Abstract
Male breast cancer remains understudied despite evidence of rising incidence. Using a co-ordinated multi-centre approach, we present the first large scale biomarker study to define and compare hormone receptor profiles and survival between male and female invasive breast cancer. We defined and compared hormone receptor profiles and survival between 251 male and 263 female breast cancers matched for grade, age, and lymph node status. Tissue microarrays were immunostained for ERα, ERβ1, -2, -5, PR, PRA, PRB and AR, augmented by HER2, CK5/6, 14, 18 and 19 to assist typing. Hierarchical clustering determined differential nature of influences between genders. Luminal A was the most common phenotype in both sexes. Luminal B and HER2 were not seen in males. Basal phenotype was infrequent in both. No differences in overall survival at 5 or 10 years were observed between genders. Notably, AR-positive luminal A male breast cancer had improved overall survival over female breast cancer at 5 (P = 0.01, HR = 0.39, 95% CI = 0.26–0.87) but not 10 years (P = 0.29, HR = 0.75, 95% CI = 0.46–1.26) and both 5 (P = 0.04, HR = 0.37, 95% CI = 0.07–0.97) and 10 years (P = 0.04, HR = 0.43, 95% CI = 0.12–0.97) in the unselected group. Hierarchical clustering revealed common clusters between genders including total PR–PRA–PRB and ERβ1/2 clusters. A striking feature was the occurrence of ERα on distinct clusters between genders. In female breast cancer, ERα clustered with PR and its isoforms; in male breast cancer, ERα clustered with ERβ isoforms and AR. Our data supports the hypothesis that breast cancer is biologically different in males and females suggesting implications for clinical management. With the incidence of male breast cancer increasing this provides impetus for further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to figures from Cancer Research UK, there were 45,695 cases of female breast cancer (FBC) and 277 cases of male breast cancer (MBC) diagnosed in the UK in 2007 [1]. In the US it was estimated that 1,970 men and 207,090 women would be diagnosed with breast cancer in 2010 [2]. Whilst MBC accounts for less than 1% of breast cancer diagnoses worldwide, the overall improvements in survival and mortality observed in FBC has not been seen to the same extent in MBC, as demonstrated in a recent interrogation of the Surveillance Epidemiology and End Results (SEER) database [3]. Moreover, the incidence rate of MBC is rising steadily [4–7].
The etiology of MBC is poorly understood with most of our current knowledge regarding its biology, natural history, and treatment extrapolated from FBC. Retrospective studies are generally weakened by the small numbers of cases available from any one centre with studies published on as few as 15 cases [8], making it hard to draw biologically meaningful conclusions. It is, therefore, a challenge to accrue sufficiently large numbers to allow comparative analysis of possible prognostic or predictive biomarkers. Many articles imply a general similarity of MBC to FBC and this has resulted in MBC patients being treated in exactly the same way as females in the clinic, which may not be optimal. Survival rates for MBC are generally assumed to be lower than FBC, probably as a result of later diagnosis and the assumption that treatments which are proven in FBC through clinical trials will have the same impact in men [9].
A 40-year review of records of 759 cases from invasive MBC from the US Armed Forces Institute of Pathology database showed that the frequency of histological subtypes in men was comparable to that of FBC, with the exception of papillary carcinoma which was twice more common in MBC [10]. To date, modern molecular subtyping has been reported in a single study of MBC where only luminal A (35/42) and luminal B (7/42) subtypes were observed [11].
Using a co-ordinated multi-centre approach, the aim of this study was to conduct the first large scale study to address and compare the expression profile of hormone receptors and their effect on survival in FBC and MBC.
Methods
Patient cohorts
Following ethical approval from the Leeds (West) Research Ethics Committee (06/Q1205/156), 514 formalin-fixed paraffin-embedded blocks of male (251) and matched female (263) breast cancers were obtained retrospectively. The latter were all from Europe and the former from Europe (n = 196) and Canada (n = 55). Informed consent was not required as the anonymised material pre-dated September 2006, came from a Tissue Bank approved by the UK Human Tissue Authority (or equivalent) or were from non-UK patients. Patients had not received any therapy before surgery. Details on adjuvant therapy were not extensively available; where available this was predominantly endocrine therapy (tamoxifen). Patient characteristics are presented in Table 1. Cases were reviewed by specialised breast consultant histopathologists (AMH, AMS, RAB) to confirm histology and marked up for assembly into tissue microarrays (TMAs) using 3 × 0.6 mm tissue cores per case taken from formalin-fixed paraffin-embedded material as previously described [10].
