Abstract
The cancer stem cell hypothesis asserts that malignancies arise in tissue stem and/or progenitor cells through the dysregulation or acquisition of self-renewal. In order to determine whether the dietary polyphenols, curcumin, and piperine are able to modulate the self-renewal of normal and malignant breast stem cells, we examined the effects of these compounds on mammosphere formation, expression of the breast stem cell marker aldehyde dehydrogenase (ALDH), and Wnt signaling. Mammosphere formation assays were performed after curcumin, piperine, and control treatment in unsorted normal breast epithelial cells and normal stem and early progenitor cells, selected by ALDH positivity. Wnt signaling was examined using a Topflash assay. Both curcumin and piperine inhibited mammosphere formation, serial passaging, and percent of ALDH+ cells by 50% at 5 μM and completely at 10 μM concentration in normal and malignant breast cells. There was no effect on cellular differentiation. Wnt signaling was inhibited by both curcumin and piperine by 50% at 5 μM and completely at 10 μM. Curcumin and piperine separately, and in combination, inhibit breast stem cell self-renewal but do not cause toxicity to differentiated cells. These compounds could be potential cancer preventive agents. Mammosphere formation assays may be a quantifiable biomarker to assess cancer preventive agent efficacy and Wnt signaling assessment can be a mechanistic biomarker for use in human clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cancer stem cell hypothesis asserts that malignancies arise in tissue stem and/or progenitor cells through the dysregulation or acquisition of self-renewal [1]. Stem cells are long lived and capable of acquiring multiple mutations over time to transform to malignancy, while differentiated cells turn over rapidly [2]. Clonal expansion of stem cell populations through dysregulated self-renewal is hypothesized to be an early step in carcinogenesis [3]. This hypothesis is supported by recent studies demonstrating that breast tissue from women who carry germline BRCA1 mutations contains islands of cells that uniformly express the stem cell marker ALDH1. These expanded stem cell colonies display loss of heterozygosity for the normal BRCA1 allele [4, 5]. Experimental knockdown of BRCA1 in normal mammary cells leads to an increase in the ALDH-positive stem cell population in vitro and in mouse models [5].
If primitive breast cells are the targets for transforming events, then interventions aimed at reducing this cell population may provide novel risk reduction strategies. Current strategies, which include prophylactic mastectomy for BRCA1 or BRCA2 carriers, as well as the antiestrogens tamoxifen and raloxifene, are all associated with potential toxicities. These toxicities have limited the widespread utilization of tamoxifen and raloxifene in cancer prevention [6, 7]. Furthermore, hormonal interventions selectively prevent estrogen receptor (ER) positive breast cancers, but are less effective, and may actually increase the development of ER negative breast cancers [8–10]. This highlights the important need to develop non-toxic strategies to effectively prevent both ER negative and ER positive breast cancer. If the cancer stem cell hypothesis is valid, then strategies aimed at targeting stem cell self-renewal pathways represent rational approaches for cancer prevention. One such pathway is the Wnt signaling pathway, which is dysregulated in breast cancer, as well as many other malignancies [11–13]. Although the development of specific pharmacologic Wnt inhibitors has proven a challenge, there is evidence that curcumin, a dietary polyphenol found in spices, is able to downregulate the Wnt signaling pathway [14]. Interestingly, there is substantial evidence in preclinical models that curcumin is a potent chemopreventive dietary agent [15–19]. This suggests that the protective effects of curcumin might be due to Wnt inhibition of self-renewal in breast stem/progenitor cells.
Another dietary polyphenol, isolated from black and long peppers, which has been reported to reduce cancer incidence in chemical rodent models of lung cancer is piperine [20–24]. It has been suggested that piperine may also enhance the bioavailability of curcumin if these chemopreventive agents are given in combination [25]. The cancer preventive effects of piperine, as a single agent, in breast carcinogenesis have not yet been explored.
In order to determine whether the dietary polyphenols, curcumin, and piperine are able to modulate the self-renewal of normal and malignant breast stem cells, we examined the effects of these compounds on mammosphere formation and expression of the breast stem cell marker aldehyde dehydrogenase (ALDH). We demonstrate that curcumin and piperine are able to inhibit breast stem cell self-renewal and provide evidence that these effects are mediated through inhibition of Wnt signaling. These studies suggest that stem cell self-renewal assays may serve as biomarkers for cancer prevention studies. Such assays also provide potential mechanisms for the action of dietary polyphenols as cancer preventive agents.
