Abstract
Cytokines are low molecular weight regulatory proteins or glycoprotein that modulates the intensity and duration of immune response by stimulating or inhibiting the activation, proliferation, and/or differentiation of target cells. Different cytokines are known to have diverse role in breast cancer initiation and progression. Interleukin-10 (IL-10), a pleiotropic anti-inflammatory cytokine, induces immunosuppression and assists in escape from tumor immune surveillance. Like several other cytokines, IL-10 also can exert dual proliferative and inhibitory effect on breast tumor cells indicating a complex role of IL-10 in breast cancer initiation and progression. In this review, we tried to put together a comprehensive current view on significance of IL-10 in promotion, inhibition, and importance as prognosticator in breast cancer based on in vitro, in vivo, and clinical evidences. For literature collection, we conducted PubMed search with keywords “IL-10” and “breast cancer”.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer among women, comprising about one-fourth of all female cancers worldwide [1, 2]. It is well-recognized that the functional status of immune system has direct bearing on breast cancer. But, the exact biological mechanism involved in breast cancer pathophysiology is still not clearly understood [3]. Numerous reports suggest that modulation of the innate and adaptive immune response through B cells, T cells, macrophages, dendritic cells (DC), natural killer (NK) cells, and other mediators is critical in initiation and progression of breast cancer. Role of immune system and inflammation in breast cancer has been extensively reviewed elsewhere [4, 5]. Cancer immunotherapy employs a variety of options, including enhancement of stimulatory signals required for T cell activation, genetically engineered cells to secrete cytokines to enhance the intensity of anti-tumor immune response, and direct exogenous therapeutic use of cytokines. Immunoregulatory cytokines are an important component of biological milieu associated with breast cancer [6]. Several cytokines including Interferon (IFN)-α, β, and γ; Interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α) are known to play important role in coordinated manner in breast carcinogenesis [7, 8].
IL-10, initially known as cytokine synthesis inhibitory factor, is primarily a potent anti-inflammatory cytokine that inhibits gene expression and T cell/macrophage cytokine synthesis and inhibits their antigen-presenting capacity [9]. It suppresses production of IL-1α, IL-1β, TNF-α, IL-6, IL-8, IL-12, IL-18, granulocyte–macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein-1α (MIP-1α), RANTES (Regulated upon activation, normal T cell expressed, and secreted), leukemia inhibiting factor, and IL-10 itself [9]. IL-10 also inhibits IFN-γ synthesis by activated Th-cells and peripheral blood mononuclear cells (PBMC) and induces mast cell proliferation [10]. IL-10 is also a strong stimulator of B cell differentiation for immunoglobulin secretion [9]. IL-10 inhibits nuclear factor-κB (NF-κB) translocation considered as a mechanism for inhibiting immediate-early pro-inflammatory response [11].
IL-10 is located on chromosome 1 and mature human IL-10 consists of 160 amino acids with molecular weight of approximately 18 kDa in monomeric form. The homodimeric protein with single transmembrane domain subunits binds to class II cytokine receptor [12]. Human IL-10 contains four exons which show 73% amino acid homology with murine IL-10 [10]. IL-10 structure, its receptor along with their role in physiological functions and pathological conditions like inflammatory diseases have been widely reviewed elsewhere [13–20].
IL-10 production and signaling mechanism
IL-10 is an acid-labile cytokine produced by almost all leukocytes [21], including T cells, Ly+1 B cells, monocytes, macrophages, and keratinocytes [10]. Major sources in vivo are mainly monocytes, macrophages and Th cells; but it is also secreted by DC, B cells, cytotoxic T cells, γδ-T cells, NK-cells, mast cells, as well as neutrophilic and eosinophilic granulocytes [20]. IL-10 secretion in defined situation is dependent on the kind of stimulus, type of affected tissue, and time point in an immune process [22].
IL-10 was originally described as a cytokine produced by Th2 cells, but later on it became evident that IL-10 is also produced by Th1 cells [23]. IL-10 activity is mediated by its specific cell surface receptor complex, which is expressed on a variety of cells, particularly in immune cells [19]. The IL-10/IL-10R interaction activates tyrosine kinases, Jak1 and Tyk2, which are associated with IL-10R1 and IL-10R2, respectively [15]. These kinases are responsible for the phosphorylation of tyrosine residues within the intracellular domain of IL-10R1 which serve as docking sites for Signal Transducer and Activator of Transcription (STAT) molecules [24]. The receptor engagement and tyrosine phosphorylation activates the cytoplasmically localized inactive transcription factors STAT-1, STAT-3, and STAT-5, resulting in translocation and gene activation [25]. IL-10R has a strong preference for STAT-3 and cause transcriptional induction via gene regulatory elements [26]. IL-10 rapidly activates STAT-3 and it remains phosphorylated over a sustained period. This is in contrast to IL-6 mediated STAT-3 activation which is transient [27].
IL-10 expression is regulated by the balance of STAT-3 and SOCS3 (Suppressor of Cytokine Signaling-3). In a recent study, STAT-3 silencing found to reduce IL-10 expression significantly, while SOCS3 silencing induced [28]. Recently, NRDG2 (N-myc downstream regulated gene 2) expression has been shown to modulate SOCS3 and STAT-3 activity, eventually leading to inhibition of IL-10 production [29]. Signaling mechanism particularly MAP-kinase pathways involved in anti-inflammatory action of IL-10 is reviewed by Haddad et al. [30]. Although our knowledge about IL-10 production and IL-10 mediated signaling is expanding, still many aspects remain unexplained.
