Abstract
Purpose
Women ≤ 35 years old with breast cancer constitute a special group. Considering the impact of the disease and its prognosis, these patients face some specific problems that are not present in older women. What are the prognostic features of the survival rate in very young women with breast cancer?
Methods
Retrospective analysis of very young women with breast cancer from the Surgical-Oncologic Breast Cancer Department at “Theagenio” Anticancer Hospital, 2003–2016. Patient and tumor characteristics, treatment options and follow-up information were collected. Univariate–multivariate analyses were conducted and survival rates were calculated.
Results
The median age was 34 years old. 53 patients (41%) had T1, 36 (28%) had T2, 7 (5.4%) had T3 and 33 (25.6%) had T4 stage tumors. Most women, 114 (88.4%), had ductal carcinoma in their histology. Furthermore, positive axillary lymph nodes were present in 62 women (48%). In the immunochemistry report, 91 patients (70.5%) were hormone receptor positive, HER2 was overexpressed in 32 patients (24.8%) and 27 patients presented with triple-negative subtype. Out of 65 patients tested for Ki-67, 51 (78.5%), had a high expression (cut-off value of 20%). After adjusting for all possible factors, the risk of recurrence and death was six times higher in the positive lymph node group, (p < 0.001). The median disease-free and overall survival was 133 and > 173 months, respectively.
Conclusion
Breast cancer in very young women appears with large size and high-grade tumors, high incidence of infiltrated axillary lymph nodes, high Ki-67 expression and intrinsic subtypes with poor prognosis. As a result, these women need to be treated by a multidisciplinary team.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second most common cancer in the world and the most frequent malignancy in women. Its incidence rates vary across the world and increase with age [1]. However, very young females represent a special group of patients having special needs and requirements in their management, because of its aggressive behavior and association with poor prognosis [2,3,4]. Among them, there is a special and rare group of patients that includes the very young women (≤ 35 years old) with breast cancer. Despite this fact, there is no specific screening program for them [5], worldwide. In addition to the unpleasant course of disease and its prognosis, these young women face some specific problems, which were discovered with standardized Quality of Life questionnaires. These problems include disruption of their career, inability of child bearing/family completion, fulfilling the ongoing family responsibilities, the negative impact of different therapeutic modalities on sexuality and body image and also the psychosocial stress of facing a life-threatening illness at such a young age [6]. Hence, it is of high importance to take into consideration and discuss with them any possible fertility, sexuality, genetics, psychological and emotional problem, before deciding the treatment plan [7,8,9]. The aim of the current study was to describe the clinico-pathological characteristics, the applied treatment and determine the possible prognostic factors affecting the disease-free and the overall survival in very young women with breast cancer.
Methods
Study characteristics
We retrospectively reviewed the medical records of all women with breast cancer, who were treated in the Surgical-Oncologic Breast Cancer Department at “Theagenio” Anticancer Hospital, Thessaloniki, Greece from January 1, 2003 until December 31, 2016 and identified those that were ≤ 35 years old. Out of 6000 patients, approximately, diagnosed with breast cancer during this period of time, 159 were ≤ 35 years old. A written approval was received from the Head of the Department and the Scientific Committee of the hospital.
Patients
Inclusion criteria:
-
Histological confirmation of invasive breast cancer.
-
≤ 35 years old at the time of the diagnosis (January 1, 2003–December 31, 2016).
Exclusion criteria:
-
Missing important registry data after the diagnosis of breast cancer.
-
Stage IV disease at the time of the diagnosis.
-
Prior treatment for breast cancer in another.
As a result of the above-mentioned criteria, out of the 159 women ≤ 35 years old with breast cancer, 19 were excluded due to important missing registry data. Moreover, five women were excluded, because they had stage IV disease at the time of the diagnosis, because those patients have a worse prognosis irrespective of the age of women. Another six women, because in the final histological report no invasive breast cancer was found (only in situ carcinoma). Hence, finally 129 women ≤ 35 years old with breast cancer were identified as eligible for further analysis, with no duplicate data and important missing values. The flowchart of the patient selection is shown in (Fig. 1).
