Abstract
Aromatase inhibitors (AIs) are considered the gold standard for endocrine therapy of estrogen receptor (ER) positive postmenopausal breast cancer patients. The therapy may enhance therapeutic response and stabilize disease but resistance and disease progression inevitably occur in the patients. These are considered at least partly due to an emergence of alternative intratumoral estrogen production pathways. Therefore, in this study we evaluated effects of exemestane (EXE) upon the enzymes involved in intratumoral estrogen production including estrogen sulfatase (STS), 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1), and estrogen sulfotransferase (EST) and correlated the findings with therapeutic responses including Ki67 labeling index (Ki67). 116 postmenopausal patients with invasive ductal carcinoma, stage II/IIIa, were enrolled in JFMC34-0601 clinical trials between March, 2006 and January, 2008. EXE of 25 mg/day was administered according to the protocol. Pre- and posttreatment specimens of 49 cases were available for this study. Status of STS, EST, 17β-HSD1, ER, progesterone receptor (PgR), human epidermal growth factor receptor type 2 (Her2), and Ki67 in pre- and post-specimens were evaluated. Specimens examined before the therapy demonstrated following features; ER+ (100%), PgR+ (85.7%), and Her2+ (77.6%). After treatment, the number of Ki67, PgR, and ER positive carcinoma cells demonstrated significant decrement in clinical response (CliR) and pathological response (PaR) groups. Significant increment of 17β-HSD1 and STS immunoreactivity was detected in all groups examined except for STS in PaR. EST showed significant increment in nonresponsive groups. Alterations of Ki67 of carcinoma cells before and after therapy were subclassified into three groups according to its degrees. Significant alterations of intratumoral enzymes, especially 17β-HSD1 and STS, were correlated with Ki67 reduction after neoadjuvant EXE therapy. This is the first study demonstrating significant increment of STS and 17β-HSD1 following AI neoadjuvant therapy of postmenopausal ER positive breast carcinoma patients. This increment may represent the compensatory response of breast carcinoma tissues to estrogen depletion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy among women worldwide and the leading cause of cancer-related death in many countries [1, 2]. Approximately 60% of premenopausal and 75% of postmenopausal patients have sex steroid hormone-dependent breast carcinoma [3, 4]. Among these sex steroids, estrogens, especially estradiol or E2, a biologically potent estrogen, play pivotal roles in cell proliferation, development, and invasion of these hormone-dependent breast carcinoma cells [3–5].
Intratumoral estrogen production in breast carcinoma tissues has been advocated by Miller et al. in 1974 [6]. Its clinical significance was in dispute but the presence of local production of estrogens in breast carcinoma tissues has been subsequently reported by others [7, 8]. Aromatase, one of the enzymes involved in estrogen production, was subsequently demonstrated in adipocytes, stromal cells, and carcinoma cells of breast cancer tissues [9–13]. In addition, the other enzymes involved in intratumoral estrogen production (steroid or estrogen sulfatase; STS, estrogen sulfotransferase; EST and 17β-hydroxysteroid dehydrogenase type 1; 17β-HSD1, etc.) have been also reported to be overexpressed in human breast carcinoma tissues by a number of laboratories [10, 12, 14, 15].
Among these enzymes above, aromatase is the one catalyzing the rate limiting step in the biosynthetic pathway for estrogen [16] and has been considered an important critical target for pharmacological inhibitors which may cause estrogen deprivation for the postmenopausal patients with estrogen receptor (ER) positive breast carcinoma [3, 13, 15]. An introduction of aromatase inhibitors (AIs) in the treatment algorithms of these breast cancer patients has actually been considered one of the major achievements in breast cancer therapy through the last decades [17, 18]. Especially, third-generation AIs suppressed the aromatase activity in the magnitudes of more than 98%, which subsequently resulted in clinical benefits and relatively lower incidences of adverse effects [16, 19]. This therapy has been established as gold standard of endocrine therapy for all stages of ER positive postmenopausal breast cancer patients in numerous countries including Japan.
