Abstract
The cancer stem cell (CSC) concept has important implications not only for our understanding of carcinogenesis, but also for the development of cancer therapeutics. There is a growing body of preclinical evidence that cancer stem cells contribute to chemotherapy and radiation resistance in breast cancer. The use of drugs that interfere with stem cell self-renewal represents the strategy of choice, but also a great challenge because cancer stem cells and their normal counterparts share many pathways. Dietary compounds have been used in cancer prevention for decades, and some of these compounds target specific mechanisms that control CSC self-renewal. However, to date, no significant impact of CSCs on clinical outcome has been identified. The new paradigm imposed by the CSC model may change the way therapeutic effects are measured in clinical trials, stressing the effect on overall survival over just rapid tumor size reduction. In this chapter, we present the concept of cancer stem cell, mechanisms of conventional anticancer treatment resistance, and how dietary compounds may be used to target the self-renewal capability of CSCs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cancer is the second most frequent cause of death in developed countries. The standard of care for systemic cancer treatment usually involves conventional chemotherapy where the choice of drugs is based upon tumor phenotype, patient condition, and whether the patient has previously responded to treatment, in the case where the tumor relapsed after a first line of treatment. Although most chemotherapeutic treatments induce tumor shrinkage, very often the tumor develops resistance and relapses. In recent years, it has become clear that most solid tumors show a hierarchical organization at the cellular level with a small population of cancer stem-like cells responsible for tumor initiation and maintenance, the so-called cancer stem cells (CSCs) or tumor initiating cells (TICs) for their ability to initiate tumors in immune-compromised animal models. The presence of cancer stem cells in tumors is likely one of the main reasons why current oncologic therapies are not very effective in preventing tumor progression, metastasis, and recurrence (Shafee et al. 2008; Tanei et al. 2009; Cirenajwis et al. 2010) . Common chemotherapeutic drugs and radiotherapy often fail to eliminate these cells. Therefore, elimination of CSCs may become a necessary step for an effective cure, making CSCs as ultimate therapeutic target. Because CSCs are more resistant to conventional treatments than the bulk of differentiated tumor cells, the combination of CSC specific targeting agents with conventional chemotherapy will likely overcome tumor resistance and prevent tumor relapse, thus eventually will improve patient survival.
Medicinal plants have served as the source of therapeutic agents for many kinds of diseases including cancer. Natural compounds derived from fruits and vegetables (here onward referred as phytochemicals) have demonstrated their effectiveness in reducing the proliferation of tumor cells, both in vitro and in vivo. Epidemiological evidence has shown an association between certain dietary elements and a reduction of the incidence of cancer. In fact, some of the most common chemotherapeutic drugs are derived originally from plants, such as taxanes (paclitaxel, docetaxel, and others, derived from Taxus brevifolia), camptothecin (derived from Camptotheca acuminate), or vinca alkaloids (such as vincristine, derived from Catharanthus roseus). Some of the effects of phytochemicals may be directly related to the ability of the compounds to target cancer stem cell self-renewal . The aim of this chapter is to describe the current knowledge about the origin of cancer stem cells and how phytochemicals may target these rare cell populations, with special attention to breast cancer.
Cancer Stem Cells and Cancer Treatment
Tumors comprise heterogeneous populations of cells that have varying degrees of tumorigenic potential. Increasing evidence suggests that a biologically unique population of cancer stem cells exists in most neoplasms and may be responsible for tumor initiation, progression, metastasis, and relapse. Evidence that tumors arise from stem/progenitor cells has been obtained from leukemia (Bonnet and Dick 1997) , breast (Al-Hajj et al. 2003) , brain (Singh et al. 2003) , colon (Ricci-Vitiani et al. 2007), and most other tumors (pancreas, melanoma, glioblastoma, ovary, liver, and prostate). However, the target cell for transformation that originates CSCs remains unknown. This could be a stem cell, a progenitor cell, or a terminally differentiated cell that acquires, through mutations and epigenetic changes, the stem cell self-renewing property. It is possible that any cell in the tissue cell hierarchy with proliferative capability could serve as a cancer-originating cell upon acquiring the changes that promote self-renewal and prevent postmitotic differentiation.