Data collection
Data were collected during a period of one month. In order to avoid inconsistencies among different dates of data collection, a uniform data collection sheet was used, during the retrospective mining of the patient’s medical records. The data sheet included the following information:
-
Patient’s identifiers:
-
o
Name
-
o
Hospital identification number
-
o
-
Patient’s age
-
Parity
-
Family history
-
Tumor characteristics:
-
o
Tumor size
-
o
Histological type
-
o
Grade
-
o
-
Axillary lymph node status:
-
o
Number of positive lymph nodes
-
o
Number of total dissected lymph nodes
-
o
-
Metastasis status and site
-
Disease stage (TNM staging)
-
Immunochemistry evaluation:
-
o
Estrogen receptor
-
o
Progesterone receptor
-
o
Human epidermal growth factor receptor 2 expression
-
o
Proliferation marker
-
o
-
Intrinsic subtypes:
-
o
Luminal A
-
o
Luminal B
-
o
HER2 positive
-
o
Basal-like
-
o
-
Treatment:
-
o
Surgery
-
o
Chemotherapy
-
o
Radiotherapy
-
o
Endocrine therapy
-
o
-
Time related data:
-
o
Date of diagnosis
-
o
Date of recurrence
-
o
Date of last follow-up
-
o
-
BRCA testing
-
Breast reconstruction
Statistical analysis
All analyses were performed using RStudio. For descriptive statistics of qualitative variables, the frequency distribution procedure was run with calculation of the number of cases and percentages. On the other hand, for descriptive statistics of quantitative variables, the mean, median, range, and standard deviation were used to describe central tendency and dispersion. Univariate and multivariate analyses were performed. Disease-free (DFS) and overall survival (OS) analyses were performed using the Kaplan–Meier curves and groups were compared using the log-rank test. Disease-free survival was defined as the time interval between date of diagnosis and date of first recurrence. A p value of < 0.05 was considered as statistically significant.
Results
This retrospective cohort study included 159 very young women with breast cancer, representing 2.65% of the 6.000 women who were treated during the period of the study for histologically proven breast cancer in the Surgical-Oncologic Breast Cancer Department. After screening the patients based on the inclusion and exclusion criteria, 129 patients were eligible for further analysis in this study.
Patients’ characteristics are outlined in (Table 1). The median age of the women at the time of the diagnosis was 34 years old, with a range of 21–35. Out of the 129 patients, 20 women (15.5%) had positive family history and BRCA mutations were identified in 17 patients (13.2%) among 32 women, who underwent genetic testing. Genetic testing was proposed to all women, due to the young age, and especially to those with positive family history or intrinsic subtypes with poor prognosis. Regarding parity, none of women that were included in this study had ≥ 4 children.
Regarding tumor location, they were almost equally located in both breasts, with no bilateral cases. The size of the tumor ranged from 1 to 85 mm and the median tumor size was 20 mm, with 20 cases (15.5%) of multifocal tumors. Moreover, according to the TNM staging system: 53 patients (41%) had T1, 36 (28%) had T2, 7 (5.4%) had T3 and interestingly 33 (25.6%) had T4 stage tumors. The most common histopathological type was invasive ductal carcinoma, 114 women (88.4%); associated in situ components were present in 70 patients (54.3%). According to tumor grading, more than the half of the patients, 82 (63.6%) had grade three tumors. On the other hand, all women underwent either axillary lymph node dissection or sentinel lymph node biopsy (SLNB). The number of total dissected lymph nodes ranged from 1 to 31 and the median number of excised nodes was 12. Positive axillary lymph nodes were present in 62 women (48%). According to TNM staging system, 67 patients (52%) had N0, 35 patients (27%) had N1, 18 patients (14%) had N2 and 9 patients (7%) had N3 stage lymph nodes. Tumor pathologic characteristics are summarized in (Table 2).
