Abstract
Young breast cancer patients are more likely than old patients to experience ipsilateral breast tumor recurrence (IBTR) after breast conserving surgery (BCS). However, the pathological processes underlying this relationship have not been elucidated. We investigated the effect of young age on IBTR in a Korean cohort of women with different molecular subtypes of breast cancer. We analyzed data of 2,102 consecutive breast cancer patients who underwent BCS and post-surgical radiation therapy (RT) at two Korean institutions between 2000 and 2005. Patients were classified as young (≤40 years; N = 513) or old (>40 years; N = 1,589). Breast cancer subtype was determined by estrogen receptor (ER), progesterone receptor (PR), and HER2. Median follow-up duration was 61 months. The 5-year IBTR rate was 3.4% in young patients and 1.1% in old patients (P < 0.001). Univariate analysis indicated that IBTR rate in young patients with luminal A and HER2 subtypes was significantly greater than in old patients with these subtypes (P = 0.015 and P < 0.001, respectively). Multivariate analysis, which used luminal A subtype in old patients as reference, indicated that HER2 subtype in young patients was associated with increased risk of IBTR (hazard ratio, HR = 12.24; 95% CI: 2.54–57.96). Among old patients, HER2 subtype was not associated with increased IBTR. In conclusion, young women had a higher rate of IBTR after BCS and RT than old women. This difference is mainly among women with HER2 subtype. Aggressive local control and adjuvant therapy should be considered for young women with HER2 subtype breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer tends to be more aggressive in young women than in old women [1–3]. A previous study of breast cancer patients younger than 35 years indicated that the risk of death increases by 5% for every 1-year reduction in age [4]. Young age has a stronger effect on poor survival in women with hormone receptor-positive breast cancers [5]. Young age is also an independent risk factor for ipsilateral breast tumor recurrence (IBTR) following treatment by breast conserving surgery (BCS) and post-surgical radiation therapy (RT) [6–11]. The high rate of IBTR in young women is a significant problem for surgeons, because young women often prefer BCS over more radical surgery.
The pathological processes underlying the relationship of young age and IBTR have not yet been elucidated. Gene expression profiling studies have established that breast cancers can be divided into four major subtypes that have different incidences, survival rates, and responses to therapy: luminal A (estrogen receptor, ER+ or progesterone receptor, PR+ and HER2−), luminal B (ER+ or PR+ and HER2+), HER2-enriched (ER−, PR−, and HER2+), and triple-negative (ER−, PR−, and HER2−) [12, 13]. However, the impact of breast cancer subtype on local control has not been well established. Several recent studies showed that patients with the luminal A subtype had a low rate of IBTR and that those with the HER2 subtype had a high rate of IBTR [14–16].
In this study, we investigated the effect of patient age and breast cancer subtype on IBTR after treatment by BCS and RT in a cohort from Korea.
Patients and methods
Study population
The Asan Medical Center (AMC) and the Seoul National University Hospital (SNUH) are the two largest hospitals in Korea and have maintained prospective web-based databases that include information on all patients who underwent surgery for breast cancer. Between 2000 and 2005, 7,496 patients underwent breast cancer surgery at these two hospitals. Among them, we analyzed the patients who had invasive breast cancer with tumors less than 5 cm in diameter and were treated by BCS and RT. Patients with the following conditions were excluded from our analysis: inflammatory breast cancer, distant metastasis, unclear T or N status, unavailable immunohistochemistry (IHC) data for ER, PR, and HER2 expression, and treatment by preoperative systemic therapy. Patients who did not receive adjuvant RT after BCS and those with positive surgical margins were also excluded. A total of 2,102 patients were included in this study. A total of 513 patients (24.4%) were 40 years or younger and 1,589 (75.6%) were older than 40 years.
Treatment
Local treatment of breast cancer consisted of BCS followed by external beam radiation of the entire breast. The most common doses were 50.4 Gy in 1.8 Gy fractions to the whole breast, plus a tumor-bed boost to 60.4 Gy. A separate supraclavicular or axillary field was not usually added after axillary dissection unless the patient had four or more positive nodes. Patients received adjuvant systemic chemotherapy according to St. Gallen and/or NCCN guidelines. None of the patients received adjuvant trastuzumab. Standard adjuvant hormone therapy was added for patients with hormone receptor-positive breast cancer according to the above guidelines.
Tissue microarrays
Tumor tissues were stained by IHC with antibodies directed against ER, PR, and HER2. Tumors were considered HER2-positive only if they scored 3+ on IHC or if they were 1+ or 2+ on IHC and had at least twofold amplification of the HER2 gene based on fluorescence in situ hybridization (FISH). In the absence of positive FISH data, tumors scored 1+ or 2+ by IHC were considered HER2-negative. Based on IHC results, tumors were classified as luminal A (ER+ or PR+ and HER2−), luminal B (ER+ or PR+ and HER2+), HER2 (ER−, PR−, and HER2+), or triple-negative (ER−, PR−, and HER2−).