There are a number of reports using in vitro culture of tumor cells and animal models showing that CSCs are more resistant to conventional cancer therapies, thereby placing these cells at the root of tumor recurrence and metastases. Several preliminary reports have indeed shown that this is also the case for human cancer patients. In breast cancer, Li et al. (Li et al. 2008) showed that conventional chemotherapy increased the fraction of CD44high CD24−/low cells in a neoadjuvant setting of advanced breast cancer patients. Tanei et al. (Tanei et al. 2009) have shown that paclitaxel and epirubicin-based chemotherapy enriches for aldehyde dehydrogenase-1 positive cells in breast tumors; another marker for CSCs (Ginestier et al. 2007) . CSCs from brain tumors expressing the neural stem cell surface marker CD133+ were resistant to standard chemotherapeutic drugs (Singh et al. 2004) . Current therapeutic agents for the management of the cancer patient are directed towards rapidly proliferating cells, failing to address the mechanisms of self-renewal and tumor initiation, which are the mechanisms that define stem cell activity. Therefore, if CSCs have intrinsic different sensitivity to these agents, then treatment would not succeed in complete cancer eradication, and tumor shrinkage reflects the effect in rapid proliferating non-CSC cells. On the other hand, targeting just CSCs may not be sufficient as a cancer therapy because proliferating cells could also give rise to CSCs. Thus combined elimination of CSCs and non-CSCs may be the way to go for a complete cancer treatment. But, how are CSCs less sensitive to the conventional anticancer therapies? Several studies show that CSCs are relatively resistant to conventional antineoplastic agents, both in vitro and in vivo in animal models. For example, after treatment of TM40D breast cancer cells with paclitaxel/epirubicin, a common first-line treatment for breast cancer, most of the surviving cells expressed the CSC markers CD44+/CD24low, also evidenced in tumor biopsies from treated breast cancer patients (Creighton et al. 2009) . In colorectal cancer, human primary tumors transplanted into mice after treatment with oxaliplatin or irinotecan showed an increased fraction of cells with a CSC phenotype, compared with tumors before treatment or untreated tumors, and increased tumorigenicity (Dylla et al. 2008) .
Mechanisms of Drug Resistance in CSCs
Stem Cells are not Actively Dividing Cells
Normal stem cell longevity is ensured by prolonged exit from the cell cycle, a mechanism that prevents the exhaustion of the replicative potential and limits DNA damage (Wilson et al. 2008) . A similar mechanism is presumed to operate in CSCs, making these cells less sensitive to antiproliferative drugs, minimizing the exposure to DNA-damaging metabolic products. However, the existence of dormant CSCs has not been directly demonstrated and the cell cycle status of CSCs in homeostasis is still controversial.
Increased Expression of Antiapoptotic Proteins
Two major apoptosis -inducing pathways coexist in cancer cells. The extrinsic or receptor-mediated pathway is initiated upon engagement of one or several of the death receptor (DR) family, promoting the assembly of a multiprotein complex that ultimately activates the initiator caspase-8, that subsequently will activate the effector caspases (-3, and -7). The intrinsic pathway is initiated by the loss of outer mitochondrial membrane permeability and the release to the cytosol of proapoptotic mediators, mainly cytochrome c and Smac proteins. Cytochrome c binds to the scaffolding protein Apaf-1 that assembles a protein complex for the activation of the initiator caspase-9. Smac proteins are inhibitors of the inhibitors of apoptosis (IAPs) family , preventing their role as caspase activation blockers. The intrinsic pathway is engaged by a plethora of intracellular stimuli, mainly reflecting cell stress. Both pathways are tightly controlled by a complex network of proapoptotic and antiapoptotic proteins, such as the Bcl2 family proteins. CSCs have been reported to harbor multiple defects in the apoptosis -inducing machinery. For example, CD133-positive glioblastoma stem cells were reported to be resistant to Fas-induced apoptosis (extrinsic pathway), which was associated with the expression of a monomeric form of Fas protein (Bertrand et al. 2009) . These cells also show higher expression of the inhibitor of apoptosis proteins XIAP and cIAPs compared to the CD133 negative population (Liu et al. 2006) . CD133 positive cells in several tumor models showed increased expression of FLIP, an inhibitor of TRAIL (one of the DR) activation, making them more resistant to TRAIL-induced apoptosis (Zobalova et al. 2008) . Increased expression of antiapoptotic Bcl2 family members have been described in glioma, breast, and colon cancer stem cells (Madjd et al. 2009; Kemper et al. 2012; Qiu et al. 2012) , suggesting an alternative target to overcome treatment resistance in these tumors.