In the immunochemical profile, 91 (70.5%) were hormone receptor positive. HER2 was overexpressed in 32 patients (24.8%) and fluorescence in situ hybridization (FISH) was necessary to identify HER2 expression in 31 cases (24%). Half of the patients, 65 (50.4%), were tested for the proliferation marker Ki-67, because some of the patients were treated before Ki-67 became a standard marker in the immunochemistry report. 20% was the cut-off point for high and low expression [10]; 51 out of 65 patients (78.5%) had a high Ki-67. Based on the aforementioned immunochemistry markers, the following intrinsic subtypes of breast cancer were identified: 31 patients (24%) presented with Luminal A type, 60 patients (46.5%) presented with Luminal B type, 11 patients (8.5%) presented with HER2-positive type and interestingly 27 patients (21%) presented with triple-negative type. Tumor biomarker characteristics are summarized in Table 3.
Regarding treatment options (Table 4), all 129 patients underwent surgical treatment. Almost half of the patients, 66 (51.2%), were offered breast-conserving surgery (BCS) and 63 (48.8%) mastectomy, both combined either with SLNB or axillary lymph node dissection. Out of 63 patients with mastectomy, 18 (28.6%) underwent breast reconstructive surgery. Furthermore, chemotherapy was offered to almost all women, 121 (93.8%). Out of the 121 women, 42 (34.7%) had neo-adjuvant and 79 (65.3%) adjuvant chemotherapy, while in 29 (24%) anti-HER2 therapy was co-administrated. The criteria to offer neo-adjuvant chemotherapy were immunochemical profile (e.g., triple-negative type), tumor size (> T4), positive axillary lymph nodes. Radiotherapy was offered to 111 patients (86%) and endocrine therapy to 91 patients (70.5%).
All women that were included in the study had a frequent follow-up, from 6 to 173 months, with a median of 62 months. Tumor recurrence occurred in 34 patients (26.4%): 16 (47.1%) had a locoregional recurrence and 18 (52.9%) a distant metastasis. The first site of distant recurrence was bone metastasis in seven patients (38.9%), liver metastasis in 6 (33.3%), lung metastasis in 3 (16.6%), infiltration of cervical lymph nodes in 1 (5.6%) and brain metastasis in 1 (5.6%). Unfortunately, the mortality rate was approximately 20%.
In order to identify which factors were associated with DFS and OS, univariate and multivariate analyses were conducted and compared with literature [11]. Regarding DFS (Table 5), the univariate analysis revealed that axillary lymph node status was the only statistically significant variable (p < 0.001), which increases the risk of recurrence by 415% (HR: 5.142). But, in the final model (with factors with a p < 0.2) for the multivariable analysis we included both the intrinsic subtypes (p = 0.0272) and the axillary lymph node status. The risk of recurrence was decreased in the Luminal A type by 70% (HR: 0.3016), in the HER2 positive by 48% (HR: 0.5204) and in the Luminal B by 37% (HR: 0.6314), but it was increased in the Basal-like by 490% (HR: 5.8897), after adjusting for the other factors.
On the other hand, regarding OS (Table 6) in the univariate analysis, the results indicate that the correlation of tumor size and risk of death is statistically significant (p = 0.017) and for every 10 mm increase the risk of death is increased by 25% (HR: 1.237). The other variable that was found statistically significant was lymph node status (p < 0.001) and the risk of death was increased by 480% (HR: 5.835) in patients with positive axillary lymph nodes. All the other variables were not statistically significant related to the OS. In the multivariate analysis, we included in the final model the type of operation (p = 0.06743) and the axillary lymph node status. The risk of recurrence was increased by 110% (HR: 2.09) in the mastectomy group and by 500% (HR: 6.037) in the positive axillary lymph node group.
