Abstract
The Generations trial, a multicenter, placebo-controlled, double-blind trial, compared arzoxifene 20 mg/day and placebo in 9,354 postmenopausal women with osteoporosis (N = 5,252) or low bone mass (N = 4,102). Primary outcomes were vertebral fracture in the osteoporotic population and invasive breast cancer in all study participants. Here, we report the detailed breast cancer findings from the trial. Breast cancers were detected by annual mammograms and clinical examination. After 48 months follow-up, breast cancer incidence was compared between treatment groups by estrogen receptor (ER) and progesterone receptor (PR) status and baseline risk factors. Baseline breast cancer risk factors, including age, estimated Gail risk, and bone mineral density, were well balanced between treatment groups. A total of 75 breast cancers occurred 53 in the placebo group and 22 in the arzoxifene group (HR 0.41, 95 % CI 0.25–0.68, P < 0.001). There were 62 invasive breast cancers, 39 identified as invasive ER-positive (placebo 30, arzoxifene 9; HR 0.30, 95 % CI 0.14–0.63, P = 0.001) and 30 identified as invasive PR-positive (placebo 23, arzoxifene 7; HR 0.30, 95 % CI 0.13–0.71, P = 0.003). Breast cancer risk reduction with arzoxifene was similar between Gail risk groups (P interaction = 0.31) and between low bone mass and osteoporosis groups (P interaction = 0.35). Although generally well tolerated, there was a significant increase in venous thromboembolism, vasomotor symptoms, muscle cramps, and some gynecological events with arzoxifene. These findings demonstrate that in this study arzoxifene reduced the risk of ER-positive breast cancer in this population of postmenopausal women with low bone mass or osteoporosis, an effect similar to that seen with other SERMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is now good clinical evidence that some selective estrogen receptor modulators (SERMs) can substantially reduce the incidence of breast cancer, a disease which is widespread and for which prevention strategies are much needed. By virtue of their mechanism of action, however, SERMS also have effects on various other tissues. Trials have demonstrated tamoxifen efficacy in preventing breast cancer in healthy pre- and post-menopausal women together with favorable effects in reducing serum cholesterol and bone loss in postmenopausal women [1–4]. These trials have also shown unfavorable effects of tamoxifen including endometrial thickening, atypia and cancer, uterine fibromyomas, cataracts, venous thromboembolism (VTE), and a nonsignificant increase in stroke. Furthermore, there was no observed reduction in incidence of coronary events or of non vertebral fractures with tamoxifen.
The 4-year Multiple Outcomes of Raloxifene Evaluation (MORE) trial followed the tamoxifen trials, and was designed to evaluate the benzothiophene SERM, raloxifene, as a treatment to reduce fracture risk in postmenopausal women with osteoporosis [5]. The results clearly showed a reduction in vertebral fracture risk; while there was an increase in VTE risk, there was no increase in the risk of endometrial hyperplasia or stroke. In the MORE trial, there was also a significant reduction in the incidence of estrogen receptor (ER) positive invasive breast cancer [6], an effect that persisted in the Continuing Outcomes Relevant to Evista (CORE) trial, a follow up to MORE with invasive breast cancer as the primary outcome [7]. In the head-to-head NSABP-P2 trial (Study of Tamoxifen and Raloxifene, STAR), the primary analysis in 2006 indicated that the risk reduction of breast cancer by raloxifene was the same as tamoxifen, but raloxifene use was associated with a lower risk of gynecological toxicity, cataracts, and VTE; the risk of other cancers, fractures, ischemic heart disease, and stroke were similar for both drugs [8]. Based on findings from these studies, raloxifene has been approved in the United States and Mexico for invasive breast cancer risk reduction in postmenopausal women with osteoporosis or at increased risk for invasive breast cancer.