Increased Expression and Activity of Multifunctional Drug Efflux Channels from the ATP-Binding Cassette (ABC) Gene Family
Hematopoietic stem cells were described to express increased concentration of the transporters p-GP (MDR1, ABCB1) and BCRP (ABCG2; Lou and Dean 2007) , and this feature has been exploited to isolate the stem cell population on the basis of dye exclusion (side population). However, stem cells from other tissues lack overexpression of these molecules. Besides, even if some transporters are overexpressed in stem cells, this may only explain resistance to the specific drugs that can be effluxed by them, not the wide resistance response observed including resistance to ionizing radiation.
Increased Expression of Detoxifying Machinery
The family of enzymes aldehyde dehydrogenase (ALDH) is involved in detoxification of intracellular aldehydes. In particular, ALDH1A1 and ALDH3A1 isozymes have been shown to play important functional roles in normal stem cells able to metabolize chemotherapeutic agents, such a cyclophosphamide (Sladek et al. 2002) . ALDH activity, detected by the conversion of the metabolic substrate Aldefluor®, is commonly used as a marker for CSCs (Charafe-Jauffret et al. 2009) and high ALDH1 expression has been shown to correlate with poor prognosis in breast cancer patients (Ginestier et al. 2007) .
Lack of Hormone Receptors
CSCs in hormone-dependent cancers, such as breast cancer or prostate cancer, have been shown not to express hormone receptors [estrogen receptor (ER) and progesterone receptor (PR) for breast cancer; and androgen receptor (AR) for prostate cancer]. Therefore, CSCs are not being affected by drugs targeting hormone receptors (such as tamoxifen).
Targeting CSCs seems the right approach to cure the patient effectively, assuming that the CSC population is stable over time and that the CSC phenotype is intrinsic cell autonomous features not attainable by differentiated non-CSC cells. Results from our group and others, however, suggest that this may not be the case and point towards a more flexible and dynamic CSC population (Mani et al. 2008; Iliopoulos et al. 2011; Leis et al. 2012) . If this is the case, then therapy must be directed not towards CSCs, but towards the molecular mechanisms responsible for the activation of CSCs at tumor initiation and during tumor progression. In particular, stemness-associated pathways, such as those involved in the induction and maintenance of pluripotency, are promising targets for anti-CSCs drug development.
Functional Assessment of Cancer Stem Cell Activity
Application of stem cell biology to cancer research has been limited by the lack of simple methods for identification and isolation of normal and malignant stem cells. Assays commonly used to assess stem cell activity in tumors are described below.
Cell Surface Markers
In 1997 Dick et. al. demonstrated that human leukemias are driven by a small population of cells with the CD34+ and CD38− phenotype. Transplantation to humanized NOD/SCID mice at a number as few as 100 cells are capable of regenerating the original tumor (Bonnet and Dick 1997) . Clarcke and Wicha extrapolated this concept to solid tumors demonstrating that human breast tumors have a population of cells with stem cell properties. Using flow cytometry based on cell surface markers they differentiated the tumorigenic (tumor initiating) from the nontumorigenic cancer cells, identifying the tumorigenic cells as CD44+ CD24−/low Lin-. This population has capacity to generate the phenotypic heterogeneity found in the initial tumor when transplanted to humanized NOD/SCID mice (Al-Hajj et al. 2003) . Since then, specific surface markers expressed on CSCs but not on the bulk of the tumor have been identified on a variety of cancers, including brain cancers (CD133+), prostate cancer, melanoma, multiple myeloma, colon (CD133+), pancreatic, and head and neck cancers. Nevertheless, there is still not a single CSC specific marker, likely due to functional plasticity of this population. Thus, a cell that shows CSCs activity may not express a CSC-designated marker although functionally capable of initiating tumors. This complicates pathological evaluation of CSC content from natural tumor samples.