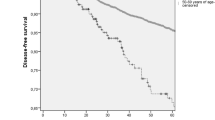
The median DFS was 130 months (Fig. 2) and the median OS was > 173 months (Fig. 3). Furthermore, in the group analysis, by using log-rank tests, there was no statistically significant difference in the DFS or the OS between the molecular subtypes (p = 0.377–0.42), the timing of the chemotherapy (p = 0.926–0.887), the grade (p = 0.743–0.633), the type of the surgery (p = 0.731–0.089), the tumor stage (p = 0.542–0.664) or the TNM staging (p = 0.0536–0.0524). However, in the axillary lymph node status group, there was a statistically significant difference in the median DFS (positive lymph nodes: 70 months vs. negative lymph nodes: 134 months) and median OS (positive lymph nodes: 95 months vs negative lymph nodes: > 173 months), between negative and positive lymph node groups (p < 0.001– < 0.001). These results are shown in (Figs. 4, 5).
Discussion
Breast cancer is the most common malignancy in women and it may occur at any age. In the last decades, a lot of effort has been made to thoroughly study breast cancer in young women, mainly due to the fact that most countries do not have an established screening program for women < 40 years old. Another problem is that the definition of young women varies across published studies with no cut-off age. Recently, the European Society of Breast Cancer Specialists (EUSOMA) and the ESO-ESMO Consensus published recommendations for the management of young women suffering from breast cancer and defined the age 40 years old as the upper limit [12, 13]. They also defined the age 35 years old as the upper limit for very young women and stated that this group consists another special population of patients that needs further investigation [12]. There are only few studies in the literature evaluating this specific topic [14,15,16].
Among our study population, the incidence of breast cancer in very young women was 2.65%. In the literature, this rate varies widely among the different populations, 1% in Finland [17], 2.7% in Japanese patients [18], 2.4% in American women [19], but 11.1% in China [20]. The median age was 34 years old, which is in accordance with the other studies [16, 21, 22]. Childbearing did not seem to affect the incidence of breast cancer, since approximately 60% of our patients were multiparous. Other risk factors that were analyzed is positive family history or inherited breast cancer. It was found that the incidence was higher in our study as compared with a recent study from Egypt [14].
As expected, breast cancer tumors were equally located in both sides. Furthermore, in accordance with the literature [14,15,16, 21, 22], our study results showed that breast cancer in very young women was associated with larger tumors (1/4 were T4), higher grade (2/3 were grade 3) and positive axillary lymph nodes (1/2 had lymph node metastasis). The ductal invasive carcinoma was the dominant histopathological type (≈90%), which is in agreement with the literature [15]. Regarding the immunochemistry, in our study, over 2/3 of the patients had positive hormone receptors, and 1/4 had HER2 overexpression. It is worthy to mention that, despite the retrospective character of our study, almost half of our patients were further tested for Ki-67. Nearly 80% of our very young women had a high Ki-67 expression (> 20%), which is an indicator of tumor aggressiveness at this age group. Other factors related to tumor aggressiveness are the intrinsic subtypes of breast cancer. Specifically, Luminal B and triple-negative subtypes were found quite frequent (≈50–≈20%) in our study population, which is also described in the literature [23]. Although the aforementioned subtypes are associated with poor prognosis in the published literature, our results did not show a statistically significant difference in survival, among the intrinsic subtypes.
All of our patients underwent surgical treatment, half of the patients (51.2%) underwent breast-conserving surgery (BCS) and the other half (48.8%) mastectomy. Our results differ from the published literature [14, 16], where the mastectomy rates are even higher. A possible explanation is that the studies include patients that where treated before the acknowledgement that mastectomy is not inferior to BCS. In our opinion, the mastectomy group percentage should be even lower, because BCS is the treatment of choice, when indicated, but as mentioned above locally advanced breast cancer is presented more often in very young women, in contrast to older women [12]. After conducting a survival analysis, we concluded that BCS can be offered to very young women with breast cancer with the same oncological results as mastectomy, which is in accordance with two recently published studies [24, 25].
Furthermore, almost all of our patients underwent chemotherapy and in 1/3 of them it was neoadjuvant and because these very young women have a longer life-expectancy, if selected properly they may benefit from the increased prognosis of neoadjuvant chemotherapy. The main criteria for this selection are infiltrated axillary lymph nodes, locally advanced tumors and certain intrinsic subtypes (e.g., triple negative). Moreover, nearly 90% of the patients underwent radiotherapy. This high percentage is explained by the fact that post mastectomy radiation therapy was needed in 46 patients (73%) due to large tumors and/or infiltrated axillary lymph nodes.