Since then, more potent SERMs, such as lasofoxifene, bazedoxifene, and arzoxifene, have been developed and evaluated for vertebral and non vertebral fracture risk reduction. Lasofoxifene has shown to reduce the risks of vertebral fractures, non vertebral fractures, ER-positive breast cancer, and stroke with no increase in risk of endometrial cancer [9, 10]. Bazedoxifene has been demonstrated to reduce the risk of vertebral fracture, with no evidence of breast or endometrial stimulation [11, 12].
In preclinical studies, arzoxifene was reported to be effective for the prevention and treatment of mammary tumors [13], and to have a endometrial safety profile similar to raloxifene [14, 15]. In phase 2 studies of women with advanced breast cancer [16, 17], tumor responses were reported with both doses studied (20 mg and 50 mg/day), with a higher response rate with the lower dose; both doses were well tolerated. Development of arzoxifene for the treatment of invasive breast cancer was stopped when it was shown that arzoxifene was not superior to tamoxifen in the treatment of locally advanced and metastatic disease [18]. However, in pre-surgical studies in pre and postmenopausal women with newly diagnosed ductal carcinoma in situ (DCIS) and Stage I invasive cancer, arzoxifene, given for an average of 1 month between initial biopsy and subsequent surgical excision, resulted in a favorable modulation of serum and tissue markers of cancer risk [19]. Together, these data supported the hypothesis that arzoxifene may reduce the risk of invasive breast cancer in postmenopausal women.
The placebo-controlled Generations trial was designed to assess the effect of arzoxifene on the incidences of vertebral fracture and invasive breast cancer as well as safety endpoints in postmenopausal women with osteoporosis or low bone mass. The primary results have been recently reported [20] to show a significant reduction in vertebral fracture but not in nonvertebral fracture incidence, together with a significant reduction in invasive breast cancer incidence. The previous report included detailed safety data for arzoxifene in the Generations trial.
In this paper, we report the detailed breast cancer findings from the Generations trial.
Methods
The Generations trial (Study H4Z-MC-GJAD) was a Phase 3, multicenter, double-blind, placebo-controlled, randomized study of postmenopausal women with osteoporosis or low bone mass (osteopenia) conducted at more than 200 sites in 22 countries. The protocol was approved by the ethics review board at each investigative site. All participants gave written informed consent for participation in accordance with the principles of the declaration of Helsinki. The study methodology has been described in detail previously [20].
Briefly, eligible women were at least 2 years postmenopausal and 60–85 years of age, inclusive. Women were enrolled with osteoporosis (femoral neck or lumbar spine T-score ≤ −2.5, with or without a prevalent vertebral fracture) or low bone mass (osteopenia) (femoral neck or lumbar spine T-score between > −2.5 and ≤1.0 with both skeletal sites > −2.5 and no prevalent vertebral fracture). Study exclusion criteria have been described previously and included a history of breast cancer, VTE or stroke.
Between July 2004 and May 2005, 9,354 postmenopausal women were randomly assigned to receive either oral arzoxifene HCl 20 mg or placebo for up to 60 months. Randomization was performed using a block size of 4 controlled by a computerized interactive voice response system. Treatments were identical in appearance. All participants were also provided with daily supplements containing ~500 mg of elemental calcium and 400–600 IU of vitamin D.
Women were permanently discontinued from the study if they did not meet enrolment criteria (e.g., inadvertently enrolled). Women were discontinued from study drug, but not study participation, if they experienced clinical events that included invasive breast cancer, DCIS, or lobular carcinoma in situ (LCIS), or if they used estrogen-containing products or SERMs. Use of topical vaginal estrogens was allowed at the lowest possible doses and for a total duration of 3 months or less.
The Generations trial was a 5-year study. Primary database lock occurred when the last enrolled participant had the opportunity to complete the 4-year mammograms when approximately 60 invasive breast cancer cases were expected.