Side Population (SP)
ATP binding cassette (ABC) transporters represent a family of proteins with the capacity to bind ATP as an energy source to transport endogenous or exogenous molecules across the cellular membrane). Some of these proteins, such as the proteins encoded by MDR, MRP, and BCRP1, contribute to drug resistance and subsequent recurrence in cancers (Hadnagy et al. 2006) . BCRP1 excludes the fluorescent dye Hoechst 33342 that universally binds to the AT-rich regions of the minor groove of DNA, identifying a side population (SP) of cells, which is enriched for cells with stem cell characteristics. A variety of established cancer cell lines, which have been maintained in culture for decades, and also tumors contain a small SP. These SP cells, but not non-SP cells, self-renew in culture, are resistant to anticancer drugs, have the capacity to form tumors when transplanted in vivo and can be identified as the “side” of the bulk of the Hoechst 33342 positively stained cells in fluorescence-activated cell sorting (FACS) analysis plots. However, this staining is technically challenging and not always reproducible; in addition, DNA intercalating agents affect the viability of the cells in subsequent cultures, limiting the application of this procedure. On the other hand, this is a functional parameter not limited to the expression of a particular marker on the cell surface.
Aldehyde Dehydrogenase 1
Aldehyde dehydrogenase 1 is an intracellular enzyme whose functions include the oxidation of toxic aldehyde metabolites to carboxylic acids like those formed during alcohol metabolism. It has been shown that ALDH1 activity enriches for cells with stemlike properties in a variety of solid malignancies (Ginestier et al. 2007) . This enhanced detoxifying activity, besides its use as a marker for stem cells, may relate to the lower sensitivity of stem cells to certain chemotherapeutics, such as cyclophosphamide (Sladek et al. 2002) . Interestingly, ALDH activity does identify a different population from, for example, CD44+ CD24−/low in breast cancer, pointing to the existence of different CSC populations or several functional states on CSCs. As ALDH1 activity has been used as a common marker for both normal and malignant stem and progenitor cells, commercial kits have been released to identify and isolate cells with high ALDH1 activity.
Sphere Formation Assay
Derived from the neural stem cell field , CSCs when cultured in serum-free restricted medium with proper growth factors preventing attachment to a substrate, can form floating spheroidal aggregates (tumorospheres) that are enriched in CSC (Dontu and Wicha 2005) . Hepatoma cell lines, squamous cell carcinoma cell lines, or head and neck squamous carcinoma cell lines, among others, form nonadherent tumor spheres in culture that possess CSC properties. In breast carcinoma cell line MCF7, the mammosphere assay has been demonstrated to enrich and propagate cells with enhanced tumor initiating ability (Deleyrolle et al. 2011) . This assay is used as a stem-cell–like functional assay that allows the propagation of mammary epithelial and breast tumor cells in an undifferentiated state based on their ability to proliferate in suspension and as a functional in vitro assay for cancer stem-like specific drug screening. A limitation of the sphere assay relates to whether this assay properly identifies the frequency of in vivo quiescent stem cells as opposed to measuring cells that adapt or can act as a proliferating mammary stem cell in vitro. Furthermore, not every cell line, despite its tumor-initiating ability, can form tumor spheres in culture, raising questions about the restrictions imposed on cell growth in this assay.
Mice Xenografts
Currently, the gold standard functional assay to demonstrate tumor-initiating ability consists of hetero-transplantation of human cancer cells into immunodeficient mice. This xenograft model has been used to study cancer pathogenesis and drug development for several decades (Morton and Houghton 2007) , and with the development of FACS analysis, self-renewal capacity of a subpopulation with a given cell surface phenotype is commonly assessed using limiting dilution cell transplantation into immune-deficient mice and then scored for tumor engraftment. Mice xenograph models can also be utilized to recapitulate a primary tumor from biopsy samples. Primary tumors are minced and enzymatically digested. Then primary tumor-derived cells are transplanted into mice, either under the skin or into the organ type in which the tumor originated, at varying cell densities. The developing time of the tumor will depend on the number of cells inoculated. This assay is costly and very low throughput, limiting its use to laboratories with dedicated animal facilities.