IL-10 is also secreted by tumor cells [31]. Numerous human cancer cell lines have been shown to secrete IL-10 into their supernatant [32]. The level of expression was more in tumor and breast cancer cell co-culture environment [33]. Breast tumor cells express high level of IL-10 mRNA [34]. However, source of increased serum level of IL-10 in cancer patient is mostly tumor infiltrating suppressor macrophages instead of tumor cells. [35].
Effects of IL-10 on immune cells and cytokines production
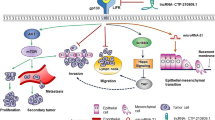
IL-10 interacts with a number of cytokines, mostly causing inhibition. IL-10 primarily acts on DCs and macrophages, and inhibits antigen presentation [36, 37]. Haddad et al. [30] reviewed inhibitory action of IL-10 on various cytokines and possible signaling mechanism involved. Figure 1 depicts few known interactions of IL-10 with various immune and tumor cells.
Role of IL-10 relevant to breast cancer initiation, progression, as well as regression. Light colored arrow shows cells secreting IL-10, dark arrow shows cells stimulated by IL-10, dotted arrow shows differentiation T-shaped arrow shows process blocked by IL-10, and lightning arrow shows detrimental effect on tumor cells
IL-10 inhibits major histocompatibility complex class II (MHC-II) expression as well as up-regulation of co-stimulatory molecules CD80 and CD86. IL-10 inhibits DC maturation and differentiation from monocyte precursors [38]. Thus, immuno-inhibitory properties of IL-10 is mainly due to their effect on antigen-presenting cells (APC) to prevent production of Th1 and Th2-associated cytokines [39]. IL-10 inhibits both proliferation of CD4+ T cells and production of IL-2 and IFN-γ by Th1 as well as IL-4 and IL-5 by Th2 [40]. IL-10 inhibits release of pro-inflammatory mediators from monocytes/macrophages and inhibits LPS (lipopolysaccharide)- and IFN-γ-induced secretion of TNF-α, IL-1β, IL-6, IL-8, G-CSF and GM-CSF [41]. IL-10 prohibits human monocytes from producing IL-1α, IL-1β, IL-6, IL-8, TNF-α, and G-CSF [42]. IL-10 also inhibits monokine synthesis more efficiently than same concentrations of IL-4 [43].
IL-10 acts as a co-stimulator for the proliferation of mast cells and peripheral lymphocytes. Optimal mast cell growth is achieved by a combination of IL-3, IL-4, and IL-10. IL-10 alone has no effect on mast cell proliferation [30]. IL-10 in humans exerts pro-inflammatory effects by enhancing IL-1 production [20]. IL-10 has also been shown to inhibit LPS-induced IL-1β production in human monocytes. IL-10 resulted in an increase in the ratio of IL-1RA to IL-1β in both neutrophils and monocytes [44].
Th17 cells are considered as developmentally distinct population from Th1 and Th2 cells and secrete IL-17. Recently, several studies reported interactive role of IL-10 and IL-17 suggesting importance of IL-10 signaling in direct inhibition of Th17 cells [45, 46].
IL-10 inducing and inhibiting agents
Anti-CD3 antibody and LPS are classically known to induce IL-10 production [47]. Similarly, IgA can also induce IL-10 expression in human monocytes [48, 49]. TGF-β and IL-6 induces IL-10 and IL-17 in normal conditions and IL-10 in turn helps in regulation of actions of IL-17 [50]. IL-27 and TGF-β induce IL-10 production and stimulate IL-10 producing cells [51, 52]. Stimulation with LPS, IL-1α or TNF-α weakly activates IL-10 expression [53].
Recently, CLA (conjugated linoleic acid) has been reported to induce IL-10 which in turn has an immunostimulatory effect on PBMCs via up-regulation of TNF-α production. But, IL-10 causes down-regulation of TNF-α production and exerts anti-inflammatory effect in LPS-stimulated PBMCs [54]. Another report showed G-1, a G protein coupled estrogen receptor (GPER) agonist and Thalidomide can induce IL-10 expression directly acting on Th17 or hybrid T cell populations [55]. Thalidomide also induces IL-10 expression in stromal population of bone marrow derived from patients with myelodysplastic syndromes [56]. Similarly, suppression of histone deacetylase 11 promotes IL-10 expression in Kupffer cells and induces tolerance following orthotopic liver transplantation in rats [57]. Apoptotic cells selectively affect IL-10 production induced by zymosan, a crude β-glucan used as fungal surrogate [58]. Transcription factors such as, cMaf also regulates IL-10 expression and T-effector development [59].
Several immunomodulators and other drugs are reported to inhibit IL-10. Immunosuppressive agent cyclosporin blocks IL-10 production [60]. 15-Deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) inhibits IL-10 mediated activation of STAT3 and blocks IL-10 signaling [61]. AS101 (ammonium trichloro (dioxoethylene-o,o′) tellurate), an immunomodulator also inhibits IL-10 signaling [62]. Recently, agents other than immunomodulators are also reported to inhibit IL-10 production. For example, Rituximab inhibits IL-10 and induces lymphoma cell apoptosis [63].
