Analysis of SOCS protein expression showed that survival was significantly (p < 0.04) poorer with greater numbers of SOCS1 expressing macrophages, but no correlation with SOCS3 macrophages (Fig. 3e, f).
Multivariate analysis for survival
Multivariate analysis was undertaken to identify independent factors for survival (Table 4). This was performed by entering variables into the model significant in univariate analyses: age, tumour grade, axillary lymph node status after completion of chemotherapy, oestrogen receptor status (positive or negative), chemotherapy treatment (anthracycline vs anthracycline plus docetaxel), CD3+, CD68+, SOCS1 cell number (expressed as below vs above median cell counts). Factors independently indicating better survival were use of anthracycline plus docetaxel (ExpB = 1.166; p = 0.006), a better pathological chemotherapy response (ExpB = 0.309; p = 0.009) and a low number of macrophage SOCS1+ macrophages (ExpB = 13.465; p = 0.044).
Discussion
Our analysis of patients treated with primary chemotherapy demonstrates a complex system. First, we show that numbers of infiltrating macrophages correlate with tumour grade in the diagnostic biopsy, response to chemotherapy in the excision biopsy and, in seeming contradiction, with poorer long-term survival. Second, we demonstrate that TIMs are heterogeneous and differentially express the SOCS family proteins that control macrophage activation. Specifically, SOCS3 expression in TIMs (a pro-inflammatory phenotype) correlates with a positive early response to chemotherapy, whereas SOCS1 expression does not. By contrast, SOCS1 expression in TIMs (an anti-inflammatory phenotype) correlates with poor long-term survival, whereas SOCS3 expression does not. These results support the concept that different subsets of TIMs facilitate tumour destruction after chemotherapy or support long-term tumour growth and survival.
The infiltrating macrophages originate from circulating monocytes recruited to tumours by chemokines and factors from necrotic cells and in response to hypoxia [26]. Once localised, monocytes mature into macrophages and respond to tumour microenvironments, developing properties favouring tumour growth and spread [40]. This has been shown in murine models [27, 30] and supported by clinical studies showing that intensity of macrophage infiltrate correlates with poor long-term outcome in cancers [29, 31, 40, 41]. In mice, TIMs express the transcriptomic signature of M2 (or alternatively activated) macrophages and enhance tumour growth and dissemination through multiple pathways [27]. These include growth factors' secretion, increasing angiogenesis and suppressing host anti-tumour immunity [42]. Thus, it is unsurprising that the intensity of macrophage infiltration in our study correlated with long-term patient survival.
In specific circumstances, TIMs facilitate tumour destruction and display properties of M1 (classically activated) macrophages promoting inflammation and tissue damage. Thus, appropriately activated macrophages destroy tumours in murine models [43–45] and large numbers of macrophages correlate with improved response to therapy [35, 41, 46]. This is consistent with our findings that macrophage numbers correlated with response to chemotherapy. Our study is exceptional in demonstrating the divergent outcomes in the same patient cohort—something only possible because of our study design.
Clinical data [47] suggest that different macrophage activation states are responsible for the distinct influence of macrophages in different tumours. Despite this, characterising macrophage activation in vivo presents a challenge. This is not only because of extreme functional plasticity, but also because macrophage infiltrates are heterogeneous, displaying different characteristics. Some panels of markers have been used to characterise M1 destructive and M2 reparative macrophages in vitro and to a limited extent in mice [42], but there is no means of doing so in patient biopsies. Therefore, we chose to correlate macrophage SOCS1 and SOCS3 with prognostic markers because of their well-characterised effects on macrophage pro- and anti-inflammatory functions [48].
SOCS3 inhibits STAT3 signalling downstream of the IL-6 receptor and also indirectly inhibits anti-inflammatory and pro-fibrotic functions of IL-4 and IL-13. In rodents, it is expressed uniquely in macrophages incubated with classical (IFN-γ/LPS) [42] and non-classical [43] M1 activation, and SOCS3 has an indispensable role in maintaining M1 phenotype [44, 49]. Regardless of whether it is an authentic marker of M1 activation, macrophages expressing SOCS3, but not SOCS1, have profoundly suppressed anti-inflammatory properties and enhanced pro-inflammatory properties. By contrast, SOCS1 inhibits pro-inflammatory macrophage properties by suppressing signalling pathways engaged by ligation of IFN-γ and TLR receptors. SOCS1 expression increases both as a direct effect of incubation with IL-4 when it is essential for sustaining the M2 phenotype and also as a negative feedback loop that prevents superactivation after IFN-γ [50]. Consequently, unique expression of SOCS1, but not SOCS3 expression, cannot be used to distinguish M1 from M2 activation, but does indicate a macrophage with attenuated pro-inflammatory properties.