It is, however, also true that resistance to these endocrine therapies still occurs, which has resulted in serious clinical problems in the management of these patients above. The mechanisms of this endocrine resistance have been examined by many investigators from the standpoints of either de novo or intrinsic and acquired resistances. Mechanisms of intrinsic or de novo resistance were evident at an initial exposure to endocrine therapy even in some ER abundant tumor cases [4]. The exact mechanisms for this type of resistance have still remained unknown at this juncture. Acquired resistance usually develops during the course of endocrine therapy of the patients who initially respond to the AIs treatment. This mode of resistance has been, in general, explained by a possible adaptation of carcinoma cells to acquire the potential to proliferate despite the inhibition or suppression of aromatization or in situ depletion of estrogens, i.e., de novo acquirement of novel signaling mechanisms to develop a state of estrogen hypersensitivity in breast carcinoma cells, which subsequently circumvent the clinical effects of AIs [4, 16].
Multiple clinical trials have been recently designed in order to examine these resistance mechanisms of AIs [4, 20–24]. A number of putative theories have been proposed to explain the development of this resistance to AIs during the treatment but an adaptation of hormone-dependent breast carcinoma cells to estrogen withdrawal or depletion and develop estrogen hypersensitivity is considered to represent the common biological features [4, 23, 24]. It is also important to note that enzymes other than aromatase are indeed involved in intratumoral estrogen synthesis in human breast carcinoma tissue as described above. However, alterations of these enzymes before and after AIs therapy have not been known at all to the best of our knowledge. Therefore, in this study, we evaluated the changes of the enzymes involved in intratumoral estrogen production including STS, 17β-HSD1 and EST in breast carcinoma tissues before and after the neoadjuvant exemestane (EXE) treatment using immunohistochemistry (IHC). We then correlated the findings with the therapeutic responses of individual patients including clinical and pathological responses and alterations of Ki67 before and after the therapy of individual patients. We also correlated the findings with changes of ER, Progesterone receptor (PgR) and human epidermal growth factor receptor type 2 (Her2) in breast carcinoma.
Materials and methods
Breast carcinoma cases
116 Japanese postmenopausal patients (55–75 years old), in whom the operable primary breast tumors had been histological diagnosed as primary invasive ductal carcinoma, TNM stage II-IIIA, had been enrolled into the study of JFMC34-0601[25] of Japanese Foundation for Multidisciplinary Treatment of Cancer between March, 2006 and January, 2008. The menopausal status was defined by natural menopause: at least 1 year since the last menstrual period with the serum level of Follicle-stimulating hormone (FSH) and plasma E2 within the postmenopausal range (FSH ≥30 IU/L, E2 <10 pmol/l). None had received prior treatment with hormonal agents, chemotherapy or endocrine therapy for breast cancer nor were taking any medications including hormonal preparations at the time of study. None had the past history of breast cancer. All patients provided written informed consents to this study, which had been approved by the local ethics committee or institutional review board.
JFMC34-0601 trial was a multicenter phase II study by Japanese Foundation for Multidisciplinary Treatment of Cancer performed from March 2006 to January 2008. The study was conducted to evaluate the possible efficacy and safety of EXE treatment for 24 weeks administration in Japanese patients with breast carcinoma. 116 Japanese postmenopausal patients had been enrolled into this study and all the patients had been diagnosed as primary operable invasive ductal carcinoma of the breast. Primary clinical endpoints were objective response rates and safety after 24 weeks of the treatment. This trial study demonstrated that 24 weeks EXE treatment was more effective than 16 weeks administration.
According to the protocol of JFMC34-0601 multicenter phase II trial study, all 116 patients initially received EXE as an oral dose of 25 mg daily for 16 weeks and then additional 8 weeks treatment was subsequently given after clinical evaluation done at week 16. Tumor size was serially monitored by calipers and breast ultrasound before treatment, at 16 weeks and at 24 weeks after receiving the neoadjuvant therapy. At week 16, clinical response was assessed and if the patients were evaluated as clinical responders (complete response or partial response or stable disease), 8 weeks of the same treatment was subsequently added until reaching the total treatment period of 24 weeks. However, if the patients were classified as clinical nonresponders (progressive disease), these patients either underwent surgery or received another modes of treatment. All the patients but ten patients (6 patients discontinued the treatment because of adverse effects and 4 patients had been classified as nonresponders) continuously received the therapy until reaching the total period of 24 weeks treatment. At week 24, clinical response was reevaluated and all the patients underwent definitive surgery. The specimens available for examinations in this study were pretreatment core needle biopsies and post treatment surgical specimens which were obtained after the surgery at week 16 or 24. The pre- and posttreatment specimens of 49 patients among these patients were available for this study of pathological response and the Immunohistochemical evaluation. ER, PgR, and Her2 status was performed by individual institutions by means of standard procedures and retrieved the data for central review and analysis.