IL-10 and cancer
Role of IL-10 in cancer though well accepted is vaguely understood. IL-10 is known to exhibit both pro and anti-tumor activities. IL-10 exhibits tumor regression activity [64]. Some proposed that IL-10 antitumor effect is due to enhanced NK cell activity [64], while others have demonstrated that anti-tumor effect depends on CD8+ or CD4+ T cell function [65]. But, in contrast several studies proposed that IL-10 may reduce immune response against cancer [34, 66]. IL-10 possibly acts as a negative mediator in the cross-talk between innate and adaptive antitumor immunity. The other mechanism of immunosuppression of anti-cancer immunity is thought to be mediated via tumor-derived factors inducing DC dysfunction and particularly alteration of DC differentiation, maturation and longevity as a mechanism for immune suppression. IL-10 is potent inhibitor of DC and thus possibly involved in reduction of anti-cancer immunity [66]. Recently, Mocellin et al. [65] reviewed both aspects of IL-10 in cancer immunity.
Cancer is illustrated by few common manifestations like, evasion of apoptosis, insensitivity to growth signals, induction of angiogenesis as proposed by Hanahan and Weinberg [67]. Various studies have reported effect of IL-10 on most of these hallmarks of tumorogenesis. IL-10 suppresses peripheral blood T cell apoptosis in vitro by increasing Bcl-2 expression [68]. IL-10 also prevents T cell apoptosis on IL-2 withdrawal and Epstein-Barr virus infections through upregulation of Bcl-2 expression [69]. Bcl-2 overexpression is attributable to activation of signal transducer and activator of transcription 3 (STAT 3) by IL-10 through autocrine or paracrine loops in lymphoma cells [70]. IL-10 could also promote Bax mRNA expression in culture-activated hepatic stellate cells [71]. Recently, IL-10 has been implicated in resistance to apoptosis in lung cancer [72]. p53 is an important tumor suppressor having inhibitory activity on IL-10 [73]. High IL-10 production is also observed in lymphocytes from p53-deficient mice with experimental autoimmune encephalomyelitis [74]. Cytokine promoters can be repressed by p53, acting as a negative regulator of these cytokines [75]. Contradictory results have been observed in relation to role of IL-10 in angiogenesis in tumor. IL-10 has been reported to exert anti-angiogenic activity in several cancers [76, 77]. Whereas some other report suggest that IL-10 may promote angiogenesis [78, 79].
IL-10 as breast tumor inhibiting cytokine
Although tumor promoting activities of IL-10 are known, it is predominantly reported to have anti-tumor property. Some of the proposed mechanisms of anti-cancer activity of IL-10 includes- activation of NK-cells [64, 79], synergistic activation of cytotoxic T lymphocyte for maintenance of CD8+ [80] and CD4+ mediated [81] anti-tumor response, enhancement in surface expression of MHC antigen for maintaining susceptibility of cancer cells to NK-cells [82], enhancement in tumor infiltration by neutrophil and macrophages, and finally modulation of angiogenesis and invasiveness through inhibition of metalloproteinase [77, 79, 83, 84].
Kundu et al. [64] studied anti-tumor and anti-metastatic properties of IL-10 in murine model and observed that tumorigenicity in immunocompetent mice was significantly abrogated by IL-10. Later, it was found that mice subjected to immunization with IL-10 expressing tumor cells promoted the loss of tumorigenicity and induced a protective anti-tumor immune response which was mediated either by NK cells or CD8+ T cells [85]. Table 1 summarizes various studies with IL-10 which describes its tumor inhibiting action particularly in breast cancer.
IL-10 as breast tumor promoting cytokine
IL-10, a well-established suppressor of immunity reduces the antigen presentation capacity of macrophages and inhibits production of several cytokines which have important role in tumor immunosurveillance. Therefore, higher IL-10 level may facilitate tumor immune escape. This observation has been reported in various types of cancers and is supported by in vivo IL-10 knock out studies. IL-10 knockout mice show increased survival and more bladder tumor rejection compared to normal mice [90] indicating inhibitory effect of endogenous IL-10 on tumor immunosurveillance system, thus, help in tumor initiation and growth. But, only few studies in breast cancer cell line or patient are available that supports direct pro-tumor action of IL-10 in breast cancer. These studies reporting breast tumor promoting activities of IL-10 are highlighted in Table 1.
IL-10 as prognostic indicator for breast cancer
Tumor progression is determined by intricate interaction of tumor cells, stromal cells and T lymphocytes. IL-10 produced by tumor cells and immune cells play an important role in tumor cell growth and proliferation in tumor microenvironment. Increased IL-10 concentration is frequently detected in serum of breast cancer patients. It is proposed that IL-10 is secreted at a higher rate by metastatic cancer cells for down-regulating inflammatory response of cell-mediated immunity [91]. IL-6 is known to promote tumor growth by up regulating anti-apoptotic and angiogenic protein in tumor cells [92–94] and elevated IL-10 may inhibit tumor growth by suppressing IL-6 production. This is supported by observation of inverse correlation of IL-10 and IL-6 levels in breast cancer patients [95]. But, IL-6 level reported both as positive and negative prognosticator of breast cancer [92]. Therefore, such inverse correlations with IL-6 level may raise question on any definitive role of IL-10 in breast cancer prognosis.
IL-10 is over expressed in estrogen receptor (ER)-negative breast tumor in comparison to ER-positive tumors [96]. Breast cancer patients with prolactin receptor (PR) positive tumor have lower IL-10 level. AP-1 expression is higher in ER-negative tumors than ER-positive tumors and higher AP-1 expression correlate with high IL-10 level [96].
Significant differences in IL-10 cytokine level reported in breast cancer patients and negative breast biopsy group. IL-10 showed more than 50% difference among cancer and negative biopsy group [97].