Our analysis of macrophage SOCS3 expression demonstrated heterogeneity and provides some explanation for correlation of intensity of macrophage infiltration with early treatment response and poorer long-term survival. The number of SOCS3 expressing pro-inflammatory and cytotoxic macrophages correlated with early response to therapy, but not with poorer survival. Conversely, greater numbers of SOCS1 expressing anti-inflammatory macrophages correlated with poor long-term survival. Accordingly, SOCS3 expression may identify macrophages with enhanced tumour killing, whereas SOCS1 expressing macrophages may favour tumour survival.
Our study focused primarily on macrophages infiltrating the breast carcinomas and the influence imposed on them by the microenvironmental cues they encountered. The results demonstrate the heterogeneity of the macrophage infiltrate and confirm the suggestion that patient outcomes might reflect this heterogeneity [51]. We have no data on the nature of cues responsible for the macrophage recruitment or differential SOCS protein expression. These could arise from tumour or other stromal cells including the infiltrating lymphocytes and indeed the bi-directional interactions between them [21]. Recent large studies have shown that both total infiltrating lymphocytes [20] and CD8+ cells [51] correlate with better patient outcomes. By contrast, we failed to detect significant differences between total (CD3+) T cells or its CD4+ and CD8+ subsets in our Cox’s proportional hazard analysis. Similarly, there was no correlation with CD25+ lymphocytes, which is consistent with the recent study demonstrating no influence of infiltrating FoxP3 positive T regulatory cells [22]. Evidence against this apparent conflict could be due to the lower numbers of subjects in our study or because we restricted our analysis to intra-tumour lymphocytes—indeed this result agrees with the previous study which also reported that intra-tumour infiltration with CD8 cells did not correlate with patient outcome [22].
In summary, this study highlights TIM heterogeneity and provides insight into complex interactions within the tumour. The results emphasise the importance of characterising activation status of infiltrating macrophages and provide proof of principle for using macrophage SOCS protein expression in breast cancer. Finally, the apparent impact of macrophage subsets on overall survival underlines the therapeutic potential of manipulating macrophage activation in cancer.
Abbreviations
- CD:
-
Cluster of differentiation
- IFN:
-
Interferon
- STAT:
-
Signal transducer and activator of transcription
- SOCS:
-
Suppressor of cytokine signalling
- TIM:
-
Tumour-infiltrating macrophage
References
Heys SD (1991) Evolution of breast cancer management: focus on neoadjuvant chemotherapy. Breast Cancer 8:339–350
Kaufmann M, von Minckwitz G, Bear HD et al (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18:1927–1934
Heys SD, Sarkar T, Hutcheon AW (2005) Primary docetaxel chemotherapy in patients with breast cancer: impact on response and survival. Breast Cancer Res Treat 90:169–185
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21:4165–4174
Bear HD, Anderson S, Smith RE et al (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24:2019–2027
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778–785
Heys SD, Sarkar T, Hutcheon AW (2004) Docetaxel as adjuvant and neoadjuvant treatment for patients with breast cancer. Expert Opin Pharmacother 5:2147–2154
Colleoni M, Bagnardi V, Rotmensz N et al (2010) A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer 46:2216–2224
Colleoni M, Viale G, Zahrieh D et al (2008) Expression of ER, PgR, HER1, HER2, and response: a study of preoperative chemotherapy. Ann Oncol 19:465–472
Miglietta L, Vanella P, Canobbio L et al (2009) Clinical and pathological response to primary chemotherapy in patients with locally advanced breast cancer grouped according to hormonal receptors, Her2 status, grading and Ki-67 proliferation index. Anticancer Res 29:1621–1625
Ring AE, Smith IE, Ashley S et al (2004) Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer 91:2012–2017
Bonnefoi H, Potti A, Delorenzi M et al (2007) Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00-01 clinical trial. Lancet Oncol 8:1071–1078
Straver ME, Glas AM, Hannemann J et al (2010) The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 119:551–558
Sekine I, Shimizu C, Nishio K et al (2009) A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with breast cancer. Int J Clin Oncol 14:112–119
Tewari M, Krishnamurthy A, Shukla HS (2008) Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg Oncol 17:301–311
Martin-Richard M, Munoz M, Albanell J et al (2004) Serial topoisomerase II expression in primary breast cancer and response to neoadjuvant anthracycline-based chemotherapy. Oncology 66:388–394
