Abstract
Inspired by the model by Walter and Day for risk of cervical cancer following negative screens, one might hypothesize that women in a mammography screening programme with a certain number of negative screens had a lower remaining breast cancer risk than that of women in general. We studied whether number of negative screens was a predictor for a low remaining breast cancer risk in women participating in the mammography screening programmes in Stockholm, Copenhagen and Funen. Data were collected from the mammography screening programmes in Stockholm, Sweden (1989–2012), Copenhagen, Denmark (1991–2009) and Funen, Denmark (1993–2009), and linked to the respective cancer registries. We calculated cumulative hazard rates for breast cancer in women in cohorts defined by age at entry and number of negative screens for the maximum follow-up period in each screening centre. For all centres and cohorts, the cumulative hazard were parallel for all number of negative screens, from after the time, when the women were scheduled to be invited for the next screen. This means that the remaining breast cancer risk is similar no matter how many negative screens a woman have had. Number of negative screens was not a predictor of a low remaining breast cancer risk in women participating in the mammography screening programmes in Stockholm, Sweden, Copenhagen and Funen, Denmark. The history of previous negative screens is therefore not suitable for personalisation of mammography screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of randomized trials of mammography screening have shown a 20–30 % reduction in breast cancer mortality at population level [1]. Following these randomized trials, national screening recommendations as well as guidelines from the European Commission [2] were issued and aided in establishing organised mammography screening programmes in several European countries.
Within the organized programmes, screening is offered equally to all women in the targeted age groups. As knowledge concerning breast cancer risk factors has accumulated [3, 4], efforts are now focused on improving screening by stratifying the screening schedule by risk factors. Several studies have for instance modelled the expected screening outcome by polygenic risk score and other known breast cancer risk factors [5–9]. Inspired by the model developed by Walter and Day [10] for evaluation of cervical cancer screening, we hypothesized that women with a certain number of negative screens belong to a particular group of women with a remaining breast cancer risk lower than that of women in general. We studied whether a certain number of negative screens are a predictor for a low remaining breast cancer risk in women participating in the mammography screening programmes in Stockholm, Copenhagen and Funen.
Materials and methods
Service mammography screening started in Stockholm, Sweden in 1989, and in Copenhagen and Funen, Denmark in 1991 and 1993, respectively, with biennial screening offered to women aged 50–69 years. All targeted women were personally invited to screening, however, in Funen invitation stopped in non-attending women. In order to take account of age, calendar year and number of offered screens, we studied a fixed cohort of women aged 50–64 years and living in Stockholm/Copenhagen/Funen, at the first date of the first invitation round of the respective programme, Fig. 1 shows the Copenhagen example. Women aged 65–69 at the first date of the first invitation round was not included as these women would have very few screens before leaving the programmes at age 70.
Data sources
In Stockholm, data were collected from the mammography screening register which includes data originating from the Swedish Population Register and the Stockholm Gotland Regional Cancer Register [11]. All women invited for any of the invitation rounds were included in the Stockholm mammography screening register, as well as dates of screening, dates of diagnosis, screening diagnosis, and all procedures following each screen. The Stockholm screening register includes all women resident in the county since 1989 and is updated with vital status and emigration on a weekly basis. Cancers diagnosed outside the screening programme (but within the region) were identified from the regional cancer register. Data linkage was made using the unique identification number issued to all Swedish residents. Women were observed from the first date of the first invitation round until date of breast cancer, death, emigration, or end of follow-up, being 31 December 2012 for Stockholm, whichever of these dates came first.
In Denmark, data were retrieved from the Mammography Screening Database [12]. This database includes data from the Copenhagen and Funen programmes coupled with data from the Danish Civil Registration System (CRS), the Danish Cancer Registry, the Danish Breast Cancer Cooperative Group, and the National Pathology Register. The database includes information on dates of invitation, participation, outcome of each screen, interval cancers, vital status and emigrations. Data were linked using the unique identification number issued to all Danish residents. Women were observed from the first date of the first invitation round until date of breast cancer, death, emigration, or end of follow-up, being 30 November 2009 for Copenhagen and 31 December 2009 for Funen, whichever of these dates came first.
Definitions
A negative screening outcome was defined as a screening session without identified abnormalities (equal to BI-RADS 1, 2 and part of 3), which included women with a positive screen and a negative assessment, i.e. women with false positive tests.
A breast cancer was an invasive breast cancer, equal to ICD-10 C50, or a carcinoma in situ, equal to ICD-10 D05. We distinguish between screen-detected breast cancers and non-screen-detected breast cancer, in the following called other breast cancers.
An invitation round was defined as a period of approximately 2 years in accordance with the administration of the respective programme.