Akbulut et al. [98] in a tumor model generated with, NT-2 cell (Neu transgenic spontaneous mouse mammary tumor-derived cell line) found that IL-10 level is higher in serum than tumor tissue and reduced by vaccination with tumor associated antigen (TAA) vector vaccine as well as chemotherapy.
Kozlowski et al. [95] found that serum IL-10 is strongly associated with breast cancer. In their study with 45 breast cancer patients and 25 normal subjects, mean IL-10 level in control samples was 5.7 pg/ml in comparison to 24.7 pg/ml in patient samples. Serum level of IL-10 was elevated in 35 (77.8%) patients. However, serum IL-10 level was not associated with any of the stages of breast cancer patient grouped using TNM classification [95]. Another study in 90 breast cancer patients and 15 healthy volunteers found no differences in baseline IL-10 levels between cancer patients and healthy volunteers [99]. However, 45% of patients had measurable plasma IL-10 level and weekly paclitaxal treatment caused transient increase. But other report indicates that paclitaxel treatment significantly decreases IL-10 levels [100]. A recent study have also reported lack of significant correlation of IL-10 level with either pathologic or clinical response in locally advanced breast cancer patients treated with neoadjuvant chemotherapy [101].
Llanes-Fernándeza et al. [102] reported that 85% (23 out of 27) of breast cancer tissue examined showed strong IL-10 expression. IL-10 was significantly associated with apoptosis markers. An inverse association of IL-10 with p53 and a positive association between IL-10, Bcl-2, and Bax were observed. 89% of patients negative for p53 were found to express IL-10. Presence of IL-10 and higher expression of Bcl-2 family proteins in tumor microenvironment are proposed to represent an increase in breast tumor aggressiveness [102].
Merendino et al. [103] reported correlation between IL-10 levels and clinical stages of breast cancer. Neoplastic metastatic disease was found to be associated with higher IL-10 levels (1002.8 ± 425.7 pg/ml, N = 10) compared with patients with non-metastatic disease (238.3 ± 103.8 pg/ml, N = 10). IL-10 level of both groups of cancer patients were also significantly higher (P < 0.05) than those of healthy donor (7.6 ± 5.7 pg/ml, N = 10). It was proposed that presence of IL-10 in sera of cancer-bearing patients possibly contributes in decreasing immune surveillance favoring tumor development. It has been suggested that IL-10 suppresses Th-1 cell development through down-regulation of IL-12 production by monocytes and favor generation of Th-2 developmental pathways, inducing humoral response and immunosuppression of cell-mediated immunity [103]. In a study conducted later by the same group [104], reported that mononuclear cells obtained from breast cancer patients exhibit a defective IL-12 production capability and generate higher amounts of IL-10.
Son et al. [105] in a recent study investigated methylation status of IL-10 gene in 30 normal, 31 benign, and 72 breast cancer paraffin-embedded tissue specimens from the National Cancer Center, Korea. Significantly lower methylation rates of the IL-10 gene in malignant tumors were observed than that of benign and normal tissues. Tissues with aberrant methylation of IL-10 gene showed significantly lower rates of mRNA expression. Whereas unmethylated IL-10 showed approximately 10,000-fold upregulated mRNA expression compared to those with IL-10 methylation. IL-10 methylation also showed significant association with lower Ki-67 expression. It was proposed that hypomethylation influences IL-10 gene activation and the process of breast carcinogenesis [105].
In another recent study [106] on adipose derived stem cells (ASC) isolated from breast cancer patients, significantly high mRNA expressions of IL-10 and TGF-β1 were reported than those from normal individuals. The culture supernatant of ASCs induced upregulation of mRNA expression levels of IL-4, TGF-β1, IL-10, CCR4, and CD25 in peripheral blood leukocytes. When the same culture supernatant was added to ASCs isolated from normal subjects augmentation of mRNA expressions of IL-4, IL-10, IL-8, MMP2, VEGF, and SDF-1 in normal ASCs was also observed [106]. This report for the first time suggest that resident derived stem cells in breast cancer tissue have crucial roles in inducing regulatory molecules like IL-10 and thereby promoting anti-inflammatory reaction within the tumor microenvironment favorable for tumor growth.
IL-10 transcripts were also found to be expressed in 14/15 primary breast adenocarcinomas and in 5/8 established breast tumor lines [107]. This supported by immunohistochemistry and immunoprecipitation from lysates and supernatants showed that established breast tumor lines produces IL-10 protein. IL-10 is localized not only in tumor cells of primary breast adenocarcinomas but also in occasional infiltrating mononuclear cells.
These findings of increased serum and transcript level of IL-10 and altered methylation pattern in breast cancer suggest that it may have utility in describing cancer prognosis along with other existing panel of molecular prognosticator. However, more elaborate multicentre-validated studies will be required before any such consideration. Various studies on expression and level of IL-10 in breast cancer and their significance in relation to breast cancer are summarized in Table 2.
IL-10 as genetic biomarker of disease risk
Genetic polymorphic variants have been recently investigated as genetic marker of possible risk for initiation and progression of common and complex diseases like breast cancer. Several candidate genes have been identified which may correlate with risk of breast cancer and its progressive stages. IL-10 contains several well-characterized polymorphic sites which have been screened in breast cancer patients to find if any of these are involved with breast cancer risk. IL-10-1082 (A>G); -819 (C>T); -592 (C>A) SNPs are most commonly studied in breast cancer patients. Majority of IL-10 polymorphism studies observed significant association of IL-10 with breast cancer with exception of one study [110] and a recent meta-analysis [111] which could not find association of IL-10 with breast cancer. Genetic polymorphism studies on IL-10 in breast cancer conducted till today are summarized in Table 3.