Breast cancer assessment
Bilateral mammograms and breast examinations were performed at baseline screening for the study and yearly thereafter, as well as at early termination if >6 months had passed since the previous mammogram. Women who had a mammogram performed within 6 months before baseline screening, with no abnormalities detected did not require further mammography before study entry. Mammograms were considered abnormal if written report suggested that follow-up imaging procedures were required, a lesion requiring sampling was identified, or if the investigator deemed findings to be clinically significant for any other reason. Initial assessment of mammograms was performed locally. If the local reading diagnosed a potential incident breast cancer (invasive, DCIS, or LCIS), all related data were collected and adjudicated. External adjudication was performed by members of an independent clinical events committee that was blinded to treatment group assignments to confirm all cases of breast cancer (both invasive and noninvasive) and atypical ductal hyperplasia. The adjudicated diagnosis of breast cancer was to be based on central review of pertinent clinical and diagnostic information, including original mammogram films, related radiology reports, reports from any additional studies (e.g., ultrasound), local pathology and surgical reports, clinical information related to cancer staging, and treatment reports. Tissue blocks from the tumor or microscopic slides representing the primary diagnosis of the tumor were sent to the central laboratory for histological diagnosis and for estrogen receptor/progesterone receptor (ER/PR) status.
Statistical analysis
The two primary outcomes were new vertebral fractures at 36 months and invasive breast cancer when all participants had the opportunity to complete 48 months of follow up. This report is confined to breast cancer outcomes in all participants at 48 months.
Comparisons of baseline characteristics between treatment groups were performed by one-way analysis of variance for continuous variables. For categorical variables, a Pearson’s χ2 test was used if the total number of women within each category was ten or more, or a Fisher’s exact test otherwise.
The primary analysis of invasive breast cancer efficacy was assessed using data available at the primary database lock at 48 months. Analyses were based on the intention-to-treat principle. Time-to-event analysis methods were used and treatment group differences on the incidence of breast cancer were assessed with a log rank test at a significance level of 0.04. Hazard ratios (HRs) and 95 % confidence intervals (CIs) were calculated from a cox proportional hazards regression model. Counts and proportions of subjects with an invasive breast cancer were also provided. Subgroup analyses were conducted for subgroups of interest, including Gail score [21], if the number of outcomes was at least 50. Treatment by subgroup interactions were tested at the liberal 0.10 significance level. No adjustments were made for multiple comparisons. Statistical analyses were performed with the use of SAS software, version 8.2 (update version at later date) (SAS Institute), and all data were analyzed by Eli Lilly and Company. With a two-sided alpha of 0.04, the trial sample size provided 80 % power to detect a 55 % relative reduction in risk of invasive breast cancer.
Results
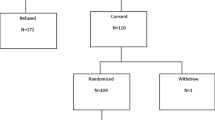
Of the 16,948 postmenopausal women screened, 9,354 women were eligible and consented to randomization (4,678 to placebo and 4,676 to arzoxifene). A total of 5,252 had osteoporosis and 4,102 had a low bone mass. Overall, 78 % completed the study (Fig. 1). Both treatment groups were evenly balanced for characteristics associated with risk of breast cancer including age, previous benign breast biopsies, family history of breast cancer, estimated Gail risk, and BMD (Table 1).
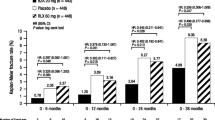
At the time of primary database lock (48 months), a total of 75 breast cancers had occurred and been adjudicated (placebo 53, arzoxifene 22, HR 0.41, 95 % CI 0.25–0.68, P < 0.001) (Table 2, Fig. 2). There were 62 invasive breast cancers (placebo 43, arzoxifene 19; HR 0.44, 95 % CI 0.26–0.76, P = 0.002) (Table 2, Fig. 2). Of those invasive breast cancers with known receptor status, 39 were invasive ER-positive (placebo 30, arzoxifene 9; HR 0.30, 95 % CI 0.14–0.63, P = 0.001) and 30 were invasive PR-positive (placebo 23, arzoxifene; HR 0.30, 95 % CI 0.13–0.71, P = 0.003) (Table 2, Fig. 2). Only 13 noninvasive cancers occurred during the 48 month follow-up (placebo 10, arzoxifene 3, HR 0.30, 95 % CI 0.08–0.1.09, P = 0.053) (Table 2).