Zebrafish
The mice xenograft model presents several caveats at a practical level, such as expensive animal facilities, number of animals used in each experiment, and the length of time to tumor formation. Zebrafish have been widely used in preclinical tests and drug screening, as well as toxicity assays for a variety of reasons: fish are inexpensive to maintain, breed in large numbers (100–300 embryos per week/couple), develop rapidly ex vivo, embryos are transparent, have short generational cycles (2–3 months), are immunodeficient until day 11 postfertilization, require a small amount of drugs per experiment, small in size, optically clear during development, and amenable to genetic manipulation. Recently, tumor cell xenografts into 2 days-postfertilization zebrafish embryos have proved useful to assess stem cell features (Eguiara et al. 2011) . Therefore, zebrafish xenografts may represent a better alternative to medium throughput drug screening in vivo, not achievable using mice.
Molecular Targets of Phytochemicals in Cancer Stem Cells
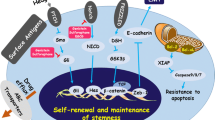
The molecular mechanisms that control self-renewal of cancer stem cells are essential elements for tumor survival and propagation. Multiple signaling pathways (Fig. 1) have been identified including the Wnt/β-catenin, Hedgehog (Hh), and Notch and PI3K-Akt signaling pathways (Beachy et al. 2004) . Although genes involved in these pathways are expressed in normal stem cells, they are frequently mutated or aberrantly activated in almost all cancers. As mentioned in Fig. 1, dietary phytochemicals are natural products target multiple signaling pathways in CSCs, such as Wnt signaling in breast cancer (Kakarala et al. 2010) or side population in brain tumors (Fong et al. 2010) . It would be interesting to determine if these compounds have differential effects on CSCs, and if so, understanding the mechanism of action of phytochemicals would lead to the development of novel therapeutic drugs for cancer treatment. Some of the phytochemicals possessing anti-CSCs activities are mentioned below.
Curcumin
Curcumin is a well-known dietary polyphenol present in an Indian spice called Curcuma Longa usually used in the preparation of curry. It has anticancer activity both in vitro and in vivo models (Epstein et al. 2010) . Unfortunately, it also affects cell proliferation through cell cycle arrest and cytotoxicity in both normal and transformed cells (Karmakar et al. 2006) . It has been described that curcumin affects many signaling pathways (Fig. 1) related to apoptosis , proliferation, stem cell self-renewal, and epithelial-to-mesenchymal transition (EMT), as well as Wnt/B-Cathenin and Notch pathways (Yan et al. 2005; Karmakar et al. 2006; Ryu et al. 2008; Kakarala et al. 2010; Yang et al. 2012) .
Piperine
Piperine is a dietary polyphenol, isolated from black and long peppers, which has been reported to reduce cancer incidence in animal models (Pradeep and Kuttan 2002; Selvendiran et al. 2004) . It was reported that piperine altered cancer stem cell self-renewal by inhibiting the ability of stem cells to grow as floating mammospheres and reducing the cell population that shows increased ALHD activity (Kakarala et al. 2010) , without affecting the differentiated cells in the culture. The specific mechanisms operating in cancer stem cell self-renewal targeted by piperine are not currently known.
Resveratrol
Resveratrol , another polyphenol is an ingredient of red wine, stops breast cancer cell growth by blocking growth stimulating effect of estrogen (De Amicis et al. 2011) . This paper suggests that resveratrol is able to counteract the malignant progression by inhibiting the proliferation of hormone-resistant breast cancer cells. This has important implications for the treatment of women with breast cancer resistant to hormonal therapy. It has also been described as a DNA demethylating agent in breast tumors and breast carcinoma cell lines (Zhu et al. 2012) . It has recently been described that resveratrol synergizes with curcumin to inhibit colon cancer growth in mouse models, suggesting a better response to chemopreventive agents (Majumdar et al. 2009) .
Cruciferous Vegetable Derived Compounds
Cruciferous vegetables such as broccoli, cabbage, kale, Brussels sprouts, and radish, have been shown to contain absorbable 3,3’-diindolylmethane (DIM) , which prevent cancer (Bradlow et al. 1999) . Sulphoraphane is another bioactive compound that is abundant in cruciferous vegetables and was shown to block mammosphere formation in breast carcinoma cell lines in vitro and decrease tumor size in mouse xenograft models, associated with a reduction of the stem cell marker ALDH (Li et al. 2010) , although it is not clear what pathways are targeted.