Although polymorphism studies are influenced by several factors like ethnicity of study population, sample size and inclusion, exclusion criteria, etc., studies indicating association of IL-10 with breast cancer may further confirm the role of IL-10 in breast cancer. IL-10 gene promoter polymorphism is already known to influence IL-10 production [117–119]. In breast cancer patient also, enhanced IL-10 level and correlation with advancing stages were reported. However, studies with larger population size of patients from different racial and ethnic origin will be required for deriving definitive conclusions. Besides, stringent application of in silico analysis for genome-wide SNP interactions of combinations of genes would be required to address current limits of polymorphism studies in breast cancer [120].
In summary, growing evidences suggest that IL-10 has important role in initiation and progression of breast cancer, although much of the intricate mechanism involved are not thoroughly investigated. IL-10 predominantly exerts tumor inhibiting action on breast cancer, however, it also has potential to promote tumor. This dual nature of IL-10 may further be dependent on temporo-spatial expression of IL-10 and level available. Majority of genetic studies points toward a significant correlation of IL-10 and breast cancer. This is also supported by elevated serum IL-10 level in breast cancer patients. Due to its complex dual role in tumor initiation and progression, any attempt to manage tumor with IL-10 as therapy would not be that simple and straight forward. However, IL-10 may serve as a crucial biomarker with certain amount of prognostic significance. Further studies on IL-10 are expected to shed more light on these contentious areas so as to convincingly establish its true nature of association with breast cancer.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Parkin DM, Fernandez LM (2006) Use of statistics to assess the global burden of breast cancer. Breast J 12(Suppl. 1):70–80
Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y (2004) Cytokines in cancer immunity and immunotherapy. Immunol Rev 202:275–293
Standish LJ, Sweet ES, Novack J, Wenner CA, Bridge C, Nelson A, Martzen M, Torkelson C (2008) Breast cancer and the immune system. J Soc Integr Oncol 6(4):158–168
DeNardo DG, Coussens LM (2007) Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 9(4):212
Rao VS, Dyer CE, Jameel JK, Drew PJ, Greenman J (2006) Potential prognostic and therapeutic roles for cytokines in breast cancer (Review). Oncol Rep 15(1):179–185
Carpi A, Nicolini A, Antonelli A, Ferrari P, Rossi G (2009) Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr Cancer Drug Target 9(8):888–903
Konwar R, Chaudhary P, Kumar S, Mishra D, Chattopadhyay N, Bid HK (2009) Breast cancer risk associated with polymorphisms of IL-1RN and IL-4 gene in Indian women. Oncol Res 17(8):367–372
Moore KW, O’Garra A, de Waal MR, Vieira P, Mosmann TR (1993) Interleukin-10. Annu Rev Immunol 11:165–190
Fitzgerald KA, O’Neill LAJ, Gearing AJH, Callard RE (2001) The cytokine facts book, 2nd edn. Academic Press, London
Lentsch AB, Shanley TP, Sarma V, Ward PA (1997) In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest 100(10):2443–2448
Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S (1997) Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J 16(19):5894–5903
Mosmann TR (1994) Properties and functions of interleukin-10. Adv Immunol 56:1–26
Howard M, O’Garra A (1992) Biological properties of interleukin 10. Immunol Today 13(6):198–200
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765
Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB (2004) Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22:929–979
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109
de Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, de Vries JE (1992) Interleukin-10. Curr Opin Immunol 4(3):314–320
Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 therapy—review of a new approach. Pharmacol Rev 55(2):241–269
Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21(5):331–344
Wolk K, Kunz S, Asadullah K, Sabat R (2002) Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168(11):5397–5402
Couper KN, Blount DG, Riley EM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180(9):5771–5777
O’Garra A, Vieira P (2007) TH1 cells control themselves by producing interleukin-10. Nat Rev Immunol 7:425–428
Ho AS, Wei SH, Mui AL, Miyajima A, Moore KW (1995) Functional regions of the mouse interleukin-10 receptor cytoplasmic domain. Mol Cell Biol 15:5043–5053
Finbloom DS, Winestock KD (1995) IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol 155(3):1079–1090
Lai CF, Ripperger J, Morella KK, Jurlander J, Hawley TS, Carson WE, Kordula T, Caligiuri MA, Hawley RG, Fey GH, Baumann H (1996) Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. J Biol Chem 271(24):13968–13975
Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM (2004) Interleukin-10 suppression of myeloid cell activation—a continuing puzzle. Immunology 113(3):281–292
Braunschweig A, Poehlmann TG, Busch S, Schleussner E, Markert UR (2011) Signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling (SOCS3) balance controls cytotoxicity and IL-10 expression in decidual-like natural killer cell line NK-92. Am J Reprod Immunol 66(3):329–335
Lee EB, Kim A, Kang K, Kim H, Lim JS (2010) NDRG2-mediated modulation of SOCS3 and STAT3 activity inhibits IL-10 production. Immune Netw 10(6):219–229
