Abstract
Purpose
Two types of macrophages are present in tumor microenvironment. M1 macrophages exhibit potent anti-tumor properties, while M2 macrophages play the pro-tumoral roles. The presence of M2 macrophages is associated with worsened overall survival in triple-negative breast carcinoma (TNBC) patients. However, the relationship between M2 macrophages and response to neoadjuvant chemotherapy (NAC) is unknown.
Methods
M2 macrophages were investigated on biopsy whole sections from 66 TNBCs treated with NAC by CD163 together with other immune checkpoint markers (PD1, PD-L1 and CD8) using a multi-color immunohistochemical multiplex assay.
Results
Incomplete response was significantly associated with older age, lower PD-L1 expression (tumor and stroma), lower levels of CD8-positive TILs in stroma, but higher level of CD163-positive macrophages, with the level of CD163-positive M2 macrophages in peritumoral area as the strongest factor.
Conclusions
Our data have demonstrated that the level of CD163-positive M2 macrophages was significantly higher in TNBC patients with incomplete response than patients with complete response, suggesting M2 macrophages’ important role in predicting TNBC patients’ response to NAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is an aggressive breast cancer subtype, which is estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative [1]. Neoadjuvant chemotherapy (NAC) has been increasingly given to TNBC patients with improved long-term outcomes in TNBC patients [2, 3]. Response to NAC is commonly evaluated by microscopically examining surgically resected specimen and pathologic complete response (pCR) is defined as no residual invasive carcinoma.
The presence of tumor-infiltrating lymphocytes (TILs) in tumor microenvironment is associated better response to NAC in breast cancer patients including TNBC patients, suggesting that the immune defense system may augment NAC-induced tumor cell death [4,5,6,7,8]. Additionally, the expression of programmed cell death 1 ligand (PD-L1) has been demonstrated to be associated with response to immunotherapy and/or chemotherapy and overall survival in breast cancer patients, especially TNBC patients. [9,10,11,12,13].
Tumor microenvironment also contains tumor-associated macrophages (TAMs), which play important roles in tumor progression [14,15,16,17]. There are two types of TAMs: M1 macrophages are pro-inflammatory and suppress tumor cells, while M2 macrophages are immunosuppressive and promote tumor growth[14, 16]. M2 macrophages are related to hormonal status, stage, lymph node status, and poor survival in breast cancers [18,19,20,21,22,23].
To understand the relationship between M2 macrophages and response to NAC, we undertook an evaluation of M2 macrophages together with other immune markers (CD8+cytotoxic T cells and PD-L1) expression in TNBCs and its association with pCR after NAC.
Patients and methods
Patients, specimens and pathological assessment of the response to neoadjuvant therapy
This study was approved by The Ohio State University institutional research board. Informed consent was obtained from all individual patients included in the study. Sixty-six triple-negative invasive breast carcinoma patients treated with neoadjuvant chemotherapy and follow-up surgical resection (39 lumpectomy specimens and 27 mastectomy specimens) were included in this study. For neoadjuvant chemotherapy, all patients received four cycles of AC (doxorubicin+cyclophosphamide) together with Taxol (paclitaxel or docetaxel).
Surgical resection specimens were carefully examined grossly and microscopically to search any residual tumor. When residual invasive tumor was not detected in breast tissue and metastasis was not detected in lymph node, pCR was rendered. The entire tumor bed(s) was submitted for histological examination for cases with pCR.
