Abstract
It has been shown in several studies that antihormonal compounds can offer effective prophylactic treatment to prevent breast cancer. In view of the low participation rates in chemoprevention trials, the purpose of this study was to identify the characteristics of women taking part in a population-based mammography screening program who wished to obtain information about the risk of breast cancer and then participate in the the International Breast Cancer Intervention Study II (IBIS-II) trial, a randomized double-blind controlled chemoprevention trial comparing anastrozole with placebo. A paper-based survey was conducted in a population-based mammography screening program in Germany between 2007 and 2009. All women who met the criteria for the mammography screening program were invited to complete a questionnaire. A total of 2,524 women completed the questionnaire, and 17.7% (n = 446) met the eligibility criteria for the IBIS-II trial after risk assessment. The women who wished to receive further information about chemoprevention were significantly younger (P < 0.01) and had significantly more children (P = 0.03) and significantly more relatives with breast cancer (P < 0.001). There were no significant differences between the participants with regard to body mass index or hormone replacement therapy. Normal mammographic findings at screening were the main reason (42%) for declining to participate in the IBIS-II trial or attend risk counseling. The ultimate rate of recruitment to the IBIS-II trial was very low (three women). Offering chemoprevention to women within a mammography screening unit as part of a paper-based survey resulted in low participation rates for both, the survey and the final participation in the IBIS-II trial. More individualized approaches and communication of breast cancer risk at the time of the risk assessment might be helpful to increase the participation and the understanding of chemopreventive approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies in recent years have demonstrated that tamoxifen is effective as a prophylactic drug in the prevention of breast cancer [1–4]. The tamoxifen prevention trials showed a reduction in the incidence of breast cancer by 38% (95% CI, 28–46; P < 0.0001), but the rates of endometrial cancer, thromboembolic events and gynaecologic symptoms increased with tamoxifen treatment [5]. These side effects show that there is a continuing need to identify an optimal drug treatment for preventing breast cancer.
Other studies have analyzed the effectiveness of raloxifene as a preventive agent [6, 7]. The National Surgical Adjuvant Breast and Bowel Project Protocol (NSABP) Study of Tamoxifen and Raloxifene (STAR) P-2 trial examined the effects of tamoxifen versus raloxifene on the risk of developing invasive breast cancer and other disease outcomes. It was shown that raloxifene was as effective as tamoxifen in reducing the risk of invasive breast cancer and was associated with a lower risk of thromboembolic events and cataracts. However, there was a higher risk of noninvasive breast cancer with raloxifene, although the difference was not statistically significant. The risks of other cancers, fractures, ischemic heart disease and stroke were similar with the two drugs [7]. Cuzick et al. [5] have provided an overview of prevention studies.
Third-generation aromatase inhibitors have been shown to be more effective than tamoxifen in preventing contralateral breast cancer when administered as an adjuvant treatment for breast cancer [8–12]. Recent publications have confirmed the long-term safety and have clearly established the long-term efficacy of aromatase inhibitors such as anastrozole (ATAC Trialists’ Group), letrozole (BIG 1–98 Collaborative Group) and exemestane (Intergroup Exemestane Study, IES) in comparison with tamoxifen as an initial adjuvant treatment for postmenopausal women with hormone-sensitive early breast cancer [9–11, 13–15].
There is currently a lack of data regarding the efficacy of aromatase inhibitors for chemoprevention of breast cancer. Each of the aromatase inhibitors has been included in the design of a phase 3 randomized breast cancer chemoprevention trial based on hypothesis-generating contralateral breast cancer data from a corresponding adjuvant trial. A large prospective and randomized study on the use of anastrozole as a preventive agent is therefore being conducted—the International Breast Cancer Intervention Study II (IBIS-II) trial [16]. The Mammary Prevention 3 (MAP.3) [17] is examining the benefit of exemestane in chemoprevention, and the “Study to Evaluate Letrozole and Raloxifene” (STELLAR) trial [18] was supposed to investigate letrozole as chemopreventive medication using raloxifene as the control, but never started recruitment. This trio of current aromatase inhibitor prevention trials has been reviewed by Dunn and Ryan [19].