The incidence of invasive breast cancer in the placebo group was higher in the women who had a Gail score ≤1.66 compared to >1.66 (1.6 per 1,000 woman-years vs. 3.8 per 1,000 woman-years, P = 0.004), but similar in those with low bone mass versus osteoporosis (2.8 per 1,000 woman-years vs. 1.8 per 1,000 woman-years, P = 0.13) and in those aged ≤65 years versus >65 years (2.2 per 1,000 woman-years vs. 2.4 per 1,000 woman-years, P = 0.79) (Table 3). However, the risk reduction with arzoxifene was similar in both Gail risk groups (HR 0.32 vs. 0.57, P value for interaction = 0.31), in the low bone mass and osteoporosis groups (HR 0.34 vs. 0.57, P value for interaction = 0.35) and in both age groups (HR 0.38 vs 0.49, P value for interaction = 0.67) (Table 3).
The histological features of the 75 breast cancers are summarized in Table 4. There were no significant differences in mean tumor size or stage or in incidence of lymph node involvement between treatment groups.
Discussion
The primary objectives of the Generations trial were to evaluate the effectiveness of arzoxifene 20 mg/day in preventing vertebral fractures in post menopausal women with osteoporosis and preventing invasive breast cancer in postmenopausal women with osteoporosis or with low bone density. We have previously reported an overall 56 % reduction in invasive breast cancer incidence (P = 0.002) [20], and here we report the details of the breast cancer outcomes. There were a total of 75 breast cancers (invasive and non invasive) identified and adjudicated, with a 59 % reduction in overall breast cancer incidence with arzoxifene compared to placebo. Of the invasive breast cancers, 39 were identified as invasive ER-positive and 30 as invasive PR-positive, with a 70 % risk reduction for both subgroups in women randomized to arzoxifene compared to placebo (P = 0.001 and 0.003). There was a low incidence of non invasive cancer in the trial with no indication of any increase with arzoxifene.
In the previous report of the main outcomes [20], it was noted that arzoxifene was generally well tolerated. Endometrial carcinoma occurred in 9 (0.2 %) women randomized to arzoxifene versus 4 (0.1 %) in the placebo group P = 0.165). There was an increase in VTE in women randomized to arzoxifene (1.3 vs. 0.6 %, P < 0.001). There was no difference in incidence of serious adverse events between the two treatment groups. There was a small absolute but significant increase in cholecystitis and chronic obstructive respiratory disease with arzoxifene. There was no difference in all cause mortality (P = 0.62).
These results are in keeping with the reported breast cancer risk reductions with other SERMs. A meta-analysis of all tamoxifen trials indicated a risk reduction of 38 % for all breast cancers, and of 48 % for ER-positive cancers [22]; a recent further analysis of the NSABP P2 study has reported a lessening of the raloxifene risk reduction of breast cancer by raloxifene compared to tamoxifen [23]. While lasofoxifene has been shown to reduce risk of invasive breast cancer [9, 10], the effect of bazedoxifene on invasive breast cancer has not been specifically studied.
It has previously been reported that breast cancer incidence in postmenopausal women increases with increasing bone mass, in keeping with the higher estrogen levels in women with higher bone mass [6]. In the Generations trial, there was a numerically (statistically nonsignificant) higher incidence of breast cancer in the low bone mass group compared to the osteoporotic group in women randomized to placebo. While arzoxifene appeared to have a significant effect on breast cancer incidence in women with low bone mass but not those with osteoporosis, the interaction P value for a treatment effect was not significant for this subgroup analysis.