Immunohistochemistry (IHC)
Antibodies, dilution, and retrieval methods are listed in Table 1. The antibody panel was focused on hormone receptors oestrogen receptor (ER)α, ERβ isoforms, progesterone receptor (PR) isoforms and androgen receptor (AR) and additional biomarkers selected to distinguish molecular subtypes of breast cancer (CK5/6, 14, 18, HER2). Each marker was run as a batch with appropriate positive (tissue known to express the biomarker of interest) and negative (no primary antibody) controls. Scoring was overseen by AMH, AMS, and RAB. Following visualisation of the signal with 3-3′diaminobenzidine chromogen, TMAs were digitised (Aperio Technologies), and hormone receptor immunoreactivity was scored using the Allred system with the following cut offs: ERα > 2, ERβ (and isoforms) > 3, AR > 2, PR (and isoforms) > 2, as validated in previous studies [12–14].
Hierachical clustering and principal components analysis (PCA)
For hierarchical cluster analysis, IHC measurements were used as inputs for all cases in each of the male and female cohorts. A Euclidian distance measure was employed with complete linkage of clusters. Clustering was conducted for the data structure of cases with the immunohistochemical parameters and for the immunohistochemical parameters as they were expressed through the population. Cluster dendrograms were plotted for both of these analyses for each gender and compared. PCA was applied to the same dataset. Analysis was based on covariances between parameters and cases in the data. Variances were computed based on the sum of squares/n − 1. Plots of the influences of variables in the factor plane of the first and second and the second and third principal components for both the male and female cases were plotted separately. As with the hierarchical clustering, both the male and female cases were combined into a single data set. The influence of variables in the factor plane of the combined cases were plotted for the first and second and the second and third principal components. The distributions of cases within the combined sets were also plotted.
Statistical analysis
Patient and disease characteristics were compared between male and FBCs using the χ 2 test (GraphPad). Associations with disease-free and overall survival (DFS and OS, respectively) were analysed by Kaplan–Meier plots and log rank test. P-values were two-sided, and P < 0.05 was considered significant.
Results
A total of 514 cases of breast carcinoma were studied, including 251 males and 263 females. The median age for the male cohort was 66 years (range 30–94) and 59 years (range 27–92) for females. Patient characteristics are shown in Table 2. As this was a matched cohort, no significant differences were observed in grade, or lymph node status between genders. Significant differences were observed in the distribution of histopathological subtypes (P < 0.0001). There was an even distribution of ductal phenotype whilst lobular carcinomas found in 9% of the female cohort was only seen in a single male case. Papillary and mucinous phenotypes were restricted to males. A significantly higher proportion of males expressed ERα compared to females (80 and 68%, respectively), although no differences in the frequency of PR was observed (71 and 72%, respectively). Follow up data was available on 183 (73%) male and 237 (90%) female cases.
Both cohorts were classified into molecular subtypes by IHC: luminal A (ERα+, and/or PR+, HER2−), luminal B (ERα+, and/or PR+, HER2+), HER2 (ERα, PR−, HER2+) and basal-like (ERα−, PR−, HER2−, CK5/6+) according to previous studies [15–17]. Representative immunoprofiles for each subgroup are shown in Fig. 1. Significant differences were observed between molecular subtypes (P = 0.0004). Luminal A was seen in 98% of males and 90% of females. Luminal B or HER2 subgroups were not observed in males but found in 6 and 2% of females, respectively. Basal-like tumours (ERα−, PR−, HER2−, CK5/6+) were infrequent in both cohorts (2% in each).
Semi-serial sections from male (a, c) and female (b, d) breast carcinoma TMAs showing immunoprofiles for each molecular subgroup. a luminal A (ERα+, PR+, HER2−), b luminal B (ERα+, PR+, HER2+), c basal (ERα−, PR−, HER2−, CK5/6+), d HER2 (ERα−, PR−, HER2+). Original magnification = ×10 (TMA core) and ×40 (insets)
We then examined the frequencies of expression between genders of other hormone receptors including AR, nuclear and cytoplasmic ERβ1 and ERβ2, nuclear ERβ5, plus the PR isoforms A and B (Table 3). AR immunoreactivity was expressed in 64% of males and 93% females, respectively, (P < 0.0001). For ERβ1 and -2, both nuclear and cytoplasmic immunoreactivity was assessed [12]. ERβ1 nuclear immunoreactivity was significantly expressed in FBC whilst cytoplasmic ERβ1 and ERβ2 immunoreactivity were associated with MBC. No associations were observed for ERβ5. Of the PR isoforms, only PRA was significantly expressed in MBC. As the male cohort contained cases of European and Canadian origin, we tested if there were differences between these; none were found.