As large sample sizes are needed in chemoprevention trials, optimal recruitment is necessary. In chemoprevention trials, recruitment is aimed at healthy patients who are to receive treatment with potentially harmful drugs. Effective planning and speedy recruitment are crucial for the successful completion of any prevention trial. For example, two studies examining the effect of goserelin with raloxifene (the RAZOR trial) and ibandronate (the GISS trial) [20, 21] had to be prematurely terminated due to poor recruitment. The main reason given by patients for declining to participate in these studies was a fear of side effects [22].
Even women at very high lifetime risk (>40%) of familial breast cancer are barely willing to participate in chemoprevention trials. In the Family History Clinic, Manchester, UK, Evans and co-workers offered such women (n = 4475) the option of entering two chemoprevention treatment trials, a magnetic resonance imaging (MRI) breast screening study, or a risk-reducing mastectomy study. Only 10% (n = 46 of 420) of eligible women have entered one of the chemotherapy trials with a similar proportion (n = 42 of 361) opting for risk-reducing mastectomy (>50% in mutation carriers) compared with 60% (n = 102 of 176) opting for MRI screening [23].
In order to learn more about participation rates in studies on chemopreventive treatment in breast cancer, the aims of this study were to identify the characteristics of women taking part in population-based mammography screening programs in Germany who are willing to obtain information about the risk of breast cancer and chemoprevention programs and to record their ultimate rate of participation in the IBIS-II chemoprevention trial.
Patients and methods
Study population and participating mammography screening units
A multicenter survey was conducted in five population-based mammography screening units in southern Germany between 2007 and 2009. The participating centers were located in Regensburg, Freiburg, Erlangen, Nuremberg and Bayreuth. At least one individual at each center was responsible for ensuring that staff in the participating institutions were informed about the study procedures and distributed the questionnaire in their institutions. Mammographic density as a possible risk factor for breast cancer was not assessed in this study.
All women who met the criteria for the mammography screening program were invited to complete a questionnaire. In accordance with the German mammography screening recommendations, these are women between 50 and 69 years of age who have no history of breast cancer, do not currently have any suspicious breast lesions, and have not undergone mammography during the previous 2 years. The procedure used in inviting women to participate in the mammography screening program in Germany has been described elsewhere [24, 25].
Questionnaire
The questionnaire was designed on the basis of the eligibility criteria for the IBIS-II chemoprevention trial. The first part requested information about the patient’s personal data (body weight, height, date of birth, number of children, menopause status and hormone replacement therapy). The second part included questions about the patient’s medical history (previous breast surgery, previous diagnosis of cancer), with special regard to a history of neoplasia in the breast. The third section covered the women’s family history of breast and ovarian cancer in relation to risk assessment.
The women were asked to indicate whether they wished to be contacted, if they were eligible for participation in the IBIS-II chemoprevention trial or wished to complete the questionnaire anonymously. The questionnaire results were recorded in an electronic data capture system, which automatically assessed eligibility for the IBIS-II chemoprevention trial. Data on mammographic density, which is an inclusion criterion for the IBIS-II chemoprevention trial, were not available for these women and did not result in any indication of increased risk; it is therefore not taken into account here.
Patient information and contact procedure
The women who requested contact if they were eligible for participation in the IBIS-II chemoprevention trial were called and provided with further information about the risk of breast cancer. In the next step, they were offered a personal interview for breast cancer risk counseling, including information about chemopreventive treatment options, with the help of the informed consent procedure for the IBIS-II chemoprevention trial (German version).
The IBIS-II chemoprevention trial
The International Breast Cancer Intervention Study Group is conducting this randomized, double-blind, controlled chemoprevention trial comparing anastrozole with a placebo. The primary aim of this study is to determine whether anastrozole is effective in preventing breast cancer in postmenopausal women at increased risk of developing the disease.
The trial is designed as a randomized, double-blind, placebo-controlled, multicenter study. Participants are randomly assigned to one of two treatment arms. In arm 1, participants receive oral anastrozole daily for 5 years, while in arm 2, they receive an oral placebo daily for 5 years. In both arms, treatment continues in the absence of the development of breast cancer (including ductal carcinoma in situ), a drop in the T-score below minus 4, or the occurrence of a new fragility fracture. Participants are followed for 5 years. The inclusion criteria relative to risk assessment for breast cancer are based on the Tyrer–Cuzick model [26]. The IBIS-II chemoprevention trial has currently recruited more that 5,000 women and will continue recruitment until the end of 2011.