Clinical response
Clinical response was based on changes in tumor volume taken at 16 weeks and/or 24 weeks after the neoadjuvant therapy. Clinical response was defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumors [26].
Pathological response
Tissue sections of the same tumors from pretreatment core needle biopsies and final surgical specimens were obtained and assessed for changes in cellularity and degree of fibrosis in hematoxylin-eosin stained slides. Pathological response was categorized, using the modified criteria described by Miller et al. [27], and assessed as follows: complete when there was no evidence of malignant cell at the original tumor site; partial response when histological decrement in cellularity and/or increment in fibrosis was detected; or no change/nonresponse, by two of the authors above (NC and MC).
Immunohistochemistry
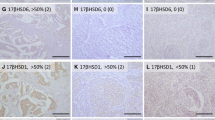
One 4-μm section of each submitted paraffin blocks of pre- and posttreatment specimens were stained with hematoxylin-eosin to verify an adequate number of invasive breast carcinoma cells and the quality of fixation in order to determine the suitability of further immunohistochemical analyses. Serial tissue sections (4-μm) were then prepared from selected blocks and immunohistochemistry was performed to immunolocalize STS, 17β-HSD1, EST and Ki67 as described previously [10, 14, 28]. In brief, IHC staining was performed by streptavidin-biotin amplification method using a Histofine Kit (Nichirei, Tokyo, Japan). The lists of primary antibodies used in this study and concentrations with the antigen retrieval method were summarized in Table1. Tissue sections of full-term placenta were used as positive controls for STS, 17β-HSD1 and EST.
The immunostained slides were independently evaluated by three of the authors (NC, TS and HS), blinded to clinical outcome of individual patients. STS, 17β-HSD1, and EST immunoreactivity was evaluated using a semiquantitative method as follows: score 2, >50% positive cells; 1, 1–50% positive cells; and 0, no immunoreactivity, as previously described by Suzuki et al. [14]. Evaluation of Ki67 was performed by counting of 1,000 carcinoma cells or more from each cases and the percentage of immunoreactivity was subsequently determined as a labeling index (LI) [28].
In addition, the Ki67 LI was then subclassified, using the criteria described by Miller et al. [27], into three different groups according to the percentage of Ki67 alterations after treatment as follows: Group1; increased group, the Ki67 LI in this group was associated with increment after therapy. Group2; no change group, the Ki67 LI demonstrated unchanged or reduction for less than 40% of the pretreatment level. Group3; decreased group, the Ki67 LI demonstrated the reduction for more than 40% of the pretreatment level. ER and PgR immunoreactivity was scored by assigning proportion and intensity scores, according to Allred’s procedure [29]. The membrane staining pattern was estimated in Her2 immunohistochemistry and scored on a scale of 0–3 [30]. ER, PgR, and HER2 were all independently evaluated by two of the authors (TS and HS).
Statistical analysis
The Mann–Whitney U test was used to compare the pretreatment IHC scores of all biological markers according to the clinical and pathological responses to EXE treatment in individual patients. The Wilcoxon matched-pairs signed-ranks test was employed in order to determine the mean differences between pre- and posttreatment IHC scores of individual biological markers in relation to the clinical and pathological responses status and alterations of Ki67 LI. The correlation among intratumoral enzymes (STS, 17β-HSD1 and EST) were analyzed using Spearman’s rank nonparametric correlation test. The statistically significance was considered for the P value < 0.05.
Results
The breast carcinoma specimens examined before the therapy demonstrated the following features after central review of the specimens; ER+ (100%), PgR+ (85.7%) and Her2+ (77.6%).
Clinical and pathological responsiveness
The relevant clinical findings of the patients were summarized in Table 2. All the patients in this study received 16 weeks of EXE treatment but 2 patients were evaluated as PD and the subsequent surgery was advocated at 16 weeks of treatment. Only 47 patients were continuously administrated for EXE treatment until 24 weeks. Clinical response was reevaluated and clinical responders were classified as PR in 27 cases or 55.1% while clinical nonresponders included 19 cases of SD (38.8%) and 3 cases of PD (6.1%) including those previously evaluated as PD (2 patients). Pathological responders and nonresponders corresponded to 22 cases (44.9%) and 27 cases (55.1%), respectively. The correlation between clinical and pathological responses was demonstrated in Table 3.