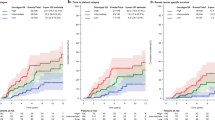
In luminal A carcinomas, no differences in overall survival were observed between genders at either 5 or 10 years (Fig. 2a, b). This was also reflected in the unselected cohorts (data not shown). When hormone receptor expression was considered, only AR significantly associated with survival. AR-positive luminal A MBC had significantly improved overall survival over the equivalent FBC at 5 (P = 0.01, HR = 0.39, 95% CI = 0.26–0.87) but not 10 (P = 0.29, HR = 0.75, 95% CI = 0.46–1.26) years (Fig. 2c, d). In the unselected group, ERα and AR-positive MBC had significantly improved overall survival over ERα and AR-negative cases at both 5 (P = 0.04, HR = 0.37, 95% CI = 0.07–0.97) and 10 (P = 0.04, HR = 0.43, 95% CI = 0.12–0.97) years (Fig. 2e, f) with ERα and AR-positive MBC also having significantly improved overall survival over the equivalent FBCs at 5 (P = 0.05, HR = 0.48, 95% CI = 0.29–1.00) but not 10 (P = 0.37, HR = 0.79, 95% CI = 0.48–1.32) years (Fig. 2g, h).
Kaplan–Meier survival curves comparing survival between genders according to different tumour classifications. No gender-related differences were observed comparing OS of MBC (blue line) and FBC (pink line) at 5 (a) and 10 (b) years. Comparison of OS of Luminal A AR-positive MBC (blue line) and FBC (pink line) showed MBC had significantly improved OS at 5 (c) but not 10 (d) years. In unselected ERα-positive MBC those which were AR positive (black line) had significantly improved over those who were AR negative (orange line) at both 5 (e) and 10 (f) years. Comparison of OS of unselected ERα and AR-positive carcinomas showed MBC (blue line) had significantly improved survival over FBC (pink line) at 5 (g) but not 10 (h) years
Hierarchical clustering based on hormone receptor profiles classified MBC and FBC into three distinct groups (Fig. 3). The cytoplasmic ERβ cluster was common to both genders. In FBC an ERα/PR cluster was observed, grouping ERα and PR isoforms, whilst ERβ isoforms clustered with AR (ERβ/AR cluster). In MBC, there were striking changes in the position of ERα; AR and ERα clustered with ERβ (ERα/β AR cluster) whilst PR isoforms formed an independent cluster (PR cluster). This was also reflected in a PCA-based plot of variable factor co-ordinates (data not shown).
Discussion
Currently, MBC is treated based on the assumption that it is essentially the same disease as FBC. In this the largest comparative study to date, directly comparing the immunohistochemical profile of matched MBCs and FBCs has revealed that whilst superficially there is similarity between genders, when probed more deeply, subtle differences are uncovered.
The histological breakdown of our cohort is in line with previous reports with the papillary phenotype and variants thereof, which are twice more common in males, only seen in the male cohort [10]. Although we did not observe any mucinous carcinomas in our female cohort the expected frequency of this phenotype is only 0.9% [18]. We observed only a single case of lobular carcinoma in males; this is to be expected given the rarity of this phenotype in men [19]. Thus, our cohort can be regarded as representative.