Statistical analysis
The primary end point was time to IBTR as a first event, including any invasive or noninvasive recurrence in the ipsilateral breast. Differences in clinicopathologic features between intrinsic subtypes were examined using the χ2 test. For univariate survival analysis, IBTR was estimated using Kaplan–Meier curves, and survival differences were assessed using the log rank test. Cox proportional hazard models that accounted for covariates were used to calculate adjusted hazard ratios (HRs). Clinicopathologic covariates included age at diagnosis (≤40 or >40 years), tumor size (T1 or >T1), tumor grade (1, 2, or 3), and lymph node status (negative or positive). Treatment covariates included chemotherapy and hormonal therapy. All statistical tests were two-sided, and a P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 12.0 (SPSS, Chicago, IL).
Results
Basic characteristics of young and old patients
The percentages of patients with large tumors, lymph node metastases, and high-grade tumors were significantly greater among young women (≤40 years) than old women (>40 years). The percentages of women with the luminal A and luminal B subtypes were greater among old patients than young patients (P < 0.005 for each, Table 1). Within each intrinsic subtype, young patients were more likely to have larger tumors and positive lymph node metastases than old patients (P < 0.005 for each, Table 2).
IBTR rates of young and old patients
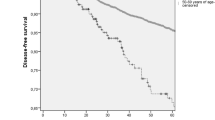
After a median follow up of 61 months, 23 of the 513 young patients experienced IBTRs, with a 5-year cumulative incidence of 3.4%; and 17 of the 1,589 old patients experienced IBTRs, with a 5-year cumulative incidence of 1.1%. This difference was highly significant (P < 0.001, Fig. 1a). Subtype analysis showed that younger patients had significantly higher IBTR rate than older patients in luminal A (P = 0.015) and HER2 (P < 0.001) subtypes (Fig. 1b, d).
IBTR rates by intrinsic subtype
Among all women, IBTR was significantly more common in patients with the HER2 subtype compared with the other subtypes (P < 0.001). However, a high rate of IBTR in the HER2 subtype was present in young patients (P < 0.001) but not in old patients (P = 0.444) (Fig. 2a, b). The 5-year cumulative incidence of IBTR in young women with the HER2 subtype was 13.9% (95% CI: 8.9–18.8), compared with 1.3% (95% CI: 0.4–2.2) in old women with this subtype (P < 0.001, Fig. 2c).
Multivariate analysis of IBTR
Multivariate analysis using the luminal A subtype as a reference indicated that only the HER2 subtype was independently and significantly associated with increased risk of IBTR (HR = 7.19; 95% CI: 1.34–38.66, data not shown). Multivariate analysis using old patients with the luminal A subtype as a reference indicated that the HER2 subtype was an independent risk factor for IBTR in young patients (HR = 12.14; 95% CI: 2.54–57.96) but not in old patients (HR = 0.55; 95% CI: 0.05–6.34) (Table 3).
Annual risk of IBTR
The pattern of annual risk of IBTR was similar among young and old patients (data not shown), but differed by intrinsic subtype. In particular, young patients with the HER2 subtype had two peak times for risk of IBTR, 2–3 years after surgery and 7–8 years after surgery (Fig. 3).
Discussion
Our results indicate that the overall 5-year rate of IBTR among Korean breast cancer patients who underwent BCS and RT was low (1.6%), but that this rate was higher in young women (≤40 years; 3.4%) than in old women (>40 years; 1.1%). Moreover, the rate of IBTR varied by breast cancer subtype, as assessed by IHC staining for ER, PR, and HER2. Multivariate analysis showed that the presence of the HER2 subtype in younger patients was significantly and independently associated with increased local recurrence.
Previous studies reported that young age, defined by various cutoffs, is associated with increased risk of local relapse in patients undergoing BCS [6, 7, 17]. A subset analysis of the European Organization for Research and Treatment of Cancer (EORTC) Trial 22881/10882 reported that the most important risk factors for local recurrence after BCS were age younger than 50 years and high-grade invasive tumor [7]. Another study reported that the relative risk of locoregional recurrence increased by 7% for every 1-year decrease in age [6].