Pretreatment evaluation of biological markers according to the responses to EXE
The means of individual biological markers, which were subjected to various responses to EXE treatment, were demonstrated in Table 4. No statistical significance was detected in all the markers examined between clinical and pathological response and nonresponse groups, except for Her2 scoring which was higher in pathological nonresponsive group than that of responsive group.
Associations between alterations of biological markers during the therapy and responses to exemestane treatment in individual patients
Alterations of immunohistochemical biomarkers examined in breast tumor tissues before and after EXE neoadjuvant treatment according to clinical and pathological responses were summarized in Tables 5 and 6. In clinical response group, the significant decrement of Ki67 LI (P < 0.0001), ER (P = 0.0098) and PgR expression (P < 0.0001) was detected. In addition, the statistically significant increment was demonstrated in STS (P = 0.0084) and 17β-HSD1 (P = 0.0015). EST also demonstrated some degrees of increment but this increase did not reach statistical significance (P = 0.375). Among clinical nonresponders, STS, 17β-HSD1 and EST were all significantly increased (P = 0.0078, P = 0.0010 and P = 0.0313, respectively). In addition, PgR and Ki67 LI demonstrated significant decrement (P = 0.0034 and P = 0.0003, respectively) but ER and Her2 status did not reveal any significant differences between before and after the therapy (Table 5). In pathological response group, the significant decrement of IHC scores were demonstrated in ER (P = 0.0186), PgR (P < 0.0001) and Ki67 LI (P < 0.0001). Among the enzymes examined, only 17β-HSD1 demonstrated statistically significant increment (P = 0.0068) (Table 6). In contrast, the intratumoral enzymes in pathological nonresponders, STS, 17β-HSD1 and EST were associated with statistically significant increment (P = 0.0002, P = 0.0001 and P = 0.0156, respectively). In addition, the significant decrement was also detected in PgR (P = 0.0004) and Ki67 LI (P = 0.0003) following the therapy among these nonresponder group.
Alterations of intratumoral enzymes and biological markers according to the changes of Ki67 labeling index
Differences of the individual enzyme between pre- and posttreatment were evaluated according to these categories of Ki67 LI described above. Immunoreactivity of STS, 17β-HSD1, EST, ER, PgR, and Her2 in pretreatment specimens was not significantly different among these three different groups of Ki67 LI changes (Nonparametric ANOVAs; Data not shown). In group 1 or those whose Ki67 LI increased after the therapy, no statistically significant differences of intratumoral enzymes and biomarkers were detected in the specimens between before and after the treatment. In group 3 or those whose Ki67 LI decreased with more than 40% of the pretreatment level, the significant increment of STS and 17β-HSD1 were demonstrated (P = 0.0008 and P = 0.0003, respectively). In addition, ER and PgR scorings following the therapy demonstrated significant decrement compared to pretreatment (P = 0.0013 and P < 0.0001, respectively) in group 3 patients. In group 2 or those whose Ki67 LI unchanged or decreased with less than 40% of the pretreatment level, only 17β-HSD1 was associated with statistically significant increment (P = 0.0313). Moreover, among the enzymes examined and among the other biomarkers examined, only PgR status was significantly decreased following the therapy (P = 0.0117) in group 2 patients. EST and Her2 scorings were not different among these three different groups of Ki67 LI alterations (Table 7).
Correlation among STS, 17β-HSD1 and EST immunoreactivity before and after EXE treatment
Results were summarized in Table 8. The status of three enzymes examined in this study was significantly correlated among each others in tissue specimens before the therapy. However, in tumor tissues following the therapy, only the status of STS and 17β-HSD1 was significantly correlated each other.
Discussion
This is the first study to demonstrate significant alterations of the enzymes other than aromatase involved in intratumoral estrogen production following aromatase inhibitor administration. Several clinical studies have been reported using exemestane as primary endocrine therapy in operable breast cancer patients but results of clinical and pathological responses to exemestane varied among these studies [31–34]. Alterations in tumor histopathological features following aromatase inhibitors administration include the changes in cellularity, degree of fibrosis, histological grading [17, 27], and treatment-related changes of cell proliferation, apoptosis, and hormone receptor expression were also described [31–37].