We used IHC to classify our breast tumours into molecular subtypes. Whilst gene array analysis is still considered the ‘gold standard’, we were unable to apply this to our 514-case cohort due to high cost. Nevertheless, molecular profiling of breast cancer based on immunohistochemical typing has now gained widespread acceptance as a surrogate method and is arguably more robust as it overcomes the limitations of gene array in that it directly identifies the cells expressing the marker of interest [15–17, 20]. When applied to TMAs, a limitation is core loss, which we experienced in this study and which may have impacted on the higher than anticipated levels of luminal A phenotype in the female population. Nevertheless, luminal A phenotype was the most common in both sexes with basal-like tumours infrequent in both. Sporadic expression of basal cytokeratins has been previously reported in MBC [21]. Interestingly, the luminal B phenotype was not seen in males. This contrasts a recent study of 42 MBCs where luminal B was seen in 17% of cases [11]. The lack of luminal B carcinomas in our male cohort reflects the absence of HER2 expression, which has been variably reported in MBC ranging from 0 to 95% [22]. It is notable that some of the earlier studies on HER2 relied solely on IHC to determine positivity, considering any degree of membrane immunoreactivity positive [8, 22–28]. The validity of studies relying on HER2 IHC without recognition of gene expression is questionable. According to ASCO/ACP and NEQAS guidelines only those scoring 3+ or above are considered HER2 positive. Equivocal cases are scored 2+ and go forward for FISH analysis and only those with HER2 gene amplification are considered positive. In studies using both IHC and FISH to detect HER2, protein expression was always higher than gene amplification [29–31]. Although we relied on IHC to detect HER2, we are confident our data is robust; we are a regional HER2 testing centre and our HER2 IHC was conducted via this service using two different antibodies and running the test according to clinical standards. Whilst we did not observe any cases which scored >2+ in our MBC cohort, we observed scores of 1+ in 22/251 cases (8%). These would be considered negative in clinical practice. Nevertheless, this provides confidence that the high frequency of HER2 negativity we observed was not simply due to antigen degradation in archival material.
It has been suggested that separation of luminal A and luminal B breast tumours should be based on the expression of proliferation markers such as Ki67, not on HER2 expression as is currently the case [32]. However, this has yet to gain widespread acceptance and we believe there are several issues that still need to be standardised before this can be implemented. These include choice of Ki67 antibody, e.g. MIB1 or SP6 [33] and how to optimally distinguish between low and high proliferation scores. Once these issues are resolved it will be interesting to determine if the difference between MBC and FBC in this series is purely the result of lack of HER2 expression, or if MBCs have a lower proliferative index as well. Contrary to the general impression, one of the most significant findings from this, the most authoritative study to date, was the observation of no differences in overall survival at 5 or 10 years between genders in either our unselected or the luminal A cohorts. Whilst there have been a number of case–control and population-based studies addressing survival in MBC using data from cancer registries [3, 4, 34, 35], direct comparative studies between genders are scarce. A Chinese study of 35 MBC and 70 matched FBC showed the latter had significantly better overall survival at 5 and 10 years, but when the comparison was restricted to female postmenopausal, outcomes were similar [36]. Despite a small number of cases in the male arm and unbalanced cohort size, a Japanese study of 14 MBC and 140 FBC showed no difference in overall survival [37]. This was also reflected in a UK study comparing outcome in 41 MBC and 123 FBC which showed that when matched for key prognostic factors (size, grade and lymph node status), outcome was similar between genders [38], agreeing with our study. Of note was the observation that when MBC was compared with an unselected FBC group, males had worse outcome [38], which may explain some of the earlier studies inferring a worse prognosis in men [39, 40]. Gender comparative information obtained from 1988 to 2003 SEER data showed worse breast cancer-specific survival in males diagnosed with stage I disease; however, the authors attributed this to in-stage migration rather than being of clinical relevance [41].
A common finding in MBC is the higher frequency of hormone receptor expression in particular ERα (reviewed in [42]), which was also reflected in this study. We have explored this further using hierarchical clustering where one of the striking features was the occurrence of ERα on distinct clusters in males and females. In FBC, ERα clustered with PR and its isoforms; as PR is oestrogen-regulated [43], this is unsurprising. In MBC, the position of ERα changed, clustering instead with ERβ isoforms and AR. The potential role of ERβ in breast cancer has been the subject of much debate and recent work in FBC by us and others shows this depends on the cell location and the type of isoforms expressed [9, 44]. However, ERβ is subject to complex regulation involving 3′UTRs [45] and microRNAs [46], and we need to further understand its biology before speculating on any role it may play in MBC.