Evaluation of M2 macrophage, cytotoxic T cells and PD-L1 expression using multi-color multiplex immunohistochemistry with CD163, CD8 and PD-L1
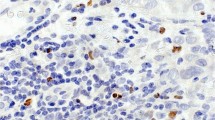
M2 macrophages are usually characterized by CD163 expression [21, 24]. We evaluated M2 macrophages, cytotoxic T cells and PL-L1 expression by applying a multi-color multiplex immunohistochemistry (IHC) with three distinct antibodies including CD163 (clone SP57, rabbit; Ventana Medical Systems, Inc), CD8 (clone MRQ26, mouse; Ventana Medical Systems, Inc,) and PD-L1 (clone SP263, rabbit; Ventana Medical Systems, Inc, Tucson, AZ, USA). IHCs were performed on freshly cut whole sections from pre-NAC biopsy specimens. M2 macrophages and cytotoxic T cells were evaluated by estimating the percentage of tumoral or stromal areas infiltrated by CD163-positive cells and CD8-positive cells, respectively. PD-L1 expression was evaluated by estimating the percentage of tumor cells or stromal (immune) cells with specific membranous PD-L1 staining. Representative images with different combinations of CD163 (red), CD8 (green) and PD-L1 (brown) expression are illustrated in Fig. 1.
Representative images of different immune reaction and PD-L1 expressions in triple-negative breast carcinoma, as detected with anti-PD-L1 multiplex immunohistochemistry (anti-CD8 in green, anti-CD163 in red, and anti-PD-L1 in brown). A, B One PCR case with high PD-L1, high CD8, but low CD163; C, D One incomplete case with no PD-L1, low CD8, but high CD163. A , C: H&E stains, B, D: multiplex immunohistochemistry, (× 200)
Statistical analyses
Statistical analysis was performed using SAS version 9.4 for Windows (SAS Institute, Inc, Cary, NC, USA). Descriptive statistics were used to summarize patient clinical and pathologic characteristics. Categorical data were summarized as frequency and percentage, and continuous variables as medians and ranges. To study the associations with pCR, Fisher’s exact was used for categorical variables. Wilcoxon rank-sum test was used to compare the continuous variables. All the continuous variables have been tested for normality using Kolmogorov–Smirnov test. A multivariable logistic regression model was used to determine the variables associated with the incidence of death as well as recurrence. Variables with a p value < 0.10 in the univariate analysis were entered into a multivariable model. Variables were removed sequentially from the multivariable model using the backward selection method.
Results
Clinicopathologic characteristics of 66 triple-negative breast carcinomas with neoadjuvant chemotherapy and subsequent resection
The clinical and pathologic findings are summarized in Table 1. The median age at diagnosis was 51 years (26–74 years). Sixty-four cases were invasive ductal carcinoma NOS, and the other two cases were metaplastic carcinoma. All cases were Nottingham grade 3 or 2 with an average of 2.85. Fifty-nine cases had negative HER2 IHC, and the other seven cases had equivocal HER2 IHC, but negative HER2 fluorescence in-situ hybridization (FISH). Twenty-eight cases (42.4%) had pCR. Among 38 cases with residual tumor, 16 had lymph nodal metastasis.
Univariate analysis of factors associated with response to NAC in 66 triple-negative breast carcinomas
Twenty-eight cases (42.4%) had complete response (pCR) and 38 (57.6%) had residual tumor. Among 66 cases, PD-L1 expression in tumor cells was identified in 32 (48.5%) cases with at least 1%. PD-L1 expression in stromal immune cells was identified in 35 (53%) cases. CD8-positive T cell infiltrate was identified in tumoral area for 56 (87.9%) cases and in peritumoral stromal area for 64 (97.0%) cases. CD163-positive M2 macrophages were found in tumoral area for 65 (98.5%) cases and in peritumoral area for all cases.
Univariate analysis was performed to examine the associations between the response to NAC and clinicopathologic and immune reaction variables, including age, grades, HER2 IHC, PD-L1, CD8 and CD163. The complete response group showed significantly younger age (47 vs 53.2, p = 0.041); increased PD-L1 expression in both tumor cells and stromal immune cells (tumoral: 7.8% vs 2.6%, p = 0.004; stromal: 7.3% vs 1.9%, p = 0.007); increased CD8-positive cells in both tumoral and stromal areas (tumoral: 11% vs 7.4%, p = 0.004; stromal: 15% vs 8.7%, p = 0.001); but decreased CD163-positive M2 macrophages in both tumoral and stromal areas (tumoral: 22.8% vs 35.9%, p = 0.003; stromal: 24.8% vs 39.2%, p = 0.003) (Table 2).