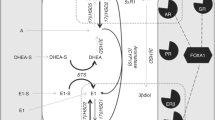
In tumor specimens following aromatase inhibitors therapy, one of the histological features most frequently or significantly affected was considered the number of mitotic figures or Ki67 positive carcinoma cells, which decreased in the great majority of cases [17, 36, 37], usually more pronounced than tamoxifen therapy [35]. In IMPACT study [36], Dowsett et al. evaluated the alterations of the number of Ki67 positive carcinoma cells using immunohistochemistry in 10% formalin-fixed and paraffin-embedded tissue sections of both pretreatment and after 2 weeks of neoadjuvant anastrozole treatment. Fifty-two out of 56 patients (93%) were associated with some degree of Ki67 labeling index reduction over the only 2-week period of therapy. Reported results of subsequent IMPACT studies further highlighted the clinical or therapeutic importance of evaluating the changes of Ki67 labeling index of carcinoma cells before and after the treatment [37]. We also demonstrated marked decrement in cell proliferation evaluated by the changes of Ki67 labeling index between before and after the therapy in all clinical and pathological response and nonresponse groups, which is also consistent with results of previously reported studies [17, 33, 36–39]. Therefore, an inhibition of aromatase activity and subsequent in situ decreased tissue estrogen availability were considered to affect the expression of molecules present in the downstream of ER signaling pathways related to cell proliferation regardless of response to treatment [3, 17, 33, 36–39] (Fig. 1).
Changes of the enzymes other than aromatase involved in intratumoral estrogen biosynthesis following aromatase inhibitor therapy have been considered or postulated important in relation to the development of treatment resistance [3] but have remained virtually unknown. To the best of our knowledge, this is the first study demonstrating a significant increment of STS and 17β-HSD1 following aromatase inhibitor neoadjuvant therapy of ER positive postmenopausal breast carcinoma patients. We hypothesized that this increment of STS and 17β-HSD1 detected in this study may be due to the compensatory response of breast carcinoma tissues to estrogen depletion and may represent an attempt of breast carcinoma to increase intratumoral estrogen concentrations using the estrogen producing or metabolizing pathways other than aromatase. In particular, a significant increment of STS and 17β-HSD1 following exemestane treatment was detected in the group associated with decreased Ki67 labeling index in our present study (Table 7). This increment of the enzymes above was not detected in the group associated with increased Ki67 labeling index, i.e., those associated with an absence of suppression of tumor cell proliferation. However, it awaits further investigations such as the intratumoral regulation of STS and 17β-HSD1 under estrogen depletion in order to substantiate this interesting hypothesis.
The simultaneous increase in STS and EST expression detected in our present study may be considered due to intratumoral metabolism and synthesis of estrogens. Both of these enzymes play pivotal roles in intratumoral estrogen production in the hormone-dependent breast carcinoma. STS hydrolyzes estrone sulfate (E1-S) to estrone, while EST sulfonates estrogens to inactive estrogen sulfates [15]. Therefore, an increment of STS levels in breast carcinoma cells may result in increased intratumoral estrogen production, but EST expression may also increase as one of the counterbalance effects or responses to an increment of intracellular estrogen, especially in nonresponder groups. However, it awaits further investigations to study the mechanisms of this simultaneous increment of both enzymes.
Among these enzymes examined, in particular, 17β-HSD1 was an only intratumoral enzyme whose expression increased regardless of clinical response, pathological response or Ki67 changes of the patients. Suzuki et al. [40] reported that the status of 17β-HSD1 immunoreactivity in carcinoma cells was significantly correlated with that of ER and PgR, suggesting estradiol, synthesized by 17β-HSD1 in carcinoma cells, act on these cells locally in breast carcinomas. In addition, reductive 17β-hydroxysteroid dehydrogenases are the last step in estrogen activation and thus play pivotal roles in biological behavior of ER positive breast carcinoma cells [41]. Sasano et al. [15] also reported that the status of intratumoral aromatase, 17β-HSD1, EST and STS in human breast cancer tissues varied markedly among different cases and, especially, no significant correlation was detected between intratumoral aromatase and 17β-HSD1. Therefore, an inhibition of 17β-HSD1 may be considered to confer clinical or therapeutic benefits upon the patients in whom over-expression of intratumoral 17β-HSD1 but not of aromatase was present in breast cancer tissues. The analysis of STS and 17β-HSD1 using immunohistochemistry is therefore considered important because these inhibitors may not work unless these enzymes or targets are not present or overexpressed in breast carcinoma cells. Therefore, an analysis of these enzymes as potential surrogate markers of treatment may be required for the successful clinical outcome of treatment when specific inhibitors against these enzymes will be clinically available.