Statistical analysis
All data are presented as means with standard deviation or as frequencies and percentages, unless otherwise noted. Survey participants who met the eligibility criteria and indicated further interest were compared with participants who did not wish to obtain further information, using appropriate statistical tests. Student’s t tests were performed for continuous outcomes, Wilcoxon rank-sum tests for discrete and ordinal-categorical outcomes and χ2 tests or Fisher’s exact test for categorical outcomes. The χ2 test was used when all expected frequencies were greater than five; Fisher’s exact test was used otherwise. Multiple logistic regression models were developed to assess overall associations between participants’ wishes (binary outcome) and patient characteristics (predictor variables). The final model was obtained by backward stepwise variable selection. All tests are two-sided, and a P value of <0.05 was considered statistically significant. All statistical analyses were carried out using the R system for statistical computing (version 2.8.1; R Development Core Team, Vienna, Austria, 2008).
Results
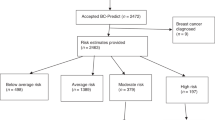
Questionnaires were distributed to 5,151 women participating in the mammography screening program in the five units mentioned above, from 2007 to 2009 (Fig. 1). A total of 2,524 women (49%) completed the questionnaire. Of these, 17.7% (n = 446) met the eligibility criteria for the IBIS-II chemoprevention trial, although it should be borne in mind that mammographic density, which is an inclusion criteria for the trial, was not part of the risk assessment. A total of 202 women (45.3%) wished to obtain further information and 35 requested personal risk counseling at the University Breast Center in Erlangen, Germany. Of these 35 women, three stated that they were interested in participating and were enrolled in the IBIS-II chemoprevention trial.
Sociodemographic data
Table 1 presents the sociodemographic data for the participants in the mammography screening program who completed the questionnaire (n = 2524). Their mean age was 59.5 years, and women with children formed the largest group (89%). The average age at first birth was 23.6 years and the median number of children was two. The women’s mean body mass index (BMI) was 27.3. They were all postmenopausal, with an average age of menopause of 49.1 years; 11.3% of them (n = 277) were receiving hormone replacement treatment. In all, 241 women (9.6%) stated that they had undergone breast surgery, while 72 (2.6%) had a medical history including preneoplastic findings in the breast. A total of 171 (6.8%) had a medical history including a cancer diagnosis of any sort. With regard to family history, 364 women (14.4%) stated that they had relatives with a history of breast and/or ovarian cancer.
Questionnaire responses
Table 2 shows the questionnaire responses of the women who were eligible for inclusion in IBIS-II (n = 446) with regard to their interest in receiving further information. The women willing to receive further information about a chemopreventive breast cancer trial were significantly younger (P < 0.01) and had significantly more children (P = 0.03) and significantly more relatives with breast cancer (P < 0.001) than women who were not interested in receiving any further information. There were no differences between the participants with regard to BMI, HRT, or history of breast surgery or cancer.
All of the patient characteristics in Table 2 were used in the full multivariate logistic regression model. In the backward stepwise selection, the variables “relatives with breast cancer” and “number of children” remained statistically significant (Table 3). In addition, these two variables had a plausible link in the model with a request for further information about breast cancer risk and chemoprevention. The dominant variable predicting a request for further information was the number of relatives with breast cancer. For each relative with breast cancer, the odds for requesting further information were multiplied by 1.7 in comparison with women with a negative family history (Table 3).
IBIS-II-eligible women’s interest in further information adjusted to the IBIS-II inclusion criteria
Table 4 shows the interest in receiving further information expressed by the women who were eligible for inclusion in IBIS-II (n = 446), relative to the adjusted characteristics of the IBIS-II inclusion criteria. The analysis of the variables confirms the strong influence of a family history of breast or ovarian cancer on awareness of breast cancer and willingness to receive further information about a chemopreventive breast cancer trial. The frequency of having more than one relative with breast cancer was significantly higher among women who were interested in receiving information about chemoprevention (P < 0.01) than in those who were not interested. The influence of parity also remained statistically significant (P = 0.02) after adjustment to the IBIS-II inclusion criteria.