Analysis
In the analysis women were divided into three fixed cohorts:
-
Cohort 1: women aged 50–54 years at first date of first invitation round and living in the screening area on this date.
-
Cohort 2: women aged 55–59 years at first date of first invitation round and living in the screening area on this date.
-
Cohort 3: women aged 60–64 years at first date of first invitation round and living in the screening area on this date.
Each of these 3 cohorts was further stratified by number of negative screens:
-
0 Negative screening outcome: Women were in this group from the date the screening programme started, until the date of their first negative screening outcome, date of breast cancer diagnosis (both screen-detected and cancers detected outside the programme), death, emigration, or end of follow-up, whichever came first, see Fig. 2.
-
1 Negative screening outcome: Women attending screening in the first invitation round and having a negative screening outcome. Women were in this group until the date of their second negative screening outcome, date of breast cancer diagnosis, death, emigration, or end of follow-up, whichever came first, see Fig. 2.
-
2 Negative screening outcome: Women attending the first two invitation rounds and at both screenings having a negative screening outcome. Women were in this group until the date of their third negative screening outcome, date of breast cancer diagnosis, death, emigration, or end of follow-up, whichever came first, see Fig. 2.
-
And so on.
For each cohort, we calculated the cumulative hazard rate of breast cancer by time since first screening date and number of negative screening outcomes.
By definition, a woman with x negative screening outcomes, who have a screen-detected breast cancer at her next screen, will remain in the x negative screening outcome group, and her screen-detected breast cancer will contribute to the cumulative hazard for the x negative screening outcome group. Since a lot of the breast cancers are screen-detected, the cumulative hazard for the x negative screening outcome group will increase around the time where the women are screened again. If the cumulative hazards from two groups are proportional from a certain point in time it means that from this point in time, the two groups have the same remaining breast cancer risk. Until women in both groups have been invited for their next screening and the cumulative hazard have increased due to this, the cumulative hazard cannot be proportional, since the increase does not happen at the same time. If the cumulative hazards from two groups (women with x and x−1 negative screening outcomes) are proportional from the time where women with x negative screening outcomes are no longer invited at next screen, then the remaining breast cancer risk is equal for women with x and x−1 negative screening outcomes, respectively.
For the Stockholm data we used STATA version 12, and for the Danish data SAS version 9.1.2.
Results
Tables 1, 2 and 3 show the data for all the three cohorts in the three screening centres, respectively. In all cohorts in all three centres, the number of women was largest in the group of 1 negative screening outcome and this number declined with increasing number of negative screening outcomes. This is due to the fact that women with 5 negative screening outcomes will necessarily have had 4 negative screening outcomes prior to the fifth one, as well as 3, 2 and 1 prior to the fourth one. However, the breast cancer cases were not passed from one group to the next, as follow-up was discontinued when a woman was diagnosed with breast cancer.
In Stockholm, the 20,808 women in cohort 1 with 2 negative screening outcomes had 85 screen-detected cancers and 106 other breast cancers from the time they had their second negative screening outcome until they had their third negative screening outcome, emigrated, died or end of follow-up. In total, the 20,808 women had 191 breast cancers when belonging to the 2 negative screening outcomes group. The 7763 women in cohort 1 with 9 negative screening outcomes had in total 195 breast cancers while being in this group. The fact that the 9 negative screening outcomes group had more breast cancers than the 2 negative screening outcomes group does not imply that the 9 negative screening outcomes group had a higher cumulative breast cancer incidence as the two groups did not have the same follow-up time.
The mammography screening programme in Funen had more screen-detected breast cancers than the programmes in Copenhagen and Stockholm, probably due to a higher proportion of women who participates in all screenings offered to them.
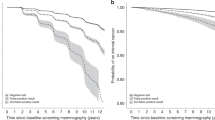
Figure 3 show the cumulative breast cancer hazard in the Stockholm, Copenhagen and Funen mammography screening programmes by number of negative screening outcomes. The figures look remarkably alike for the 3 centres. Since we calculated only cumulative hazard from the time of the start of the screening programme, and since women with a recent breast cancer diagnosis was not invited to screening, women with 1 negative screening outcome had by definition a cumulative breast cancer risk of 0 at the time of their first invitation to screening (0–2 years). Women with 1 negative screening outcome (the blue dots) will have their next screen at approximately 2 years later (2–4 years after the start of the screening programme), where the cumulative hazard curve increased dramatically due to screen-detected cancers, where after it slowed down. It should be noted that at this point in time the majority of women had left this cohort and moved on to the cohort with 2 negative screening outcomes. Exactly the same picture was seen for women with more negative screening outcomes. When the cohort of women turns so old that not everyone is invited to the next screening, then the cumulative hazard curve will not increase so dramatically at the time of the next screen.