In conclusion, results of this study indicated that an increment of STS and 17β-HSD1 may represent at least one of the mechanisms why hormone-dependent breast carcinoma cells developed resistance to AIs, which also suggest a possible adaptation of carcinoma cells in response to intratumoral estrogen depletion as a result of effective aromatase inhibitor therapy.
References
Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ (2007) Global Cancer Facts & Figures 2007. American Cancer Society, Atlanta, GA. http://www.cancer.org/docroot/STT/STT_0.asp. Accessed 19 Nov 2009
American Cancer Society (2009) Breast Cancer Facts & Figures 2009–2010. Atlanta, GA: American Cancer Society. http://www.cancer.org/docroot/STT/STT_0.asp. Accessed 19 Nov 2009
Pasqualini JR, Chetrite GS (2005) Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol 93:221–236
Chen S, Masri S, Hong YY, Wang X, Phung S, Yuan YC, Wu X (2007) New experimental models for aromatase inhibitor resistance. J Steroid Biochem Mol Biol 106:8–15. doi:10.1016/j.jsbmb.2007.05.020
Sasano H, Nagasaki S, Miki Y, Suzuki T (2009) New developments in intracrinology of human breast cancer estrogen sulfatase and sulfotransferase. Ann NY Acad Sci 1155:76–79
Miller WR, Forrest AP (1974) Oestradiol synthesis by a human breast carcinoma. Lancet 2:866–868
James VH, Reed MJ, Lai LC, Ghilchik MW, Tait GH, Newton CJ, Coldham NG (1990) Regulation of estrogen concentrations in human breast tissues. Ann NY Acad Sci 595:227–235
Larionov AA, Berstein LM, Miller WR (2002) Local uptake and synthesis of oestrone in normal and malignant postmenopausal breast tissues. J Steroid Biochem Mol Biol 81:57–64
Miller WR, Hawkins RA, Forrest APM (1982) Significance of aromatase activity in human breast cancer. Cancer Res 42:S3365–S3368
Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, Moriya T, Hirakawa H, Evans DB, Hayashi S, Ohuchi N, Sasano H (2007) Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res 67:3945–3954
Sasano H, Harada N (1998) Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocr Rev 19:593–607
Santen RJ, Leszczynski D, Tilson-Mallet N, Feil PD, Wright C, Manni A, Santner S (1986) Enzymatic control of estrogen production in human breast cancer: relative significance of aromatase versus sulfatase pathways. Ann N Y Acad Sci 464:126–137
Miller WR, Anderson TJ, Jack WJL (1990) Relationship between tumour aromatase activity, tumour characteristics and response to therapy. J Steroid Biochem Mol Biol 37:1055–1059
Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, Akinaga S, Hirakawa H, Kimura M, Sasano H (2003) Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res 63:2762–2770
Sasano H, Suzuki T, Nakata T, Moriya T (2006) New development in intracrinology of breast carcinoma. Breast Cancer 13:129–136
Santen RJ (2003) Inhibition of aromatase: insights from recent studies. Steroids 68:559–567
Miller WR, Anderson TJ, White S, Larionov A, Murray J, Evans D, Krause A, Dixon JM (2005) Aromatase inhibitors: cellular and molecular effects. J Steroid Biochem Mol Biol 95:83–89
Geisler J (2008) Aromatase inhibitors: from bench to bedside and back. Breast Cancer 15:17–26
Geisler J, Lønning PE (2005) Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res 11:2809–2821
Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S (2008) Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res 68:4910–4918
Lønning PE (2009) Lack of complete cross-resistance between different aromatase inhibitors; a real finding in search for an explanation? Eur J Cancer 45:527–535
Santen RJ, Lobenhofer EK, Afshari CA, Bao Y, Song RX (2005) Adaptation of estrogen-regulated genes in long-term estradiol deprived MCF-7 breast cancer cells. Breast Cancer Res Treat 94:213–223
Brodie A, Macedo L, Sabnis G (2009) Aromatase resistance mechanisms in model system in vivo. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2009.09.004
Flågeng MH, Moi LL H, Dixon JM, Geisler J, Lien EA, Miller WR, LØnning PE, Mellgren G (2009) Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br J Cancer 101:1253–1260