Again, all of the variables used in the single analyses were used in the full multivariate logistic regression model. Backward stepwise selection identified the variables “two or more first-degree or second-degree relatives who developed breast or ovarian cancer” and “nulliparous or age at first birth ≥30 years” as the most important predictive factors (Table 5). The dominant variable predicting a request for further information was still the number of relatives with breast or ovarian cancer. These women requested further information more than twice as often (Table 5).
Reasons for not considering chemoprevention
The reasons given by the women who were eligible for inclusion in IBIS-II for requesting further information, but declining to participate in the IBIS-II chemoprevention trial or take the opportunity of attending an information meeting (n = 199, 202 minus 3) are presented in Table 6. A normal mammogram at screening was the main reason given for declining to participate or attend risk counseling, followed by comorbid conditions. Expected organizational and time problems associated with participating in a clinical trial involving a fixed time schedule and attending study centers also emerged as a further obstacle to recruitment for chemoprevention trials.
Discussion
To the best of our knowledge, this is the first study that has investigated willingness to take chemopreventive drugs in a population-based mammography screening cohort of healthy women in a population-based screening setting. The results show that 17.7% of all women who completed the paper-based survey were at increased risk as defined by the inclusion criteria for the IBIS-II chemoprevention trial, even without taking mammographic density into consideration in the risk estimation. However, the final recruitment rate (three of 446 eligible women) is very low.
Women participating in breast cancer prevention trials are now aware that it is possible to reduce their personal risk by taking antihormonal agents. In addition, evidence of an increased risk of breast cancer and cardiovascular disease following the use of HRT has altered women’s awareness in connection with this topic. Fasching et al. [27] showed that 61.4% of participants identified HRT as a risk factor for breast cancer at a time before the publication of the data from the Million Women Study [28] and the Women’s Health Initiative (WHI) trial [29]. However, this information was not associated with greater willingness to receive chemopreventive drugs.
Analysis of factors relating to enrolment in the NSABP-P1 breast cancer prevention trial has shown that concerns about not being able to take HRT were an important factor for nonparticipation in chemoprevention trials [30]. However, the results of the Million Women Study and the WHI trial were not yet available at the time when this report was published.
These findings are in contrast to those of this study in the population-based screening, which show that use of HRT does not significantly influence women’s interest in receiving further information about chemoprevention. Of the 2,524 women who had completed the questionnaire, 11.3% (n = 277) stated that they were receiving HRT. In the group of women eligible for inclusion in IBIS-II (n = 446), 11.8% (n = 53) were receiving HRT. The analysis revealed no differences with regard to requests for further information (P = 0.94) about the risk of breast cancer or chemoprevention; 45.3% of these women (n = 24) were interested in receiving further information, while 54.7% (n = 29) were not. This is in accordance with the reasons given for declining to participate in the IBIS-II chemoprevention trial or to take the opportunity to attend an information meeting among the women eligible for inclusion in IBIS-II (n = 199, 202 minus 3), only one of whom stated that unwillingness to stop HRT was a reason for declining.
The results of the WHI trial confirmed that combined estrogen–progestin use was positively associated with an increased risk of breast cancer [31]. The early termination of the WHI trial received attention in the mass media and was followed by strong declines in HRT use in Western countries [32]. One year later, the Million Women Study, a cohort study of British women, demonstrated that past users no longer had an increased risk of breast cancer occurrence [33, 34]. Nonetheless, the publication of controversial data concerning HRT in recent years has caused a significant reduction in the use of HRT. It is therefore not surprising that concerns about not being able to take HRT lost their predictive value in relation to participation in chemoprevention trials.
Our study identified 446 of 2,524 women (17.7%) as having an increased risk of breast cancer according to the IBIS-II inclusion criteria (without the important risk factor mammographic density). Compared to previous studies with less than 10% of eligible women for chemoprevention [23], this must be considered a high number. A selection bias seems to be probable, given the fact that only 49% (n = 2524) completed the distributed questionnaire. It has to be pointed out that the completion of the survey was completely voluntary. In one earlier study, we identified an increased breast risk as the main factor correlating with the interest in the topic of chemoprevention and breast cancer risk [27].