Multivariable analysis of factors associated with response to NAC in 66 triple-negative breast carcinomas
A multivariable logistic regression model was then performed to analyze the variables with p < 0.10 in the univariate analysis (age, mitosis, PD-L1, CD8 and CD163). Variables with p > 0.05 were removed sequentially from the multivariate model using backward selection method. In this model only increased mitosis (p = 0.031), increased PD-L1 expression in stromal immune cells (p = 0.005) and decreased CD163-positive M2 macrophages in stromal area (p < 0.001) were significantly associated with complete response to NAC (Table 3).
Discussion
Achieving pCR is associated with lower risk of progression and death, and better long-term survival outcomes in TNBC patients [25,26,27]. Tumor microenvironment components, such as TILs and PD-L1 expression on immune cells, play important roles in breast cancer patients’ survival and the response to chemotherapy [4,5,6, 12, 13]. M2 macrophages, another important component within tumor microenvironment, promote tumor growth and are associated with poor survival in breast cancer patients, especially in TNBC patients [14, 16, 18,19,20,21,22,23]. CD163 is a well-known specific marker for M2 macrophages. TNBCs usually show more CD163-positive TAMs than other subtypes of breast cancers [28]. Previous studies have demonstrated CD163 expression is correlated with poor prognosis in TNBC patients [19,20,21, 28, 29]. However, studies of M2 macrophages’ effect on the response to NAC in breast cancer patients are lacking. To our knowledge, this is the first study to investigate the relationship between M2 macrophages and the response to NAC in TNBCs. Our data have demonstrated that the presence of M2 macrophages, especially in stromal area, negatively affects the response to NAC in TNBCs.
In addition to M2 macrophages, several other factors including age, mitosis, CD8-positive T cells and PD-L1 expression were found to correlate with the response to NAC in our cohort. Univariate analysis identified younger age, increased CD8-positive TILs and increased PD-L1 expression as positive predictors for pCR, while multivariable analysis identified increased mitosis and increased PD-L1 expression in stromal immune cells as positive predictors for pCR. As mentioned above, previous studies have demonstrated a positive association between TILs and pCR. The failure to identified CD8-positive TILs as a significant predictor in multivariable analysis is probably caused by the small size of our study cohort, although the average percentage of CD8-positive cells in pCR group is greater than that in incomplete response group without statistically significant difference. The relationship between PD-L1 expression and cancer prognosis is controversial. PD-L1 expression is expected to be associated with a poorer prognosis in cancer patients due to its immune evasion mechanism to suppress the immune response to tumor cells. However, previous studies have demonstrated a positive association between PD-L1 expression and favorable prognosis. [10, 30, 31] However, this may be caused by the simple association of PD-L1 expression with increased TILs during anti-tumor immune response, rather than an association with tumor immune evasion in this setting. The positive association between PD-L1 expression and pCR found in current study may be simply explained as such.
Our study has several limitations. The greatest one is perhaps its small cohort size. Based on the findings from this relatively small cohort, we could not establish the positive relationship between CD8-positive T cells and pCR in multivariable analysis with statistical significance, although the univariate analysis showed a significant correlation. However, we were able to identify the presence of CD163-positive M2 macrophages as a negative predictor for pCR. The retrospective nature of the study and lack of long-term follow-up outcome are other limitations.
In summary, we have demonstrated a negative correlation between CD163-positive M2 macrophages and the response to NAC in TNBC patients, suggesting M2 macrophages’ important role in predicting the response to NAC and/or potential target for developing future therapy.