Sato N, Masuda N, Saji S, Takei H, Yamamoto Y, Sasano H, Toi M (2009) Neoadjuvant exemestane for 24 weeks in postmenopausal women with hormone receptor positive stage II or IIIa breast cancer (JFMC34-0601) [abstract 591]. J Clin Oncol 27:15S
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Miller WR, White S, Dixon JM, Murray J, Renshaw L, Anderson TJ (2006) Proliferation, steroid receptors and clinical/pathological response in breast cancer treated with letrozole. Br J Cancer 94:1051–1056
Bouzubar N, Walker KJ, Griffiths K, Ellis IO, Elston CW, Robertson JFR, Blamey RW, Nicholson RI (1989) Ki67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer 59:943–947
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Takei H, Suemasu K, Inoue K, Saito T, Okubo K, Koh J, Sato K, Tsuda H, Kurosumi M, Tabei T (2008) Multicenter phase II trial of neoadjuvant exemestane for postmenopausal patients with hormone receptor-positive, operable breast cancer: Saitama Breast Cancer Clinical Study Group (SBCCSG-03). Breast Cancer Res Treat 107:87–94
Mlineritsch B, Tausch C, Singer C, Luschin-Ebengreuth G, Jakesz R, Ploner F, Stierer M, Melbinger E, Menzel C, Urbania A, Fridrik M, Steger G, Wohlmuth P, Gnant M, Greil R (2008) Exemestane as primary systemic treatment for hormone receptor positive post-menopausal breast cancer patients: a phase II trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-17). Breast Cancer Res Treat 112:203–213
Yamashita H, Takahashi S, Ito Y, Yamashita T, Ando Y, Toyama T, Sugiura H, Yoshimoto N, Kobayashi S, Fujii Y, Iwase H (2009) Predictors of response to exemestane as primary endocrine therapy in estrogen receptor-positive breast cancer. Cancer Sci 100:2028–2033
Barnadas A, Gil M, González S, Tusquets I, Munoz M, Arcusa A, Prieto L, Margeli-Vila M, Moreno A (2009) Exemestane as primary treatment of oestrogen receptor-positive breast cancer in postmenopausal women: a phase II trial. Br J Cancer 100:442–449
Miller WR, Dixon JM, Macfarlane L, Cameron D, Anderson TJ (2003) Pathological features of breast cancer response following neoadjuvant treatment with either letrozole or tamoxifen. Eur J Cancer 39:462–468
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G, Smith IE (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer-a study from the IMPACT trialists. J Clin Oncol 23:2477–2492
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G (2007) Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170
Anderson H, Bulun S, Ian Smith, Dowsett M (2007) Predictors of response to aromatase inhibitors. J Steroid Biochem Mol Biol 106:49–54
Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady C, Quebe-Fehling E, Borgs M (2001) Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Cli Oncol 19:3808–3816
Suzuki T, Moriya T, Ariga N, Kaneko C, Kanazawa M, Sasano H (2000) 17beta-hydroxysteroid dehydrogenase type 1 and type 2 in human breast carcinoma: a correlation to clinicopathological parameters. Br J Cancer 82:518–523
Aka JA, Mazumdar M, Lin SX (2009) Reductive 17β-hydroxysteroid dehydrogenases in the sulfatase pathway: Critical in the cell proliferation of breast cancer. Mol Cell Endocrinol 301:183–190
Acknowledgments
We thank the local hospital pathologists who performed tumor sample analysis and other clinicians who were not part of the research team, but took responsibility for patient management. Japanese Foundation for Multidisciplinary Treatment of Cancer was responsible for collating the data in a database. We also thank Kyowa medix co. Ltd Japan for providing a kit of STS antibody.
Disclosure/conflict of interest
Hironobu Sasano and Yasuhiro Miki received the educational research grant from Pfizer Japan Inc., Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chanplakorn, N., Chanplakorn, P., Suzuki, T. et al. Increased estrogen sulfatase (STS) and 17β-hydroxysteroid dehydrogenase type 1(17β-HSD1) following neoadjuvant aromatase inhibitor therapy in breast cancer patients. Breast Cancer Res Treat 120, 639–648 (2010). https://doi.org/10.1007/s10549-010-0785-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0785-3