Materials and methods
Materials
Curcumin 98% pure and piperine 99% pure were donated by Sabinsa Corporation (Piscataway, NJ). Both curcumin and piperine were prepared into 1 mM stock solutions in dimethyl sulfoxide for dissolution into cell culture media.
Human normal breast tissue dissociation
Normal breast tissue from women undergoing elective reduction mammoplasty who provided informed consent (University of Michigan IRBMED approved tissue collection protocol) was obtained through the Tissue Procurement Service with the Department of Pathology at the University of Michigan Medical School. The tissue was mechanically and enzymatically dissociated by methods detailed by Dontu et al. [2].
Mammosphere formation assay
Unsorted human breast epithelial single cells were plated in ultralow attachment plates (Corning Inc, Corning NY) at a density of 100,000 viable cells/ml in primary culture and 5,000 cells/ml in passages. Primary mammospheres were allowed to form for 7–10 days in serum-free mammary epithelial basal medium (MEBM) (Cambrex Bio Science Walkersville, Inc., Walkerville, MD) with curcumin and/or piperine treatment for 4–72 h. Primary mammospheres were centrifuged (1,000 rpm), dissociated with trypsin (Invitrogen), a 22 gauge needle (Fisher Scientific), and then sieved through a 40-μm sieve to obtain single cells. These sphere forming single cells, enriched for breast stem and early progenitor cells [2, 4], were used for serial passage (secondary and tertiary sphere formation studies) and differentiation experiments to test the functional definition of stem cells to self-renew and differentiate. Sphere number was counted manually and verified by two independent observers blinded to treatment. Each experiment was performed in triplicate wells per tissue and repeated using three separate tissues to account for intra and intertissue variability and findings were averaged with standard error.
Breast stem cell marker testing
Breast stem cells were identified using Aldefluor (Stem Cell Technologies, Newark, NJ) according to the method of Dontu et al. [2].
Curcumin and piperine’s effect on ability of primary sphere forming cells to serially passage
Unsorted single normal human breast epithelial cells were cultured in suspension in serum-free media with curcumin alone at concentrations ranging from 5 to 25 μM, piperine alone at concentrations ranging from 5 to 25 μM, or curcumin plus piperine compared to DMSO vehicle control and primary spheres collected, dissociated, and resuspended in MEBM to form secondary spheres. The secondary spheres were counted after 7 days, and then dissociated again and recultured to form tertiary spheres.
Differentiating culture conditions
Breast normal epithelial single cells dissociated from tertiary mammospheres to enrich breast stem cells were plated on collagen-coated plates at a density of 2,000 viable cells/10 cm petri dish. Curcumin, with or without piperine, or dimethyl sulfoxide vehicle control (DMSO) were added first from the time of plating for 48-h treatment with triplicate plates per dose combination. In a second set of experiments, cells were allowed to attach for 48 h prior to treatment with curcumin and/or piperine and grown in Ham’s F12 medium with FBS and growth factors as detailed elsewhere [2]. Colonies were allowed to form for 7 days, photographed, and manually counted using a microscope with a counting grid by two independent observers.
ALDH1A1 immunohistochemistry staining to assess curcumin and piperine effect on number of ALDH positive cells
We treated unsorted normal human single breast epithelial cells with curcumin, piperine, and curcumin + piperine compared to DMSO vehicle control in 6-well ultralow attachment plates for 72 h, then stained using the immunohistochemistry kit (Zymed) as per manufacturer’s instructions with ALDH1A1 primary antibody and biotinylated secondary antibody and streptavidin immunoperoxidase. ALDH1A1 cells were quantified by flourescence-activated cell sorting (FACS) with 4′-6-Diamidino-2-phenylindole (DAPI) for viability assessment.
Immunohistochemistry
To assess the lineage composition of the colonies, cells were fixed in the plates for 20 min in methanol, at 20°C, and then stained using Peroxidase Histostain-Plus and Alkaline-Phosphatase Histostain-Plus kits (Zymed), according to the manufacturer’s protocol. The primary antibodies, epithelial specific antigen (ESA) and cytokeratin 18 for luminal cells, and CD10 and cytokeratin 14 (Novocastra) for myoepithelial cells, were used at the dilutions indicated by the manufacturer.
Curcumin and piperine’s effect on Wnt signaling
Mammosphere formation [26] was also examined in unsorted MCF7 and Sum 159 breast cancer cell lines in anchorage independent, serum-free media conditions. β-catenin was detected in both human normal epithelial cells and MCF7 cell lines by immunohistochemical staining (rabbit monoclonal β-catenin primary antibody dilution 1:500 (Thermo Scientific)).