References
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13(11):674–690
Darb-Esfahani S, Loibl S, Müller BM et al (2009) Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res 11(5):R69
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281
Ono M, Tsuda H, Shimizu C et al (2012) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 132(3):793–805
Issa-Nummer Y, Darb-Esfahani S, Loibl S et al (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE 8(12):e79775
Denkert C, Loibl S, Noske A et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113
Yamaguchi R, Tanaka M, Yano A et al (2012) Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 43(10):1688–1694
Seo A, Lee H, Kim E et al (2013) Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 109(10):2705–2713
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121
Wimberly H, Brown JR, Schalper K et al (2015) PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res 3(4):326–332
Cortes J, Cescon DW, Rugo HS et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet (Lond, Engl) 396(10265):1817–1828
Mittendorf EA, Zhang H, Barrios CH et al (2020) Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (Lond, Engl) 396(10257):1090–1100
Hou Y, Nitta H, Wei L, Banks PM, Parwani AV, Li Z (2018) Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer: the association with response to anti-HER2 neoadjuvant therapy. Clin Breast Cancer 18(2):e237–e244
Liguori M, Solinas G, Germano G, Mantovani A, Allavena P (2011) Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers 3(4):3740–3761
Allavena P, Mantovani A (2012) Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol 167(2):195–205
Quatromoni JG, Eruslanov E (2012) Tumor-associated macrophages: Function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res 4(4):376–389
Hu W, Li X, Zhang C, Yang Y, Jiang J, Wu C (2016) Tumor-associated macrophages in cancers. Clin Transl Oncol 18(3):251–258
Yang M, Li Z, Ren M et al (2018) Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer 9(13):2308–2316
Ni C, Yang L, Xu Q et al (2019) CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: a retrospective study and meta-analysis. J Cancer 10(19):4463–4472
Zhao X, Qu J, Sun Y et al (2017) Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget 8(18):30576–30586
Tiainen S, Tumelius R, Rilla K et al (2015) High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 66(6):873–883
Klingen TA, Chen Y, Aas H, Wik E, Akslen LA (2017) Tumor-associated macrophages are strongly related to vascular invasion, non-luminal subtypes, and interval breast cancer. Hum Pathol 69:72–80
Jamiyan T, Kuroda H, Yamaguchi R, Abe A, Hayashi M (2020) CD68- and CD163-positive tumor-associated macrophages in triple negative cancer of the breast. Virchows Arch 477(6):767–775
Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch’ng ES (2019) Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol 9:1512
Huang M, O’Shaughnessy J, Zhao J et al (2020) Association of pathologic complete response with long-term survival outcomes in triple-negative breast cancer: a meta-analysis. Can Res 80(24):5427–5434
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (Lond, Engl) 384(9938):164–172
Von Minckwitz G, Untch M, Blohmer J-U et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804
Medrek C, Pontén F, Jirström K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306
Tang X (2013) Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett 332(1):3–10
Bae SB, Cho HD, Oh M-H et al (2016) Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer 19(3):242
Velcheti V, Schalper KA, Carvajal DE et al (2014) Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 94(1):107–116
Funding
None.
Author information
Authors and Affiliations
Contributions
Study design: All. Data collection: VA, TS, HN, AVP, ZL. Data analysis: VA, TS, HN, ZL. Statistical oversight: LW. Manuscript preparation: VA, TS, HN, ZL. Manuscript revision: VA, TS, HN, AVP, ZL. Manuscript approval: All.
Corresponding author
Ethics declarations
Conflict of interest
V. Arole, T. Shen, L. Wei, A. Parwani and Z. Li have no financial relationship to disclose. H. Nitta is an employee of Roche Tissue Diagnostics.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arole, V., Nitta, H., Wei, L. et al. M2 tumor-associated macrophages play important role in predicting response to neoadjuvant chemotherapy in triple-negative breast carcinoma. Breast Cancer Res Treat 188, 37–42 (2021). https://doi.org/10.1007/s10549-021-06260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06260-1