The ultimate recruitment rate was very low (n = 3). In view of the fact that the majority of the women eligible for IBIS-II who requested further information but did not participate in the trial (n = 199) stated that a normal mammogram at screening (42%) was the main reason for declining to participate, it appears to be doubtful whether chemoprevention assessment can be implemented in a mammography screening program. The fact of having a normal mammogram appears to outweigh the fear of an increased risk of breast cancer and the need for chemoprevention.
Further reasons given for declining to participate in the IBIS-II chemoprevention trial or to take the opportunity of attending an informative counselling among women eligible for inclusion in IBIS-II was available from 199 patients. In addition to concerns about concomitant diseases (20%), a lack of mobility in the countryside in northern Bavaria (12%) was a major reason given for declining to participate, which usually correlates with higher age. In this study, 21% of the women (19 of 91) in the group aged >64 stated that a long journey was a serious obstacle, while in the group aged <55, the figure was only 5.5% (one of 18). Expected time problems associated with participating in a clinical trial with a fixed time schedule and study centers was only stated as being an obstacle by 7% of the women.
With regard to the predictive values in this survey, the logistic model correctly classified 70% of the women who did not request further information and only 42% of the women who requested further information. If it is assumed, as was observed, that in general about half of all eligible individuals are actually interested in further information, the positive predictive value of the model is approximately 60%. However, these estimates are optimistic, as they are based on the same data that were used to fit the model.
When one attempts to summarize all of the factors analyzed in this study, an individual participant’s family history of breast cancer appears to be the key factor in her willingness to undergo treatment with chemopreventive drugs. In clinical practice, counseling patients in relation to their risk of breast cancer is a complex task. Several risk factors have to be taken into consideration. Many models have been published for different data sets of risk factors [35–38]. Some of the models tend to rely more on genetic susceptibility, while others include clinical risk factors. Current studies such as the IBIS-II chemoprevention trial use prediction models like the Tyrer–Cuzick risk calculator [26]. It is not yet clear which of these models best fits the population receiving counseling. To date, only a few evaluation studies have been published [39].
Moreover, extensive evidence has grown that high mammographic density is a risk factor for breast cancer [40]. Recently published prediction models include mammographic density as an additional risk factor [41, 42], and the incremental benefit of breast density in assessing breast cancer risk was confirmed by a metaanalysis of Cummings et al. [43]. As already pointed out, our study did not use breast density for identifying women at risk. It has to be hypothesized that including this risk factor would lead to substantially different results.
Summarizing the present results shows that women participating in a population-based mammography screening program are willing to complete a short, structured questionnaire. This can be regarded as justifying the use of this type of instrument and providing women with an opportunity to find out more about their breast cancer risk and possible chemoprevention strategies. However, the resulting recruitment rate from this screening program was disappointing. Interestingly, women’s concerns regarding HRT were not found to have any predictive value for participation in chemoprevention trials, in contrast to the findings of earlier studies.
Information regarding the factors that influence a patient’s willingness to participate in chemoprevention trials could help to improve recruitment. Evaluating the effects of a woman’s risk of breast cancer, parity and age before she enters a clinical trial could help identify potential participants. However, better information about further factors, like for example mammographic density that determine and influence patients’ attitudes to participation in prevention trials is needed in order to adapt the study design and inclusion criteria and increase participation rates and compliance in such trials.