The effect of curcumin + piperine on Wnt signaling in breast cancer cell lines cells was examined by lentivirus-delivered reporter assay systems (90% transfection efficiency) to stably infect MCF7 cells. The virus reporter assay uses a green fluorescence protein, GFP, and is driven by a Tcf/Lef promoter. The number of cells with activated Wnt pathway was quantified by flow cytometry analysis for GFP fluorescence in the presence and absence of treatment with curcumin, piperine, or curcumin + piperine compared to DMSO vehicle control.
Results
Curcumin and piperine inhibit mammosphere formation
Our group, and others, have previously demonstrated that one of the properties of stem/progenitor cells is their ability to survive under anchorage independent conditions and generate mammospheres [12]. These mammospheres are composed of a small number of stem cells which self-renew, generating mammospheres on serial passage as well as progenitor cells capable of multilineage differentiation [2]. We determined the effects of varying concentrations of curcumin on mammosphere formation. Compared to DMSO control, 5 μM curcumin inhibited mammosphere formation by 50%, while 10 μM curcumin completely inhibited. Piperine also inhibited mammosphere formation compared to controls but the effects were less pronounced than curcumin (Fig. 1b, c, d). The addition of 10 μM piperine to 5 μM of curcumin further reduced mammosphere formation compared to either agent separately. Furthermore, this decrease in mammosphere formation was not due to non-specific toxicity, since cell viability, as determined by trypan blue exclusion at 5 μM curcumin, was 89%. Piperine, when added alone, had a more modest effect on mammosphere number; but also resulted in a decrease in mammosphere size (Fig. 1a).
a Primary mammospheres formed from unsorted normal human breast epithelial cells in suspension culture for 10 days. Left picture incubation with curcumin 5 μM, right picture piperine 5 μM treatment. b Primary mammosphere number (±SEM) formed from unsorted normal human breast epithelial cells in suspension culture with curcumin (C5 = curcumin 5 μM, C10 = curcumin 10 μM); 1C piperine treatment (P5 = piperine 5 μM, and P10 = piperine 10 μM), 1D curcumin plus piperine treatment
Curcumin and piperine decrease stem cell self-renewal
We have previously shown that the ability of mammospheres to be serially passaged at clonal density is an indirect marker of stem cell self-renewal [2]. We, therefore, determined the effect of curcumin, piperine, or their combination when added during primary mammosphere formation on the ability of these cells to be serially passaged in mammospheres at clonal density. The addition of 5 μM curcumin + 10 μM piperine to primary mammospheres inhibited the ability of cells isolated from these spheres to form secondary mammospheres by more than 50% compared to DMSO control. Furthermore, unlike DMSO controls, these cells were completely unable to form tertiary mammospheres. This suggests that curcumin and piperine affect breast stem cell self-renewal and that this process is irreversible since the removal of these compounds did not restore the ability of these cells to form mammospheres upon serial passage.
Curcumin and piperine’s effect on cellular differentiation
Curcumin and piperine affect progenitor cell proliferation but not cellular differentiation. The observation that piperine affected mammosphere size to a greater extent than mammosphere number (Fig. 1a, c) suggested that this compound might affect progenitor cell proliferation. In order to examine this in more detail, as well as to determine the effects of curcumin and piperine on cellular differentiation, we treated mammospheres with these dietary polyphenols and then determined the proliferative and differentiation capacity of these cells by plating mammosphere-derived cells in attached cultures on collagen-coated dishes in the presence of fetal calf serum. We have previously shown that progenitor cells differentiate into myoepithelial and luminal epithelial cells, which can be identified by their cytokeratin expression [2]. Single cells derived from tertiary mammospheres were plated at clonal density on collagen-coated dishes. For the first 48 h of plating, 5 μM curcumin + 10 μM piperine were added, at which point they were removed and cells were then cultured in the absence of these compounds for an additional 10 days. The addition of the dietary polyphenols resulted in a 50% reduction in the number of colonies formed. In contrast, when cells were allowed to attach and differentiate for 48 h prior to treatment with curcumin and piperine, there was no difference in colony number compared to DMSO treated controls. Together with the data on mammosphere formation, these studies suggest that the effects of curcumin and piperine are primarily on undifferentiated stem and progenitor cells rather than on more differentiated cells. Colonies generated at clonal density contained cells that were identified as luminal epithelial by CD 8 (red staining) or myoepithelial as identified by CK 14 (staining blue) in mixed colonies containing both cell types. Addition of curcumin and piperine had no significant effect on the generation of these cell lineages.