Previous publications have proposed several hypotheses to account for the effect of age on IBTR [18]. First, young women may be more likely to have breast tumors with aggressive subtypes. Indeed, similar to previous studies [15, 18, 19], we found that the percentages of patients with the HER2 and triple negative subtypes were greater in young women than in old women. Second, dense breasts, which are more common in younger women, may be a risk factor for breast cancer and local recurrence [20]. The mechanisms underlying the association of dense breasts and tumor recurrence are largely unknown, although previous research indicated that circulating growth factors and proteins may influence breast density and tumor recurrence [21–23]. Moreover, dense breasts may have a masking effect on tumor detection by mammography [24]. Third, age-specific biologic differences in breast carcinomas may be highly subtype dependent [8]. We found that IBTR rates were higher in young women with the HER2 subtype, suggesting age-specific differences in breast tumor biology. On the other hand, previous studies have shown that age is an independent risk factor for poor survival [2, 18], mainly in ER-positive breast cancers [5]. Our results suggest that the pathophysiology of local aggressiveness might be different from the pathophysiology of distant metastasis and poor survival in young women with breast cancer.
The biologic subtypes of breast cancer have a profound impact on local recurrence [16, 25, 26]. A study of 793 patients, in which 3.5% were younger than 35 years, who underwent BCS showed that increased rates of recurrence were associated with the HER2 and basal subtypes [16]. A study of 2985 patients, in which 7.5% were younger than 40 years, used a six-marker IHC panel and found that the HER2 enriched type (not luminal HER2) was associated with increased IBTR after BCS [15]. Another study showed that the HER2 subtype in patients with T1a,b N0 breast cancer was predictive of locoregional recurrence [14]. In contrast, a study of 498 patients of median age 61 years showed by multivariate analysis that breast cancer subtype was not associated with IBTR [26]. Most previous studies of IBTR have only included small numbers of patients younger than 40 years, limiting the ability to assess the impact of age on local recurrence. In contrast, 24.4% of our patients were 40 years or younger, allowing us to better assess the effect of age.
The reason why the HER2 subtype is associated with IBTR in younger patients is unclear. This subtype may be relatively resistant to post-lumpectomy RT [14]. Preclinical studies have shown that EGFR expression and activation by ligands correlate with radioresistance and that signal transduction initiated by receptor activation promotes cancer cell survival and proliferation after ionizing radiation [27–30]. HER2 inhibitors can affect cellular responses to ionizing radiation by induction of apoptosis and cell cycle arrest and by impeding DNA repair [27, 31]. Previous research indicated that targeting of PI3K-AKT-mTOR signaling significantly radiosensitized HER2-activated breast cancer cells by inhibition of DNA repair. Therefore, blocking the PI3K-AKT-mTOR pathway may help patients to overcome resistance to currently available HER2 inhibitors plus irradiation [30].
It remains unknown whether mastectomy provides better survival rates than BCS for young patients with the HER2 subtype of breast cancer. Changes in breast cancer surgery over the past decades have led to a reduction in the extent of surgery [32, 33] and better quality of life for patients. Oncoplastic surgery has become important, especially for younger patients. A comparison of mastectomy with BCS and RT in younger patients showed no differences in local recurrence, disease-free survival, or overall survival [34] suggesting that extensive surgery for patients at high risk of IBTR may not reduce the likelihood of IBTR.
A previous study found that additional radiation after BCS and standard RT reduced local recurrence from 19.5 to 10.2% in breast cancer patients 40 years and younger (P = 0.002) [8]. The 10-year results of the EORTC 22991/10883 trial demonstrated that additional radiation had the largest absolute benefit on local control in younger patients [7]. In our study, most patients received a tumor-bed boost, but this did not compensate for the higher rate of IBTR in young patients. Neuschatz et al. [35] reported that close margin was associated with higher local recurrence rate only in younger patients (less than 45 years old), suggesting the importance of strict surgical local control for young patients.
Our finding of an increased local recurrence in patients with the HER2-positive subtype must be interpreted in the light of our exclusion of patients who received adjuvant trastuzumab, which is now part of the standard of care of patients with HER2-positive early breast cancer. Indeed, several large-scale trials have assessed the efficacy and safety of trastuzumab use in the adjuvant setting and found that trastuzumab reduced the number of local and regional recurrences [16, 36, 37].
Previous studies have assessed the usefulness of biomarkers, such as Ki-67 and p53, and multi-gene signatures, such as wound response signature and the Oncotype DX assay, as indicators of local recurrence [38–40]. We suggest that the interaction of patient age with these and other biomarkers be considered in future studies.
In conclusion, we have shown here that younger breast cancer patients with the HER2 subtype have an increased risk for IBTR after BCS and RT. To our knowledge, this is the largest study to establish a relationship of patient age, tumor intrinsic subtype, and IBTR. Based on our results, we suggest that young breast cancer patients, especially those with the HER2 subtype, be provided with more strict local control to reduce the probability of IBTR, and be considered for more aggressive systemic therapy including trastuzumab. Further investigations of the mechanisms underlying the high rate of IBTR in younger breast cancer patients with the HER2 subtype are warranted.