Regarding the potential relationship of AR with ERα, at a functional level, AR transfection into ERα-positive breast cancer cells inhibited ERα transactivation and oestrogen-stimulated growth through interaction with oestrogen response elements [47]. Other work indicates that oestrogen activation via ERα and ERβ can mediate AR signalling [48]. Given the recognised pro-proliferative effects of ERα and PR and the anti-proliferative effects of ERβ and AR [49] this suggests the coordinated expression of these receptors could influence survival. This was demonstrated in the current study where AR-positive luminal A MBC had improved overall survival than the equivalent FBC and was also borne out in the unselected group. This supports other recent work showing AR is an important prognostic factor in ERα-positive FBC [12]. Previous studies examining the impact of AR in MBC have been compounded by the high expression frequency of AR in some studies [22], the low number of cases in others [50], or combinations of both [51, 52] precluding meaningful survival analysis. Studies examining the effect of AR on survival in MBC are contradictory, possibly as a result of limited numbers available from single centre studies. In a series of 43 MBC Kwiatowska et al. [53] showed AR expression correlated with reduced survival. A similar-sized study (n = 47) showed no effects of AR expression in MBC on survival, however, it is noteworthy that in the study, MIB-1 expression, which detects proliferating cells, was higher in AR negative compared to AR-positive cases [26]. The association of AR positivity with better outcome in MBC in this study indicates this is a potentially important prognostic factor, paralleling observations in FBC where the potential prognostic role for AR in FBC is receiving increased attention [54] following the observation that AR is an independent prognostic marker in a large series of FBCs (n = 953; [12]). Moreover, AR expression in MBC could turn out to have both prognostic and predictive value; its presence suggests that anti-androgen therapy could be explored as a therapeutic approach. Androgen blockade is commonly used in prostate cancer treatment but so far remains inadequately tested in breast cancer [55]. The complete absence of cell line models derived from MBCs presents a challenge in being able to model this in vitro.
To our knowledge, this is the largest retrospective biomarker study directly comparing matched male and female breast carcinoma; however, we acknowledge there are some limitations. Despite our best efforts, these include lack of availability of follow up data and missing/incomplete clinicopathological data. Of note is the absence of data on germline mutations, particularly BRCA2 which is involved in MBC development and associated with reduced survival [53, 56, 57]. Nevertheless, our study has confirmed that whilst superficially similar to FBC, when studied more rigorously MBC is biologically different, echoing a hypothesis proposed by the Multidisciplinary Meeting on MBC [58] and supporting recent work at the transcriptional [59], microRNA [60, 61] and genomic [62] levels. With the incidence of MBC rising [4–7], collectively these studies provide a strong impetus for further study of this rare cancer type.
References
Breast cancer-survival statistics. Cancer Research UK Web Site. http://info.cancerresearchuk.org/cancerstats/. Accessed 26 Jan 2011
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60:277–300
Anderson WF, Jatoi I, Tse J, Rosenberg PS (2010) Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 28:232–239
Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN (2004) Breast carcinoma in men: a population-based study. Cancer 101:51–57
Hodgson NC, Button JH, Franceschi D, Moffat FL, Livingstone AS (2004) Male breast cancer: is the incidence increasing? Ann Surg Oncol 11:751–755
Speirs V, Shaaban AM (2009) The rising incidence of male breast cancer. Breast Cancer Res Treat 115:429–430
White J, Kearins O, Dodwell D, Horgan K, Hanby AM, Speirs V (2011) Male breast carcinoma: increased awareness needed. Breast Cancer Res 13:219
Clark JL, Nguyen PL, Jaszcz WB, Jatoi A, Niehans GA (2000) Prognostic variables in male breast cancer. Am Surg 66:502–511
Fentiman IS, Fourquet A, Hortobagyi GN (2006) Male breast cancer. Lancet 367:595–604
Burga AM, Fadare O, Lininger RA, Tavassoli FA (2006) Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch 449:507–512
Ge Y, Sneige N, Eltorky MA, Wang Z, Lin E, Gong Y, Guo M (2009) Immunohistochemical characterization of subtypes of male breast carcinoma. Breast Cancer Res 11:R28
Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V (2008) Nuclear and cytoplasmic expression of ERβ1, ERβ2, and ERβ5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res 14:5228–5235
Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, Adamson R, Rhodes T, Miller K, Walker R (2000) Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol 53:634–635
Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G, Sapino A (2010) Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat 124:607–617
Callagy G, Cattaneo E, Daigo Y, Happerfield L, Bobrow LG, Pharoah PD, Caldas C (2003) Molecular classification of breast carcinomas using tissue microarrays. Diagn Mol Pathol 12:27–34
Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JF, Macmillan D, Blamey RW, Ellis IO (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116:340–350
Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkilä P, Heikkinen T, Nevanlinna H, Akslen LA, Bégin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, García-Closas M, Caldas C, Pharoah PD, Huntsman D (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 Studies. PLoS Med 7:e1000279
Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW (1992) Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 20:479–489
Michaels BM, Nunn CR, Roses DF (1994) Lobular carcinoma of the male breast. Surgery 115:402–405
O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC (2010) Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 16:6100–6110
Ciocca V, Bombonati A, Gatalica Z, Di Pasquale M, Milos A, Ruiz-Orrico A, Dreher D, Folch N, Monzon F, Santeusanio G, Perou CM, Bernard PS, Palazzo JP (2006) Cytokeratin profiles of male breast cancers. Histopathology 49:365–370
Rayson D, Erlichman C, Suman VJ, Roche PC, Wold LE, Ingle JN, Donohue JH (1998) Molecular markers in male breast carcinoma. Cancer 83:1947–1955
Blin N, Kardas I, Welter C, Ryś J, Niezabitowski A, Limon J, Seitz G (1993) Expression of the c-erbB2 proto-oncogene in male breast carcinoma: lack of prognostic significance. Oncology 50:401–408
Avisar E, McParland E, Dicostanzo D, Axelrod D (2006) Prognostic factors in node-negative male breast cancer. Clin Breast Cancer 7:331–335
Shpitz B, Bomstein Y, Sternberg A, Klein E, Liverant S, Groisman G, Bernheim J (2000) Angiogenesis, p53, and c-erbB-2 immunoreactivity and clinicopathological features in male breast cancer. J Surg Oncol 75:252–257
Pich A, Margaria E, Chiusa L, Candelaresi G, Dal Canton O (1999) Androgen receptor expression in male breast carcinoma: lack of clinicopathological association. Br J Cancer 79:959–964
Bruce DM, Heys SD, Payne S, Miller ID, Eremin O (1996) Male breast cancer: clinico-pathological features, immunocytochemical characteristics and prognosis. Eur J Surg Oncol 22:42–46
Dawson PJ, Paine TM, Wolman SR (1992) Immunocytochemical characterization of male breast cancer. Mod Pathol 5:621–625
Bloom KJ, Govil H, Gattuso P, Reddy V, Francescatti D (2001) Status of HER-2 in male and female breast carcinoma. Am J Surg 182:389–392
Fonseca RR, Tomas AR, Andre S, Soares J (2006) Evaluation of ERBB2 gene status and chromosome 17 anomalies in male breast cancer. Am J Surg Pathol 30:1292–1298
Rudlowski C, Friedrichs N, Faridi A, Füzesi L, Moll R, Bastert G, Rath W, Büttner R (2004) Her-2/neu gene amplification and protein expression in primary male breast cancer. Breast Cancer Res Treat 84:215–223
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Zabaglo L, Salter J, Anderson H, Quinn E, Hills M, Detre S, A’Hern R, Dowsett M (2010) Comparative validation of the SP6 antibody to Ki67 in breast cancer. J Clin Pathol 63:800–804
Anderson WF, Althuis MD, Brinton LA, Devesa SS (2004) Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat 83:77–86
Adami HO, Hakulinen T, Ewertz M, Tretli S, Holmberg L, Karjalainen S (1989) The survival pattern in male breast cancer. An analysis of 1429 patients from the Nordic countries. Cancer 64:1177–1182
Xia LP, Zhou FF, Guo GF, Wang F, Wang X, Yuan ZY, Zhang B (2010) Chinese female breast cancer patients show a better overall survival than their male counterparts. Chin Med J (Engl) 123:2347–2352
Anan K, Mitsuyama S, Nishihara K, Abe Y, Iwashita T, Ihara T, Tamae K, Ono M, Toyoshima S (2004) Breast cancer in Japanese men: does sex affect prognosis? Breast Cancer 11:180–186
Willsher PC, Leach IH, Ellis IO, Bourke JB, Blamey RW, Robertson JF (1997) A comparison outcome of male breast cancer with female breast cancer. Am J Surg 173:185–188
Ouriel K, Lotze MT, Hinshaw JR (1984) Prognostic factors of carcinoma of the male breast. Surg Gynecol Obstet 159:373–376