Curcumin and piperine affect ALDH+ stem/progenitor cells
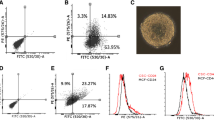
The previous experiments suggested that curcumin and piperine effects were primarily on stem/progenitor rather than differentiated cell populations. We have previously demonstrated that both normal and malignant breast stem/progenitor cells can be enriched by virtue of their expression of ALDH as assessed by the Aldefluor assay [4]. We demonstrated that ALDH+, but not ALDH-cells, are capable of mammosphere formation and of generating mammary structures in the humanized fat pads of NOD/SCID mice [2, 4]. In order to confirm that the effects of dietary polyphenols were on the primitive cell population, we isolated the ALDH+ component of primary human breast cells and examined the effect of polyphenols on mammosphere formation in this stem/progenitor-enriched population. The addition of 5 μM curcumin and 5 μM piperine inhibited mammosphere formation by 50% compared to DMSO controls in this stem/progenitor-enriched population. Mammosphere formation in these cells was completely inhibited by 10 μM curcumin and 10 μM piperine. These studies provide further evidence for the enhanced sensitivity of stem/progenitor cells to curcumin + piperine (Fig. 2).
a Secondary and tertiary sphere formation from human normal single epithelial cells in suspension with curcumin and piperine. b Number of differentiated colonies formed from secondary sphere forming human breast stem cells with curcumin and piperine from time of plating versus treatment for 48 h after plating. c Immunostaining for CK14 myoepithelial marker in blue and CK18 luminal marker in red in mixed differentiated colonies formed in the presence of curcumin and piperine
Curcumin and piperine reduce the percent of cells expressing the stem cell marker ALDH1
To provide independent evidence that curcumin and piperine are able to reduce the stem cell populations, we examined the effects of these compounds on the proportion of breast cells expressing the stem cell marker ALDH1. The known effects of piperine on cellular transport precluded the use of the functional Aldefluor assay to assess ALDH expression post treatment with piperine. However, we have previously demonstrated that the predominant Aldefluor isoenzyme expressed in breast stem cells is ALDH-1A1 [4]. ALDH1 immunochemistry can thus be utilized to assess stem/progenitor cell number in normal and malignant breast tissues [4]. We determined the proportion of cells expressing ALDH-1A1 by flow cytometry. As shown in Fig. 3b, 10 μM curcumin reduced the proportion of ALDH-1A1 expressing cells from 7.3 to 1.5%. Ten μM piperine also decreased the percentage of ALDH-1A1 positive cells to 0.89% and the combination of curcumin and piperine further reduced ALDH-1A1 positive cells to 0.20% (Fig. 3b). These results confirm the previous studies demonstrating that curcumin and piperine are able to effect mammary stem/progenitor cells.
a Primary mammosphere number formed from ALDH + presorted normal human breast stem/early progenitor cells in suspension culture with curcumin and piperine treatment (C5 = curcumin 5 μM, c10 = curcumin 10 μM, P5 = piperine 5 μM, and P10 = piperine 10 μM) compared to DMSO vehicle and no treatment (NT controls). b Percent of ALDH1A1 positive cells after treatment of unsorted normal human breast epithelial cells with curcumin and piperine treatment
Curcumin and piperine inhibit Wnt signaling
There is considerable evidence that the Wnt pathway regulates self-renewal in the breast as well as other organs. In addition to canonical Wnt signaling, other signal transduction pathways, including Akt, may also regulate stem cell self-renewal through downstream Wnt signaling [27]. Furthermore, Wnt dysregulation results in mammary carcinogenesis in animal models and there is evidence for Wnt dysregulation in human breast tumors [11, 13, 28–32]. Previous studies have shown that curcumin inhibits Wnt signaling in other systems [33, 34]. We, therefore, determined the effect of curcumin and piperine on Wnt signaling in well-characterized breast cancer cell lines utilizing the TCF-Lef Topflash reporter system. Exposure to 10 μM curcumin for 12 h reduced GFP expression from 13% in DMSO vehicle controls to 0.89%, while 10 μM piperine inhibited GFP expression to 2.39% (Fig. 4b). The combination of curcumin and piperine caused a further inhibition of GFP expression to 0.55%. These studies indicate the potent inhibitory effect on Wnt signaling of both curcumin and piperine.