References
Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Orlando L, Ghisini R et al (2006) Role of endocrine responsiveness and adjuvant therapy in very young women (below 35 years) with operable breast cancer and node negative disease. Ann Oncol 17:1497–1503
Han W, Kim SW, Park IA, Kang D, Youn YK, Oh SK et al (2004) Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer 4:82
O’Rourke MT, Ellison PT (1993) Age and prognosis in premenopausal breast cancer. Lancet 342:60
Han W, Kang SY (2010) Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat 119:193–200
Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS et al (2007) Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea—a report from the Korean Breast Cancer Society. J Clin Oncol 25:2360–2368
Bollet MA, Sigal-Zafrani B, Mazeau V, Savignoni A, de la Rochefordiere A, Vincent-Salomon A et al (2007) Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (<40 years) women treated with breast conserving surgery first. Radiother Oncol 82:272–280
Jones HA, Antonini N, Hart AA, Peterse JL, Horiot JC, Collin F et al (2009) Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol 27:4939–4947
Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I et al (2001) Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med 345:1378–1387
Botteri E, Bagnardi V, Rotmensz N, Gentilini O, Disalvatore D, Bazolli B et al (2010) Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol 21:723–728
Zhou P, Gautam S, Recht A (2007) Factors affecting outcome for young women with early stage invasive breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat 101:51–57
Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S et al (2006) Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer 106:35–41
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Albert JM, Gonzalez-Angulo AM, Guray M, Sahin A, Strom EA, Tereffe W et al (2010) Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a, bN0 breast cancer. Int J Radiat Oncol Biol Phys 77:1296–1302
Voduc KD, Cheang MCU, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28:1684–1691
Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL et al (2008) Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 26:2373–2378
Antonini N, Jones H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W et al (2007) Effect of age and radiation dose on local control after breast conserving treatment: EORTC trial 22881-10882. Radiother Oncol 82:265–271
Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N et al (2011) Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 29:e18–e20
Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y et al (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330
Cil T, Fishell E, Hanna W, Sun P, Rawlinson E, Narod SA et al (2009) Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer 115:5780–5787
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ et al (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6:798–808
Harvey JA (2004) Quantitative assessment of percent breast density: analog versus digital acquisition. Technol Cancer Res Treat 3:611–616
Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E et al (2001) Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomark Prev 10:243–248
van Gils CH, Otten JD, Verbeek AL, Hendriks JH (1998) Mammographic breast density and risk of breast cancer: masking bias or causality? Eur J Epidemiol 14:315–320
Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J et al (2006) Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24:5652–5657
Millar EKA, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL et al (2009) Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol 27:4701–4708
Sartor CI (2003) Epidermal growth factor family receptors and inhibitors: radiation response modulators. Semin Radiat Oncol 13:22–30
Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ (1999) Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 59:1347–1355
Rao GS, Murray S, Ethier SP (2000) Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. Int J Radiat Oncol Biol Phys 48:1519–1528
No M, Choi EJ, Kim IA (2009) Targeting HER2 signaling pathway for radiosensitization: alternative strategy for therapeutic resistance. Cancer Biol Ther 8:2351–2361
Sambade MJ, Camp JT, Kimple RJ, Sartor CI, Shields JM (2009) Mechanism of lapatinib-mediated radiosensitization of breast cancer cells is primarily by inhibition of the Raf>MEK>ERK mitogen-activated protein kinase cascade and radiosensitization of lapatinib-resistant cells restored by direct inhibition of MEK. Radiother Oncol 93:639–644
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Coulombe G, Tyldesley S, Speers C, Paltiel C, Aquino-Parsons C, Bernstein V et al (2007) Is mastectomy superior to breast-conserving treatment for young women? Int J Radiat Oncol Biol Phys 67:1282–1290
Neuschatz AC, DiPetrillo T, Safaii H, Price LL, Schmidt-Ullrich RK, Wazer DE (2003) Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer 97:30–39
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Koukourakis MI, Giatromanolaki A, Galazios G, Sivridis E (2003) Molecular analysis of local relapse in high-risk breast cancer patients: Can radiotherapy fractionation and time factors make a difference? Br J Cancer 88:711–717
Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Viale G, Renne G et al (2004) Minimal and small size invasive breast cancer with no axillary lymph node involvement: the need for tailored adjuvant therapies. Ann Oncol 15:1633–1639
Zellars RC, Hilsenbeck SG, Clark GM, Allred DC, Herman TS, Chamness GC et al (2000) Prognostic value of p53 for local failure in mastectomy-treated breast cancer patients. J Clin Oncol 18:1906–1913
Acknowledgments
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0004148 and 2010-0028631).
Conflict of interests
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.J., Han, W., Yi, O.V. et al. Young age is associated with ipsilateral breast tumor recurrence after breast conserving surgery and radiation therapy in patients with HER2-positive/ER-negative subtype. Breast Cancer Res Treat 130, 499–505 (2011). https://doi.org/10.1007/s10549-011-1736-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1736-3