Haddad JJ, Saadé NE, Safieh-Garabedian B (2003) Interleukin-10 and the regulation of mitogen-activated protein kinases: are these signalling modules targets for the anti-inflammatory action of this cytokine? Cell Signal 15(3):255–267
Kruger-Krasagakes S, Krasagakis K, Garbe C, Schmitt E, Hüls C, Blankenstein T, Diamantstein T (1994) Expression of interleukin 10 in human melanoma. Br J Cancer 70(6):1182–1185
Gastl GA, Abrams JS, Nanus DM, Oosterkamp R, Silver J, Liu F, Chen M, Albino AP, Bander NH (1993) Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 55(1):96–101
Joimel U, Gest C, Soria J, Pritchard LL, Alexandre J, Laurent M, Blot E, Cazin L, Vannier JP, Varin R et al (2010) Stimulation of angiogenesis resulting from cooperation between macrophages and MDA-MB-231 breast cancer cells: proposed molecular mechanism and effect of tetrathiomolybdate. BMC Cancer 10:375
Venetsanakos E, Beckman I, Bradley J, Skinner JM (1997) High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer 75(12):1826–1830
Al-Sarireh B, Sathaporn S, Robins A, Jenkin D, Vassanasiri W, El-Sheemy M, Jibril JA, Clark D, Eremin O (2010) Mononuclear phagocytes but not tumour cells are the main source of elevated inter-leukin (IL)-10 levels in human breast cancer. Surgeons in training 2000. The Royal College of Surgeons of Edinburgh. Retrieved on 02 December 2010 from http://www.rcsed.ac.uk/Journal/vol46_1/4610011.htm
Bogdan C, Vodovotz Y, Nathan C (1991) Macrophage deactivation by interleukin 10. J Exp Med 174(6):1549–1555
Hashimoto SI, Komuro I, Yamada M, Akagawa KS (2001) IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J Immunol 167(7):3619–3625
Commeren DL, Van Soest PL, Karimi K, Löwenberg B, Cornelissen JJ, Braakman E (2003) Paradoxical effects of interleukin-10 on the maturation of murine myeloid dendritic cells. Immunology 110(2):188–196
de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE (1991) Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 174(4):915–924
Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A (1991) IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146(10):3444–3451
Macatonia SE, Doherty TM, Knight SC, O’Garra A (1993) Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol 150(9):3755–3765
de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174(5):1209–1220
Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A (1991) IL-10 inhibits cytokine production by activated macrophages. J Immunol 147(11):3815–3822
Jenkins JK, Malyak M, Arend WP (1994) The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res 13(1):47–54
Huber S, Gagliani N, Esplugues E, O’Connor W Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY et al (2011) Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34(4):554–565
Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W et al (2011) Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34(4):566–578
Scott DE, Gause WC, Finkelman FD, Steinberg AD (1990) Anti-CD3 antibody induces rapid expression of cytokine genes in vivo. J Immunol 145(7):2183–2188
Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, Francis JN, Durham SR (2007) Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol 178(7):4658–4666
Geissmann F, Launay P, Pasquier B, Lepelletier Y, Leborgne M, Lehuen A, Brousse N, Monteiro RC (2001) A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol 166(1):346–352
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8(12):1390–1397
Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL (2007) A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8(12):1380–1389
Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A (2007) Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 8(12):1372–1379
Pang G, Couch L, Batey R, Clancy R, Cripps A (1994) GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol 96(3):437–443
Kim KH, Kim DI, Kim SH, Jung EM, Kang JH, Jeung EB, Yang MP (2011) Trans-10, cis-12-conjugated linoleic acid attenuates tumor necrosis factor-α production by lipopolysaccharide-stimulated porcine peripheral blood mononuclear cells through induction of interleukin-10. Cytokine; doi:10.1016/j.cyto.2011.06.019
Brunsing RL, Prossnitz ER (2011) Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology 134(1):93–106
Lazarini M, Traina F, Winnischofer SM, Costa FF, Queiroz ML, Saad ST (2011) Effects of thalidomide on long-term bone marrow cultures from patients with myelodysplastic syndromes: induction of IL-10 expression in the stromal layers. Leuk Res 35(8):1102–1107
Lian ZR, Xu YF, Wang XB, Gong JP, Liu ZJ (2011) Suppression of Histone Deacetylase 11 Promotes Expression of IL-10 in Kupffer Cells and Induces Tolerance Following Orthotopic Liver Transplantation in Rats. J Surg Res; doi:10.1016/j.jss.2010.12.035
Municio C, Hugo E, Alvarez Y, Alonso S, Blanco L, Fernández N, Sánchez Crespo M (2011) Apoptotic cells enhance IL-10 and reduce IL-23 production in human dendritic cells treated with zymosan. Mol Immunol; doi:10.1016/j.molimm.2011.07.022
Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y (2009) c-Maf regulates IL-10 expression during Th17 polarization. J Immunol 182(10):6226–6236
Durez P, Abramowicz D, Gérard C, Van Mechelen M, Amraoui Z, Dubois C, Leo O, Velu T, Goldman M (1993) In vivo induction of interleukin 10 by anti-CD3 monoclonal antibody or bacterial lipopolysaccharide: differential modulation by cyclosporin A. J Exp Med 177(2):551–555