a Number of spheres formed from MCF7 or SUM 159 cells in suspension culture with 12 h of curcumin and piperine treatment (C5 = curcumin 5 μM, C10 = curcumin 10 μM, P5 = piperine 5 μM, P10 = piperine 10 μM, C5 + P10 = curcumin 5 μM and piperine 10 μM). b Green fluorescence positivity in MCF7 Topflash assay with curcumin and piperine treatment
Discussion
The cancer stem cell hypothesis proposes that cancers arise in tissue stem cells through dysregulation of the normally, tightly regulated process of self-renewal or in progenitor cells through acquisition of this capacity [1]. If stem/progenitor cells are targets for transformation, then strategies aimed at limiting these cell populations through inhibition of self-renewal represent rational cancer preventive strategies. Furthermore, since these interventions are based on the regulation of self-renewal, rather than the induction of toxicity, they have the potential of being less toxic than compounds such as tamoxifen, which affect the bulk differentiated cell populations [10, 35]. Our data suggest that the dietary polyphenols, curcumin and piperine, may have these characteristics. We demonstrate that these dietary polyphenols affect normal stem/progenitor cells, as documented by decreased mammosphere formation upon serial passage and by decrease in the proportion of cells expressing the stem cell marker ALDH-1A1. In contrast, these compounds had little or no effect on differentiated cells. The relative lack of effect of these compounds on differentiated cells may account for their lack of toxicity in animal models and clinical studies [15, 36].
Cancer preventive agents such as curcumin and other polyphenols have been shown to have pleiotrophic mechanisms of action, including NFκB suppression, Cox2 down regulation, and Wnt and Notch pathway downregulation [34, 37–42]. We demonstrated that curcumin was able to inhibit Wnt signaling in MCF7 cells utilizing a TCF-Lef reporter assay system. These results support work in other systems showing the ability of curcumin to inhibit Wnt signaling [33, 43, 44], a pathway which we have previously demonstrated to play an important role in breast stem cell self-renewal and which is frequently dysregulated during breast carcinogenesis [11, 12, 31].
In addition to curcumin, we demonstrate that the dietary polyphenol piperine also is able to inhibit breast stem cell self-renewal and Wnt signaling. Previous studies have suggested that piperine could enhance curcumin’s effects by enhancing bioavailability through inhibition of curcumin’s efflux via P glycoprotein (ABCB1 or MDR1) efflux pump [45–47] as well as through the downregulation of NFκB release [48]. Our studies demonstrating that piperine affects mammosphere size and colony formation suggest that piperine may affect progenitor cell proliferation as well as enhance curcumin’s effects on breast stem cell self-renewal.
Conclusions
If systemic bioavailability of curcumin can be improved to allow bioactive concentrations of curcumin and piperine in vivo, then this combination may serve as an effective cancer preventive intervention to limit stem cell self-renewal, since these cells and dysregulation of self-renewal pathways may be involved in carcinogenesis. Strategies aimed at reducing stem cell number and inhibiting their self-renewal could be an effective approach in cancer prevention. If this is the case, then assays such as mammosphere formation and ALDH expression may serve as biomarkers for cancer prevention studies in clinical trials. Curcumin, even at large doses, has been demonstrated to be non-toxic in clinical trials. Piperine has been shown in a small, phase I clinical trial to enhance the systemic bioavailability of curcumin [25]. However, a more systematic phase I trial with pharmacokinetic, pharmacodynamic, and toxicity endpoints of repeated dosing of these agents in combination is still needed. If proven safe and efficacious, dietary polyphenols could be an acceptable non-toxic long-term cancer risk reduction strategy.