For all centres and cohorts, the cumulative hazards are parallel for all number of negative screening outcomes from after the time when the women were scheduled for the next screen. This means that the future risk of getting breast cancer after having participated in a screening does therefore not seem to depend on previous number of negative screening outcomes.
Discussion
We wanted to test the hypothesis that women with a certain number of negative screening outcomes constituted a subpopulation with a lower than average breast cancer risk. Our data did not support this hypothesis.
It was a major strength of this study that we had access to each individual woman’s complete screening history and follow-up data. It was furthermore a strength that the same pattern was found in 3 independent service mammography screening programmes.
One of the weaknesses in the study was the short follow-up time in Copenhagen and Funen for women with a history of many negative screening outcomes. A woman who had accumulated 7 negative screening outcomes in Copenhagen had only a follow-up time of a maximum of 6 years. With records going back to 1989, the follow-up time in Stockholm for the same group of women was longer resulting in a follow-up time of a maximum of 11 years. Since the same pattern was seen in all 3 programmes until the end of the follow-up time in Copenhagen and Funen, it seems likely that the pattern seen afterwards in Stockholm was representative for all 3 programmes.
In Stockholm, breast cancers diagnosed outside the programme in women who had moved out of the Stockholm region were not included. This is probably a small number of breast cancers, and it is unlikely to have affected the patterns of cumulative hazards, as we saw similar patterns in all 3 programmes.
We have undertaken sensitivity testing by looking at 2-year age cohorts instead of 5-year age cohorts in order to examine whether there was in fact an age effect that could be clouded by the use of a broader age span. However, this analysis did not reveal any age effect (data not shown).
A few studies have reported on the impact on breast cancer detection of the length of the screening interval [13–15]. However, we found no study focusing on the impact of repeated screening attendance on breast cancer detection. Our study showed that the remaining risk of breast cancer did not decrease with increasing number of negative screening outcomes. Based on these data it can therefore not be recommended to cease invitation to screening after a few screening rounds, and women should be encouraged to continue attendance even after repeated negative screening outcomes.
Conclusion
Our results show that women in a mammography screening programme with a high number of previous negative screening outcomes did not have a lower breast cancer risk than women with a low number of previous negative screening outcomes. Therefore it is not possible to personalize mammography screening schedules based solely on prior screening history.
References
Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–86.
Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, Von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol. 2008;19(4):614–22.
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86.
Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–30.
Darabi H, Czene K, Zhao W, Liu J, Hall P, Humphreys K. Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. Breast Cancer Res. 2012;14(1):R25.
Pashayan N, Duffy SW, Chowdhury S, Dent T, Burton H, Neal DE, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104(10):1656–63.
Pharoah PD, Antoniou AC, Easton DF, Ponder BA. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358(26):2796–803.
Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20.
Wu YY, Yen MF, Yu CP, Chen HH. Individually tailored screening of breast cancer with genes, tumour phenotypes, clinical attributes, and conventional risk factors. Br J Cancer. 2013;108(11):2241–9.
Walter SD, Day NE. Estimation of the duration of a pre-clinical disease state using screening data. Am J Epidemiol. 1983;118(6):865–86.
The National Board of Health and Welfare—Swedish Cancer Registry http://www.socialstyrelsen.se/register/halsodataregister/cancerregistret.
von Euler-Chelpin M, Lynge E, Rebolj M. Register-based studies of cancer screening effects. Scand J Public Health. 2011;39(7 Suppl):158–64.
O’Meara ES, Zhu W, Hubbard RA, Braithwaite D, Kerlikowske K, Dittus KL, et al. Mammographic screening interval in relation to tumor characteristics and false-positive risk by race/ethnicity and age. Cancer. 2013;119(22):3959–67.
van Ravesteyn NT, Heijnsdijk EA, Draisma G, de Koning HJ. Prediction of higher mortality reduction for the UK Breast Screening Frequency Trial: a model-based approach on screening intervals. Br J Cancer. 2011;105(7):1082–8.
Weiss NS, Etzioni R. Estimating the influence of rescreening interval on the benefits associated with cancer screening: approaches and limitations. Epidemiology. 2002;13(6):713–7.
Acknowledgments
This project was financially supported by the Nordic Cancer Union and the PhD-programme of the University of Copenhagen.
Conflict of interest
The authors declare no conflict of interest.
Ethical standard
This study was entirely based on data from registers. No contact was made with patients, relatives or their doctors. In Sweden, the study was carried out in accordance with the Swedish Data Protection Law. In Denmark, the study was registered with the Danish Data Inspection (number 2008-41-2191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andersen, S.B., Törnberg, S., Kilpeläinen, S. et al. Is mammography screening history a predictor of future breast cancer risk?. Eur J Epidemiol 30, 143–149 (2015). https://doi.org/10.1007/s10654-014-9972-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9972-6