Spence RA, MacKenzie G, Anderson JR, Lyons AR, Bell M (1985) Long-term survival following cancer of the male breast in Northern Ireland. A report of 81 cases. Cancer 55:648–652
Gnerlich JL, Deshpande AD, Jeffe DB, Selam S, Kimbuende E, Margenthaler JA (2010) Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol 18:1837–1844
Nahleh Z, Girnius S (2006) Male breast cancer: a gender issue. Nat Clin Pract Oncol 3:428–437
Weigel MT, Dowsett M (2010) Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 17:R245–R262
Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G (2008) Clinical importance of estrogen receptor-β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol 26:3727–3734
Smith L, Coleman LJ, Cummings M, Satheesha S, Shaw SO, Speirs V, Hughes TA (2010) Expression of oestrogen receptor β isoforms is regulated by transcriptional and post-transcriptional mechanisms. Biochem J 429:283–290
Al-Nakhle H, Burns PA, Cummings M, Hanby AM, Hughes TA, Satheesha S, Shaaban AM, Smith L, Speirs V (2010) Estrogen receptor β1 expression is regulated by miR-92 in breast cancer. Cancer Res 70:4778–4784
Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD (2009) Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res 69:6131–6140
Narita D, Anghel A, Cimpean AM, Izvernariu D, Cireap N, Ilina R, Ursoniu S (2010) Interaction between estrogens and androgen receptor genes microsatellites, prostate-specific antigen and androgen receptor expressions in breast cancer. Neoplasma 57:198–206
Conzen SD (2008) Minireview: nuclear receptors and breast cancer. Mol Endocrinol 22:2215–2228
Munoz de Toro MM, Maffini MV, Kass L, Kass L, Luque EH (1998) Proliferative activity and steroid hormone receptor status in male breast carcinoma. J Steroid Biochem Mol Biol 67:333–339
Murphy CE, Carder PJ, Lansdown MR, Speirs V (2006) Steroid hormone receptor expression in male breast cancer. Eur J Surg Oncol 32:44–47
Sasano H, Kimura M, Shizawa S, Kimura N, Nagura H (1996) Aromatase and steroid receptors in gynecomastia and male breast carcinoma: an immunohistochemical study. J Clin Endocrinol Metab 81:3063–3067
Kwiatkowska E, Teresiak M, Filas V, Karczewska A, Breborowicz D, Mackiewicz A (2003) BRCA2 mutations and androgen receptor expression as independent predictors of outcome of male breast cancer patients. Clin Cancer Res 9:4452–4459
Higgins MJ, Wolff AC (2010) The androgen receptor in breast cancer: learning from the past. Breast Cancer Res Treat 124:619–621
Folkerd EJ, Dowsett M (2010) Influence of sex hormones on cancer progression. J Clin Oncol 28:4038–4044
Thorlacius S, Olafsdottir G, Tryggvadottir L, Neuhausen S, Jonasson JG, Tavtigian SV, Tulinius H, Ogmundsdottir HM, Eyfjörd JE (1996) A single BRCA2 mutation in male and female breast cancer families from Iceland with varied cancer phenotypes. Nat Genet 13:117–119
Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL (2011) Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat 126:771–778
Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, Gelmon K, Nahleh Z, Bergh J, Cutuli B, Pruneri G, McCaskill-Stevens W, Gralow J, Hortobagyi G, Cardoso F (2010) Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 28:2114–2122
Callari M, Cappelletti V, De Cecco L, Musella V, Miodini P, Veneroni S, Gariboldi M, Pierotti MA, Daidone MG (2011) Gene expression analysis reveals a different transcriptomic landscape in female and male breast cancer. Breast Cancer Res Treat 127:601–610
Fassan M, Baffa R, Palazzo JP, Lloyd J, Crosariol M, Liu CG, Volinia S, Alder H, Rugge M, Croce CM, Rosenberg A (2009) MicroRNA expression profiling of male breast cancer. Breast Cancer Res 11:R58
Lehmann U, Streichert T, Otto B, Albat C, Hasemeier B, Christgen H, Schipper E, Hille U, Kreipe HH, Länger F (2010) Identification of differentially expressed microRNAs in human male breast cancer. BMC Cancer 10:109
Orr N, Cooke R, Jones M, Fletcher O, Dudbridge F, Chilcott-Burns S, Tomczyk K, Broderick P, Houlston R, Ashworth A, Swerdlow A (2011) Genetic variants at chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 influence the risk of breast cancer in men. Plos Genet 7:e1002290
Acknowledgments
Thanks to the Tayside Tissue Bank for kindly providing some of the MBC cases. This study was supported by the Breast Cancer Campaign (UK Charity no. 05074725).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaaban, A.M., Ball, G.R., Brannan, R.A. et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat 133, 949–958 (2012). https://doi.org/10.1007/s10549-011-1856-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1856-9