References
Cuzick J, Forbes J, Edwards R et al (2002) First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360:817–824
Fisher B, Costantino JP, Wickerham DL et al (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Powles T, Eeles R, Ashley S et al (1998) Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352:98–101
Veronesi U, Maisonneuve P, Sacchini V, Rotmensz N, Boyle P (2002) Tamoxifen for breast cancer among hysterectomised women. Lancet 359:1122–1124
Cuzick J, Powles T, Veronesi U et al (2003) Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300
Cauley JA, Norton L, Lippman ME et al (2001) Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat 65:125–134
Vogel VG, Costantino JP, Wickerham DL et al (2006) Effects of tamoxifen vs. raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Baum M, Budzar AU, Cuzick J et al (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Thurlimann B, Keshaviah A, Coates AS et al (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Jakesz R, Jonat W, Gnant M et al (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366:455–462
Mouridsen H, Giobbie-Hurder A, Goldhirsch A et al (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 361:766–776
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45–53
Coombes RC, Kilburn LS, Snowdon CF et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369:559–570
Cuzick J (2003) Aromatase inhibitors in prevention—data from the ATAC (Arimidex, tamoxifen alone or in combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study). Recent Results Cancer Res 163:96–103 (discussion 264–266)
Goss PE, Richardson H, Chlebowski R et al (2007) National Cancer Institute of Canada Clinical Trials Group MAP.3 Trial: evaluation of exemestane to prevent breast cancer in postmenopausal women. Clin Breast Cancer 7:895–900
Editorial (2007) NCI and the STELLAR trial. Lancet 369:2134
Dunn BK, Ryan A (2009) Phase 3 trials of aromatase inhibitors for breast cancer prevention: following in the path of the selective estrogen receptor modulators. Ann N Y Acad Sci 1155:141–161
Powles TJ (2002) Breast cancer prevention. Oncologist 7:60–64
Vescia S, von Minckwitz G, Loibl S et al (2008) The GISS Trial: a pilot phase randomized prevention trial of screening plus goserelin plus ibandronate, versus screening alone in premenopausal women at increased risk of breast cancer (abstract). Eur J Cancer 6:72
von Minckwitz GPB, Hofmann K et al (2002) Prevention with goserelin and ibandronate in premenopausal women with familial breast cancer risk: first experiences of the GISS study (abstract). Arch Gynecol Obstet 267(Suppl 1):S52
Evans D, Lalloo F, Shenton A, Boggis C, Howell A (2001) Uptake of screening and prevention in women at very high risk of breast cancer. Lancet 358(9285):889–890
Schulz-Wendtland R, Becker N, Bock K, Anders K, Bautz W (2007) Mammography screening. Radiologe 47:359–369 (quiz 70)
Kooperationsgemeinschaft-Mammographie (2009) Evaluationsbericht 2005–2007: Ergebnisse des Mammographie-Screening-Programms in Deutschland 2009. www.mammo-programm.de/fachinformationen/evaluation.php
Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111–1130
Fasching PA, von Minckwitz G, Fischer T et al (2007) The impact of breast cancer awareness and socioeconomic status on willingness to receive breast cancer prevention drugs. Breast Cancer Res Treat 101:95–104
The Million Women Study Collaborative Group (1999) The Million Women Study: design and characteristics of the study population. The Million Women Study Collaborative Group. Breast Cancer Res 1:73–80
The Women’s Health Initiative Study Group (1998) Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 19:61–109
Yeomans-Kinney A, Vernon SW, Frankowski RF, Weber DM, Bitsura JM, Vogel VG (1995) Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment. Cancer 76:46–56
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Majumdar SR, Almasi EA, Stafford RS (2004) Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA 292:1983–1988
Beral V (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419–427
Chlebowski RT, Kuller LH, Prentice RL et al (2009) Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 360:573–587
Gail MH, Brinton LA, Byar DP et al (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81:1879–1886
Claus EB, Risch N, Thompson WD (1994) Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73:643–651
Parmigiani G, Berry D, Aguilar O (1998) Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 62:145–158
Antoniou AC, Pharoah PP, Smith P, Easton DF (2004) The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91:1580–1590
Fasching PA, Bani MR, Nestle-Kramling C, Goecke TC, Niederacher D, Beckmann MW, Lux MP (2007) Evaluation of mathematical models for breast cancer risk assessment in routine clinical use. Eur J Cancer Prev 16:216–224
Boyd NF, Guo H, Martin LJ et al (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356:227–236
Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K (2008) Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 148(5):337–347
Chen J, Pee D, Ayyagari R, Graubard B, Schairer C, Byrne C, Benichou J, Gail MH (2006) Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst 98(17):1215–1226
Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R et al (2009) Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst 101(6):384–398
Acknowledgements
This project was partly sponsored by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christian R. Loehberg and Sebastian M. Jud contributed equally to this study.
Rights and permissions
About this article
Cite this article
Loehberg, C.R., Jud, S.M., Haeberle, L. et al. Breast cancer risk assessment in a mammography screening program and participation in the IBIS-II chemoprevention trial. Breast Cancer Res Treat 121, 101–110 (2010). https://doi.org/10.1007/s10549-010-0845-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0845-8