References
Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF (2004) Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 14:43–47
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17:1253–1270
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ (2009) Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15:907–913
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567
Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS (2008) BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA 105:1680–1685
Cella D, Land SR, Chang CH, Day R, Costantino JP, Wolmark N, Ganz PA (2008) Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat 109:515–526
Gennari L, Merlotti D, Paola VD, Nuti R (2008) Raloxifene in breast cancer prevention. Expert Opin Drug Saf 7:259–270
Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O’Brien K, Wang Y, Hilakivi-Clarke LA (2003) Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 22:7316–7339
Howell A (2008) The endocrine prevention of breast cancer. Best Pract Res Clin Endocrinol Metab 22:615–623
Powles TJ (2008) Prevention of breast cancer using SERMs. Adv Exp Med Biol 630:232–236
Lindvall C, Bu W, Williams BO, Li Y (2007) Wnt signaling, stem cells, and the cellular origin of breast cancer. Stem Cell Rev 3:157–168
Liu S, Dontu G, Wicha MS (2005) Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res 7:86–95
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN, Chen C, Liu SQ, Wu MC, Wang HY (2008) Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res 68:4287–4295
Park CH, Hahm ER, Park S, Kim HK, Yang CH (2005) The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett 579:2965–2971
Bachmeier B, Nerlich AG, Iancu CM, Cilli M, Schleicher E, Vene R, Dell’Eva R, Jochum M, Albini A, Pfeffer U (2007) The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem 19:137–152
Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Hohneke C, Jochum M, Nerlich AG, Pfeffer U (2008) Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis 29:779–789
Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL (2000) Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 21:331–335
Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH (1994) Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res 54:5841–5847
Huang MT, Newmark HL, Frenkel K (1997) Inhibitory effects of curcumin on tumorigenesis in mice. J Cell Biochem Suppl 27:26–34
Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF (2002) Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther 302:645–650
Pradeep CR, Kuttan G (2004) Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol 4:1795–1803
Pradeep CR, Kuttan G (2002) Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis 19:703–708
Selvendiran K, Banu SM, Sakthisekaran D (2004) Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta 350:73–78
Selvendiran K, Prince Vijeya Singh J, Sakthisekaran D (2006) In vivo effect of piperine on serum and tissue glycoprotein levels in benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Pulm Pharmacol Ther 19:107–111
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64:353–356
Huang MZ, Zhang FC, Zhang YY (2008) Influence factors on the formation of mammospheres from breast cancer stem cells. Beijing Da Xue Xue Bao 40:500–504
Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS (2009) Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol 7:e1000121
Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF (2008) Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells 26:364–371
Katoh M (2007) Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev 3:30–38
Kwan H, Pecenka V, Tsukamoto A, Parslow TG, Guzman R, Lin TP, Muller WJ, Lee FS, Leder P, Varmus HE (1992) Transgenes expressing the Wnt-1 and int-2 proto-oncogenes cooperate during mammary carcinogenesis in doubly transgenic mice. Mol Cell Biol 12:147–154
Turashvili G, Bouchal J, Burkadze G, Kolar Z (2006) Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73:213–223
Ugolini F, Charafe-Jauffret E, Bardou VJ, Geneix J, Adelaide J, Labat-Moleur F, Penault-Llorca F, Longy M, Jacquemier J, Birnbaum D, Pebusque MJ (2001) WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene 20:5810–5817
Jaiswal AS, Marlow BP, Gupta N, Narayan S (2002) Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 21:8414–8427
Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, Oh S (2008) Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun 377:1304–1308
Moen MD, Keating GM (2008) Raloxifene: a review of its use in the prevention of invasive breast cancer. Drugs 68:2059–2083
Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE (2006) Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6:10
Aggarwal BB, Banerjee S, Bharadwaj U, Sung B, Shishodia S, Sethi G (2007) Curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway and up-regulates cyclin-dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines. Biochem Pharmacol 73:1024–1032
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–398
Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN (2002) Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol 64:573–582
Aravindan N, Madhusoodhanan R, Ahmad S, Johnson D, Herman TS (2008) Curcumin inhibits NFkappaB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther 7:569–576
Binion DG, Otterson MF, Rafiee P (2008) Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57:1509–1517
Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH (2006) Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 106:2503–2513
Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R (2009) Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling. Chem Biol Interact 181:263–271
Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, Oh S (2008) Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun 377:1304–1308
Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P (2004) Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol 68:2043–2052
Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV (2007) Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem 296:85–95
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818
Singh S, Khar A (2006) Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem 6:259–270
Acknowledgements
This work was performed with NIH T32 grant, Innovative Concepts in Stem Cell Research Foundation Grant, and NIH KL2 grant support and support from the VA hospital. We are grateful to Sabinsa Co. for the donation of curcumin and piperine used in these experiments. The authors also wish to thank Irving L. Weissman (Stanford University School of Medicine, Stanford, California) for providing the LEF-1/TCF reporter constructs. We wish to thank Samadhi Liyanage and Dr. Shiv Kumar Dubey for their assistance with some experiments in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kakarala, M., Brenner, D.E., Korkaya, H. et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122, 777–785 (2010). https://doi.org/10.1007/s10549-009-0612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0612-x