In the Generation trial, in women randomized to placebo there was a statistically significantly higher incidence of invasive breast cancer for the subgroup of women with a Gail score >1.66 % at 5 years compared to those with a Gail score ≤1.66. While arzoxifene appeared to have a significant effect on breast cancer incidence in women at higher risk of invasive breast cancer (defined by Gail score) compared with the lower risk subgroup, the interaction P value for a treatment effect was not significant for this subgroup analysis. The invasive breast cancer risk for the group of women aged ≤65 years was similar to that in the group of women aged >65 years (placebo group comparison), and the effect of arzoxifene on invasive breast cancer risk did not differ between the two subgroups. These results are similar to that reported for tamoxifen and raloxifene and indicate that the magnitude of this benefit is independent of the level of risk.
The histological features of the 22 cancers which occurred in the arzoxifene group were similar in size and axillary node status to the 53 cancers in the placebo group indicating that use of arzoxifene did not facilitate nor compromise the radiological or clinical diagnosis of breast cancer.
The breast cancer findings of the Generations trial indicate that the risk reduction of breast cancer with arzoxifene is similar to that for other SERMs. Consequently, the usefulness of this drug for this purpose, should it have continued in clinical development, would have depended upon the spectrum of other benefits as well as the safety profile compared to other breast cancer risk reducing agents. Treatment with arzoxifene resulted in a significant 42 % reduction in vertebral fracture incidence, but no significant reduction in the incidence of non vertebral fracture, coronary events or stroke at 4 years, with no increase in overall serious adverse events. Arzoxifene was generally well tolerated but there was an increase in some gynecological-related adverse events, VTE, vasomotor symptoms, and muscle cramps [20]. For tamoxifen, the main risks associated with its use are an increased risk of endometrial atypia and cancer, VTE, vasomotor symptoms, and cataracts. Raloxifene does not have any adverse effects on the endometrium, but like other SERMs in clinical use, does increase the risk of VTE and vasomotor symptoms. Lasofoxifene 0.5 mg/day has been reported to reduce the incidence of vertebral fractures at 3–5 years, and the incidence of non vertebral fractures, and ER positive breast cancer at 5 years in postmenopausal women with osteoporosis; a lower dose of 0.25 mg/day was less effective and was associated with an unexplained increase in mortality compared to placebo [9]. There was no evidence that lasofoxifene increased the risk of major coronary events or stroke assessed as safety endpoints, with significantly fewer of these events reported in the lasofoxifene 0.5 mg/day versus placebo at 5 years.
The Generation trial has some limitations. The duration of follow up was short with no data to predict the overall long term risks and benefits. Two of the tamoxifen trials [24, 25] had longer follow up extending out to nearly 20 years, and in these studies, tamoxifen showed a continuing post treatment breast cancer risk reduction with toxicity confined for the most part to the treatment period. However, there is little information about the optimal duration of treatment with SERMs for breast cancer and fracture risk reduction or about the duration of the post treatment risk reduction benefits for outcomes in bone, breast, and blood vessels. A further limitation is that this study did not include an active control so direct comparison with other agents for breast cancer or fracture risk reduction is not possible. At the time that, the study was designed and enrolled there was no active control that was approved for both treatment of osteoporosis and for invasive breast cancer risk reduction.
In conclusion, the results from the Generations trial provide further evidence that SERMs can substantially reduce the risk of invasive ER-positive breast cancer in postmenopausal women. Unfortunately, although arzoxifene achieved the primary endpoint of invasive breast cancer risk reduction in this study, its anti-fracture efficacy compared with currently available osteoporosis treatments was not considered to be sufficient to justify further clinical development of the drug.