Ji JD, Kim HJ, Rho YH, Choi SJ, Lee YH, Cheon HJ, Sohn J, Song GG (2005) Inhibition of IL-10-induced STAT3 activation by 15-deoxy-Delta12, 14-prostaglandin J2. Rheumatology (Oxford) 44(8):983–988
Kalechman Y, Gafter U, Weinstein T, Chagnac A, Freidkin I, Tobar A, Albeck M, Sredni B (2004) Inhibition of interleukin-10 by the immunomodulator AS101 reduces mesangial cell proliferation in experimental mesangioproliferative glomerulonephritis: association with dephosphorylation of STAT3. J Biol Chem 279(23):24724–24732
Alas S, Emmanouilides C, Bonavida B (2001) Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin’s lymphoma to apoptosis. Clin Cancer Res 7(3):709–723
Kundu N, Beaty TL, Jackson MJ, Fulton AM (1996) Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J Natl Cancer Inst 88(8):536–541
Mocellin S, Marincola FM, Young HA (2005) Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 78(5):1043–1051
Pinzon-Charry A, Maxwell T, López JA (2005) Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol 83(5):451–461
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Cohen SB, Crawley JB, Kahan MC, Feldmann M, Foxwell BM (1997) Interleukin-10 rescues T cells from apoptotic cell death: association with an upregulation of Bcl-2. Immunology 92:1–5
Taga K, Cherney B, Tosato G (1993) IL-10 inhibits apoptotic cell death in human T cells starved of IL-2. Int Immunol 5:1599–1608
Alas S, Bonavida B (2001) Rituximab inactivates signal transducer and activation of transcription 3 (stat3) activity in b-nonhodgkin’s lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of bcl-2 and sensitization to cytotoxic drugs. Cancer Res 61:5137–5144
Wang XZ, Zhang SJ, Chen YX, Chen ZX, Huang YH, Zhang LJ (2004) Effects of platelet-derived growth factor and interleukin-10 on Fas/Fas-ligand and Bcl-2/Bax mRNA expression in rat hepatic stellate cells in vitro. World J Gastroenterol 10:2706–2710
Zeng L, O’Connor C, Zhang J, Kaplan AM, Cohen DA (2010) IL-10 promotes resistance to apoptosis and metastatic potential in lung tumor cell lines. Cytokine 49(3):294–302
Fox JG, Sheppard BJ, Dangler CA, Whary MT, Ihrig M, Wang TC (2002) Germ-line p53-targeted disruption inhibits helicobacter induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer Res 62:696–702
Okuda Y, Okuda M, Bernard CC (2003) Regulatory role of p53 in experimental autoimmune encephalomyelitis. J Neuroimmunol 135:29–37
Ohkuso-Tsukada K, Tsukada T, Isobe KI (1999) Accelerated development and aging of the immune system in p53-deficient mice. J Immunol 163:1966–1972
Kohno T, Mizukami H, Suzuki M, Saga Y, Takei Y, Shimpo M, Matsushita T, Okada T, Hanazono Y, Kume A, Sato I, Ozawa K (2003) Interleukin-10-mediated inhibition of angiogenesis and tumor growth in mice bearing VEGF-producing ovarian cancer. Cancer Res 63(16):5091–5094
Stearns ME, Rhim J, Wang M (1999) Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res 5(1):189–196
García-Hernández ML, Hernández-Pando R, Gariglio P, Berumen J (2002) Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology 105(2):231–243
Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M (1996) Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res 2(12):1969–1979
Fujii S, Shimizu K, Shimizu T, Lotze MT (2001) Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood 98(7):2143–2151
Segal BM, Glass DD, Shevach EM (2002) Cutting edge: IL-10-producing CD4+ T cells mediate tumor rejection. J Immunol 168(1):1–4
Garcia-Lora A, Algarra I, Garrido F (2003) MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol 195(3):346–355
Di Carlo E, Coletti A, Modesti A, Giovarelli M, Forni G, Musiani P (1998) Local release of interleukin-10 by transfected mouse adenocarcinoma cells exhibits pro- and anti-inflammatory activity and results in a delayed tumor rejection. Eur Cytokine Netw 9(1):61–68
Kaufman HL, Rao JB, Irvine KR, Bronte V, Rosenberg SA, Restifo NP (1999) Interleukin-10 enhances the therapeutic effectiveness of a recombinant poxvirus-based vaccine in an experimental murine tumor model. J Immunother 22(6):489–496
Dorsey R, Kundu N, Yang Q, Tannenbaum CS, Sun H, Hamilton TA, Fulton AM (2002) Immunotherapy with interleukin-10 depends on the CXC chemokines inducible protein-10 and monokine induced by IFN-gamma. Cancer Res 62(9):2606–2610
Allione A, Consalvo M, Nanni P, Lollini PL, Cavallo F, Giovarelli M, Forni M, Gulino A, Colombo MP, Dellabona P et al (1994) Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res 54(23):6022–6026
Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di Pierro F, De Giovanni C (1995) Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol 155(6):3112–3123
Kundu N, Dorsey R, Jackson MJ, Guiterrez P, Wilson K, Fu S, Ramanujam K, Thomas E, Fulton AM (1998) Interleukin-10 gene transfer inhibits murine mammary tumors and elevates nitric oxide. Int J Cancer 76(5):713–719
Paul S, Biswas A, Sasmal K, Mukherjee S, Biswas T, Biswas R (2010) IL-10 alters prolactin receptor activity emulating that during breast cancer. Cytokine 51(2):144–150
Halak BK, Maguire HC Jr, Lattime EC (1999) Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res 59(4):911–917
Abbas AK, Lichtman AH (2006) Cellular and molecular immunology, 5th edn. Elsevier, Amsterdam
Knüpfer H, Preiss R (2007) Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat 102:129–135
Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334(Pt 2):297–314
Kovacs E (2001) Investigation of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in sera of cancer patients. Biomed Pharmacother 55(7):391–396
Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ (2003) Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst 48:82–84
Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissière F, Laune D, Roques S, Lazennec G (2007) Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 9(1):R15
Lyon DE, McCain NL, Walter J (2008) Cytokine comparison between women with breast cancer and women with a negative breast biopsy. Nurs Res 57(1):51–58
Akbulut H, Tang Y, Akbulut KG, Maynard J, Deisseroth A (2008) Chemotherapy targeted to cancer tissue potentiates antigen-specific immune response induced by vaccine for in vivo antigen loading and activation of dendritic cells. Mol Ther 16(10):1753–1760
Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D et al (2004) Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 25(3):94–102
Lee M, Yea SS, Jeon YJ (2000) Paclitaxel causes mouse splenic lymphocytes to a state hyporesponsive to lipopolysaccharide stimulation. Int J Immunopharmacol 22(8):615–621
Nolen BM, Marks JR, Ta’san S, Rand A, Luong TM, Wang Y, Blackwell K, Lokshin AE (2008) Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res 10(3):R45
Llanes-Fernandez L, Alvarez-Goyanes RI, Arango-Prado Mdel C, Alcocer-Gonzalez JM, Mojarrieta JC, Perez XE, Lopez MO, Odio SF, Camacho-Rodríguez R, Guerra-Yi ME et al (2006) Relationship between IL-10 and tumor markers in breast cancer patients. Breast 15(4):482–489
Merendino RA, Arena A, Capozza AB, Chillemi S, Mesiti M (1996) Serum levels of interleukin-10 in patients affected by breast cancer. Immunol Lett 53(1):59–60
Merendino RA, Gangemi S, Misefari A, Arena A, Capozza AB, Chillemi S, D’Ambrosio FP (1999) Interleukin-12 and interleukin-10 production by mononuclear phagocytic cells from breast cancer patients. Immunol Lett 68(2–3):355–358
Son KS, Kang HS, Kim SJ, Jung SY, Min SY, Lee SY, Kim SW, Kwon Y, Lee KS, Shin KH et al (2010) Hypomethylation of the interleukin-10 gene in breast cancer tissues. Breast 19(6):484–488
Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A (2011) Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol 266(2):116–122
Heckel MC, Wolfson A, Slachta CA, Schwarting R, Salgame P, Katsetos CD, Platsoucas CD (2011) Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell Immunol 266(2):143–153
Rosen HR, Ausch C, Reinerova M, Zaspin E, Renner K, Rosen AC, Schiessel R, Moroz C (1998) Activated lymphocytes from breast cancer patients express the characteristics of type 2 helper cells—a possible role for breast cancer-associated p43. Cancer Lett 127(1–2):129–134
Rao VS, Alabi A, Dyer CE, Greenman J, Drew PJ (2008) IL-10 and IL-12 expression in breast cancer patients and effect of therapy—ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 26(15S):14016
Santos SCL, Ribeiro EMSF, Cavalli IJ, Lima RS, da Graça Bicalho M (2005) IL-10 gene polymorphisms and sporadic breast cancer. Hum Immunol 66(8), Supplement 1:53
Yu KD, Chen AX, Yang C, Fan L, Huang AJ, Shao ZM (2010) The associations between two polymorphisms in the interleukin-10 gene promoter and breast cancer risk. Breast Cancer Res Treat; doi:10.1007/s10549-010-1133-3
Giordani L, Bruzzi P, Lasalandra C, Quaranta M, Schittulli F, Della Ragione F, Iolascon A (2003) Association of breast cancer and polymorphisms of interleukin-10 and tumor necrosis factor-alpha genes. Clin Chem 49(10):1664–1667
Smith KC, Bateman AC, Fussell HM, Howell WM (2004) Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur J Immunogenet 31(4):167–173
Langsenlehner U, Krippl P, Renner W, Yazdani-Biuki B, Eder T, Köppel H, Wascher TC, Paulweber B, Samonigg H (2005) Interleukin-10 promoter polymorphism is associated with decreased breast cancer risk. Breast Cancer Res Treat 90(2):113–115
Kong F, Liu J, Liu Y, Song B, Wang H, Liu W (2010) Association of interleukin-10 gene polymorphisms with breast cancer in a Chinese population. J Exp Clin Cancer Res 29:72
Gerger A, Renner W, Langsenlehner T, Hofmann G, Knechtel G, Szkandera J, Samonigg H, Krippl P, Langsenlehner U (2010) Association of interleukin-10 gene variation with breast cancer prognosis. Breast Cancer Res Treat 119(3):701–705
Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV (1997) An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 24(1):1–8
Kingo K, Ratsep R, Koks S, Karelson M, Silm H, Vasar E (2005) Influence of genetic polymorphisms on interleukin-10 mRNA expression and psoriasis susceptibility. J Dermatol Sci 37(2):111–113
Crawley E, Kay R, Sillibourne J, Patel P, Hutchinson I, Woo P (1999) Polymorphic haplotypes of the interleukin-10 50 flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum 42(6):1101–1108
Yang CH, Chuang LY, Chen YJ, Tseng HF, Chang HW (2011) Computational analysis of simulated SNP interactions between 26 growth factor-related genes in a breast cancer association study. OMICS 15(6):399–407
Acknowledgments
The authors are thankful to Indian Council of Medical Research (ICMR), Govt. of India for financial support by (Grant Number 5/13/12/2007-NCDIII). Hamidullah would like to thank Department of Biotechnology (DBT), Govt. of India for Junior Research Fellowship. CDRI communication number of this manuscript is 8143.
Conflict of interest
None of the authors have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamidullah, Changkija, B. & Konwar, R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat 133, 11–21 (2012). https://doi.org/10.1007/s10549-011-1855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1855-x