Schmitt M, Sweep FC (2009) Tissue inhibitor metalloproteinase type-1 (TIMP-1), a novel cancer biomarker predicting response of adjuvant anthracycline-based chemotherapy in patients afflicted with primary breast cancer. Eur J Cancer 45:2444–2446
Smith IC, Welch AE, Hutcheon AW et al (2000) Positron emission tomography using [(18)F]-fluorodeoxy-d-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 18:1676–1688
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1:54–67
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR (2011) An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat 127:99–108
Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 14:2413–2420
Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, Tagliabue E, Balsari A (2009) FOXP3 expression and overall survival in breast cancer. J Clin Oncol 27:1746–1752
de Visser KE, Eichten A, Coussens LM (2006) Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6:24–37
Mantovani A, Allavena P, Sica A et al (2008) Cancer-related inflammation. Nature 454:436–444
Qualls JE, Murray PJ (2011) Tumor macrophages protective and pathogenic roles in cancer development. Curr Top Dev Biol 94:309–328
Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR (2012) Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 65(2):159–163
Tsutsui S, Yasuda K, Suzuki K et al (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14:425–431
Welm AL, Sneddon JB, Taylor C et al (2007) The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci USA 104:7570–7575
Steidl C, Lee T, Shah SP et al (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885
Nakano T, Oka K, Takahashi TM et al (1992) Roles of Langerhans’ cells and T-lymphocytes infiltrating cancer tissues in patients treated by radiation therapy for cervical cancer. Cancer 70:2839–2844
Ashida A, Boku N, Aoyagi K et al (2006) Expression profiling of esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy: clinical implications. Int J Oncol 28:1345–1352
Taskinen M, Karjalainen-Lindsberg ML et al (2007) A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide–doxorubicin–vincristine–prednisone. Clin Cancer Res 13:5784–5789
Kawai O, Ishii G, Kubota K et al (2008) Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113:1387–1395
Nelson BH (2008) The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev 222:101–116
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12:320–327
Coghlin CL, Smith LJ, Bakar S et al (2010) Quantitative analysis of tumor in bronchial biopsy specimens. J Thorac Oncol 5:448–452
Murray GI, Patimalla S, Stewart KN et al (2010) Profiling the expression of cytochrome P450 in breast cancer. Histopathology 57:202–211
Leek RD, Lewis CE, Whitehouse R et al (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56:4625–4629
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896
Wang YC, He F, Feng F et al (2010) Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 70:4840–4849
Kujawski M, Kortylewski M, Lee H et al (2008) Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Investig 118:3367–3377
Herrmann A, Kortylewski M, Kujawski M et al (2010) Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res 70:7455–7464
Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y et al (2003) CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 63:1555–1559
Movahedi K, Laoui D, Gysemans C et al (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6Chigh monocytes. Cancer Res 70:5728–5739
Dalpke A, Heeg K, Bartz H, Baetz A (2007) Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology 213:225–235
Liu Y, Stewart KN, Bishop E, Marek CJ, Kluth DC, Rees AJ, Wilson HM (2008) Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol 180:6270–6278
Nakagawa R, Naka T, Tsutsui H et al (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677–687
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955
Acknowledgements
This work was supported by an award (ONC-AU-061) from the Translational Medicine Research Collaboration—a consortium made up of the Universities of Aberdeen, Dundee, Edinburgh and Glasgow, the four associated NHS Health Boards (Grampian, Tayside, Lothian and Greater Glasgow and Clyde), Scottish Enterprise and Pfizer (formerly Wyeth). Keith N. Stewart and Emma J. McKenzie contributed equally to this work.
Ethical standards
The study was carried out following the ethical standards as applicable to the United Kingdom with permissions as detailed in the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heys, S.D., Stewart, K.N., McKenzie, E.J. et al. Characterisation of tumour-infiltrating macrophages: impact on response and survival in patients receiving primary chemotherapy for breast cancer. Breast Cancer Res Treat 135, 539–548 (2012). https://doi.org/10.1007/s10549-012-2190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2190-6