In our study, the median follow-up time was just over 5 years: 1/4 of the patients recurred and 1/5 died. The recurrence site was equally distributed between locoregional and distant metastasis (most frequent sites: bone, liver, lung), showing the importance of a close follow-up program in these very young women. We focused on the prognostic features associated with the risk of recurrence and death in a multivariate analysis. After adjusting for all the variables, the risk of recurrence was reduced by 70% in Luminal A type tumors, but it was six times higher in patients with positive axillary lymph nodes (both statistically significant). On the other hand, the risk of death was two times higher in the mastectomy group (not statistically significant) and it was also six times higher in patients with positive axillary lymph nodes (statistically significant).
Interestingly, the median DFS was high (130 months) and the median OS was > 173 months, much higher than the results of a resent Egyptian study [14]. This may be due to the fact that stage IV disease was not included in our study, but also in the fact that these young patients undergo more aggressive treatments due to their tolerability. Last but not least, in our study, axillary lymph node status resulted in a statistically significant difference between the two groups in both DFS and OS, but none of the other studied parameters were statistically significant.
Last but not least, the main difference of our study among the others in the literature was the fact that we excluded women with stage IV disease. The main reason that led us to use this exclusion criterion was the knowledge that these women are treated completely different from the other stages and their prognosis is poor from the beginning. Furthermore, the main goals of our study were to point out the need of a better screening program for young women, because like older women, early breast cancer detection offers higher chances of survival. Another difference—advantage of our study, compared to all the aforementioned studies of the literature, was the fact that Ki-67, which plays a deceive role in treatment selection, was absent or not tested in the majority of their study population.
Conclusions
Breast cancer in very young women (≤ 35 years old) is not as rare as some may believe. This specific age group of women has some special tumor characteristics and also some special needs and requirements. Breast cancer appears with larger size and higher-grade tumors, higher incidence of infiltrated axillary lymph nodes, higher Ki-67 expression and intrinsic subtypes with worse prognosis in these very young women. This causes important problems such as disruption of their career, inability of childbearing and family completion, difficulties on carrying out the ongoing family responsibilities, loss of their sexuality, inferior body image and prolonged psychosocial stress. Despite the aggressiveness of the breast cancer in very young women, the DFS and OS rates may be rather high, especially in patients with negative axillary lymph nodes. In addition, the biological characteristics (intrinsic subtypes) of the tumor should be considered in the treatment choice and in the prognosis evaluation. Thankfully, in the last decade many scientists are starting to analyze very young women with breast cancer, but further studies are needed, especially for the quality of life of these patients, due to their long life-expectancy.
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Hartley MC, McKinley BP, Rogers EA et al (2006) Differential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: a case-control study. Am Surg 72:1189–1194
Anders CK, Hsu DS, Broadwater G et al (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330. https://doi.org/10.1200/JCO.2007.14.2471
Rossi L, Mazzara C, Pagani O (2018) Diagnosis and treatment of breast cancer in young women. Curr Treat Options Oncol 20:5
Desreux JAC (2018) Breast cancer screening in young women. Eur J Obstet Gynecol Reprod Biol 230:208–211. https://doi.org/10.1016/j.ejogrb.2018.05.018
Tichy JR, Lim E, Anders CK (2013) Breast cancer in adolescents and young adults: a review with a focus on biology. J Natl Compr Canc Netw 11:1060–1069. https://doi.org/10.6004/JNCCN.2013.0128