References
Fisher B, Costantino JP, Wickerham DL et al (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Veronesi U, Maisonneuve P, Costa A et al (1998) Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 352:93–97
Powles T, Eeles R, Ashley S et al (1998) Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352:98–101
Cuzick J, Forbes J, Edwards R et al (2002) First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360:817–824
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Cummings SR, Eckert S, Krueger KA et al (1999) The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. [see comment] [erratum appears in JAMA 1999;282(22):2124]. JAMA 281:2189–2197
Martino S, Costantino J, McNabb M et al (2004) The role of selective estrogen receptor modulators in the prevention of breast cancer: comparison of the clinical trials. Oncologist 9:116–125
Vogel VG, Costantino JP, Wickerham DL et al (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. JAMA 295:2727–2741
Cummings SR, Ensrud K, Delmas PD et al (2010) Lasofoxifene for postmenopausal women with osteoporosis. N Engl J Med 362:686–696
La Croix A, Powles TJ, Osborn CK et al (2010) Breast cancer incidence in the PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst 102:1706–1715
Silverman SL, Christiansen C, Genant HK et al (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 23:1923–1934
Archer DF, Pinkerton JV, Utian WH et al (2009) Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 16:1109–1115
Suh N, Glasebrook AL, Palkowitz AD et al (2001) Arzoxifene, a new selective estrogen receptor modulator for chemoprevention of experimental breast cancer. Cancer Res 61:8412–8415
Palkowitz AD, Glasebrook AL, Thrasher KJ, Hauser KL et al (1998) Discovery and synthesis of [6-hydroxy-3-[4-[2-(1-piperidinyl)ethoxy]phenoxy]-2-(4-hydroxyphenyl)]benzothiophene: a novel, highly potent, selective estrogen receptor modulator. J Med Chem 40:1407–1416
Sato M, Turner CH, Wang T, Adrian MD et al (1998) LY353381.HCl: a novel raloxifene analog with improved SERM potency and efficacy in vivo. J Pharmacol Exp Ther 287:1–7
Buzdar A, O’Shaughnessy JA, Booser DJ et al (2003) Phase II, randomized, double-blind study of two dose levels of arzoxifene in patients with locally advanced or metastatic breast cancer. J Clin Oncol 21:1007–1014
Baselga J, Llombart-Cussac A, Bellet M et al (2003) Randomized, double-blind, multicenter trial comparing two doses of arzoxifene (LY353381) in hormone-sensitive advanced or metastatic breast cancer patients. Ann Oncol 14:1383–1390
Deshmane V, Krishnamurthy S, Melemed AS, Peterson P, Buzdar AU (2007) Phase III double-blind trial of arzoxifene compared with tamoxifen for locally advanced or metastatic breast cancer. J Clin Oncol 25:4967–4973
Fabian CJ, Kimler BF, Anderson J et al (2004) Breast cancer chemoprevention phase I evaluation of biomarker modulation by arzoxifene, a third generation selective estrogen receptor modulator. Clin Cancer Res 10:5403–5417
Cummings SR, McClung M, Reginster JY et al (2011) Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J Bone Miner Res 26:397–404
Gail MH, Brinton LA, Byar DP et al (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81:1879–1886
Cuzick J, Powles T, Veronesi U et al (2003) Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300
Vogel VG, Costantino JP, Wickerham DL et al (2010) Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res 3:696–706
Cuzick J, Forbes JF, Sestak I et al (2007) Long-term results of tamoxifen prophylaxis for breast cancer–96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst 99:272–282
Powles TJ, Ashley S, Tidy A et al (2007) Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99:283–290
Acknowledgments
This trial was sponsored by Eli Lilly and Company. The trial is registered at Clinicaltrial.gov number NCT00088010.
Disclosures
Powles, Wickerham, and Cummings have served in a consultant/advisory role for Eli Lilly and Company; Diem has received research funding from Eli Lilly and Company; Cox, Muram, Agnusdei, Dowsett and Amewou-Atisso are stockholders and full time employees at Eli Lilly and Company.
Conflict of interest
Conflict of interest have been declared in the attached manuscript. The study discussed in this paper was sponsored by Eli Lilly and Company
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Powles, T.J., Diem, S.J., Fabian, C.J. et al. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast Cancer Res Treat 134, 299–306 (2012). https://doi.org/10.1007/s10549-012-2041-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2041-5