Rosenberg SM, Partridge AH (2015) Management of breast cancer in very young women. J Clin Oncol. https://doi.org/10.1016/j.breast.2015.07.036
Tw-B MEE, V IJHI, D JGJ et al (2019) Counseling young women with early breast cancer on fertility preservation. J Assist Reprod Genet 36:4. https://doi.org/10.1007/S10815-019-01615-6
Vuković P, Kasum M, Raguž J et al (2019) Fertility preservation in young women with early-stage breast cancer. Acta Clin Croat 58:147–156. https://doi.org/10.20471/acc.2019.58.01.19
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol Off J Eur Soc Med Oncol 24:2206–2223. https://doi.org/10.1093/annonc/mdt303
Abdulwassi HK, Amer IT, Alhibshi AH et al (2020) Recurrence rates and long-term survival factors in young women with breast cancer. Saudi Med J 41:393–399. https://doi.org/10.15537/smj.2020.4.24987
Cardoso F, Loibl S, Pagani O et al (2012) The European Society of breast cancer specialists recommendations for the management of young women with breast cancer. Eur J Cancer 48:3355–3377. https://doi.org/10.1016/j.ejca.2012.10.004
Paluch-Shimon S, Cardoso F, Partridge AH et al (2020) ESO-ESMO 4rd International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol. https://doi.org/10.1016/j.annonc.2020.03.284
Darwish AD, Helal AM, Aly El-din NH et al (2017) Breast cancer in women aging 35 years old and younger: the Egyptian National Cancer Institute (NCI) experience. The Breast 31:1–8. https://doi.org/10.1016/j.breast.2016.09.018
Farouk O, Ebrahim MA, Senbel A et al (2016) Breast cancer characteristics in very young egyptian women ≤35 years. Breast cancer Dove Med Press 8:53–58. https://doi.org/10.2147/BCTT.S99350
Copson E, Eccles B, Maishman T et al (2013) Prospective observational study of breast cancer treatment outcomes for UK women Aged 18–40 years at diagnosis: The POSH study. JNCI J Natl Cancer Inst 105:978–988. https://doi.org/10.1093/jnci/djt134
Pukkala E, Rautalahti M (2013) Cancer in Finland: publications from the Cancer Society of Finland. Erweko, Helsinki
Kataoka A, Tokunaga E, Masuda N et al (2014) Clinicopathological features of young patients. Breast Cancer 21:643–650. https://doi.org/10.1007/s12282-013-0466-2
Anders CK, Johnson R, Litton J et al (2009) Breast cancer before age 40 years. Semin Oncol 36:237–249. https://doi.org/10.1053/j.seminoncol.2009.03.001
Tang J, Wu C-C, Xie Z-M et al (2011) Comparison of clinical features and treatment outcome of breast cancers in young and elderly chinese patients. Breast Care (Basel) 6:435–440. https://doi.org/10.1159/000332593
Banz-Jansen C, Heinrichs A, Hedderich M et al (2013) Are there changes in characteristics and therapy of young patients with early-onset breast cancer in Germany over the last decade? Arch Gynecol Obstet 288:379–383. https://doi.org/10.1007/s00404-013-2738-7
Thapa B, Singh Y, Sayami P et al (2013) Breast cancer in young women from a low risk population in Nepal. Asian Pac J Cancer Prev 14:5095–5099
Keegan THM, DeRouen MC, Press DJ et al (2012) Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res 14:R55. https://doi.org/10.1186/bcr3156
Vila J, Gandini S, Gentilini O (2015) Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 24:175–181. https://doi.org/10.1016/j.breast.2015.02.002
Quan ML, Paszat LF, Fernandes KA et al (2017) The effect of surgery type on survival and recurrence in very young women with breast cancer. J Surg Oncol 115:122–130. https://doi.org/10.1002/jso.24489
Funding
No funding for this study.
Author information
Authors and Affiliations
Contributions
DZ: Writing—original draft preparation, Data collection, Data analysis. DT: Conceptualization, Writing—review and editing. GG: Methodology, Software. MZ: Methodology, Writing—review and editing. DG. G: Supervision, Writing—review and editing. GD: Data collection. GS: Resources. GG: Validation, Supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Availability of data and material
All data are available in Excel file.
Code availability
RStudio free software was used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zouzoulas, D., Tsolakidis, D., Gitas, G. et al. Breast cancer in women younger than 35 years old. Arch Gynecol Obstet 302, 721–730 (2020). https://doi.org/10.1007/s00404-020-05695-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05695-z