Silibinin
Silymarin and its major constituent silibinin, are extracted from the medicinal plant Silybum marianum (milk thistle) and has traditionally been used for the treatment of liver diseases. Recently, these orally active flavonoid agents have also been shown to exert significant antineoplastic effects in a variety of in vitro and in vivo cancer models, including skin, breast, lung, colon, bladder, prostate, and kidney carcinomas (Hogan et al. 2007) due to induction of apoptotic death. More studies are required in order to determine whether it has any effects on CSCs.
Epigallocatechin-3-Gallate (EGCG)
(−)-Epigallocatechin-3-gallate (EGCG) is a bioactive polyphenolic compound present in green tea, which is one of the most widely consumed beverages in the world. Epidemiological studies suggest an association between green tea consumption and cancer prevention agents (Landis-Piwowar et al. 2007) . It has been extensively described as a Wnt pathway regulator, one of the key pathways controlling stem cell self-renewal in breast cancer and colon cancer (Bose et al. 2007) . EGCG induces HMG box-containing protein 1 (HBP1) transcriptional factor, which is a recognized suppressor of Wnt signaling (Kim et al. 2006) . Another described effect of EGCG is altering chromosomal structure through reduction in Bmi-1 levels (Balasubramanian et al. 2010) . Bmi-1 is highly expressed in cancer stem cells such as leukemia, neuroblastomas, and skin cancer, accompanied by the decreased expression of p16Ink4a and p19Arf tumor suppressor genes. Taken together, these studies support the further evaluation of EGCG in CSCs.
Vitamin A and D
One of the isoforms of vitamin D , cholecalciferol (Vitamin D3), was demonstrated to block Hedgehog-dependent signaling in breast cancer cell lines through binding to Smo, although it did not show effects on tumor growth in vivo (Bijlsma et al. 2006; Bruggemann et al. 2010) . Vitamin D can also interfere with the oncogenic mechanisms of β-catenin activity (the effector in the canonical Wnt signaling pathway) through a dual mechanism: vitamin D can modulate the expression of the Wnt signaling inhibitors DKK1 and DKK4 in colon cancer cells (Aguilera et al. 2007; Pendas-Franco et al. 2008) , and on the other hand promote the translocation of β-catenin from nucleus to plasma membrane and thereby inhibit the expression of β-catenin-responsive genes through association with the Vitamin D receptor (VDR) (Palmer et al. 2001) .
Genistein
Genistein is an isoflavone which is the major bioactive compound extracted from soy. Epidemiological evidence suggests that soy consumption decreases the risk of cancer (Messina et al. 1994) . As other isoflavones, genistein has been explored as an angiogenesis inhibitor . Besides, various studies have found that moderate doses of genistein have growth inhibitory effects on prostate, brain, breast, and colon cancer (de Lemos 2001; Morito et al. 2001; Hwang et al. 2009; Nakamura et al. 2009; Das et al. 2010; Sakamoto et al. 2010) . Regarding cancer stem cells , lifetime feeding of genistein (250 mg/kg per day) to rats increased expression of the Wnt signaling antagonist secreted frizzled-related protein 2 (sFRP2) and thus might account for a reduction in stem cell self-renewal (Su et al. 2007) . It is interesting that downregulation of sFRP2 is a frequent event in breast cancer (Suzuki et al. 2008) .
Clinical Trials Related to CSCs and Future Perspectives
A great proportion (70%) of drugs tested in oncology fail in randomized phase III clinical trials , despite extensive evidence in animal models showing therapeutic effect. The efficacy of antitumor agents in phase II clinical trials is commonly evaluated following RECIST (Response Evaluation Criteria In Solid Tumors) rules that define when cancer patients improve (“respond”), stay the same (“stable”), or worsen (“progression”) during treatments. Since the bulk of tumor cells (non-CSCs) constitute most of the tumor mass, efficacy mainly reflects the ability to kill those non-CSCs. Thus it is not tumor size reduction, but instead complete response (CR), that is a valid endpoint when associated with reduced recurrence rate. An agent that only targets CSCs is predicted to show only moderate effect on tumor size (therefore scored as a failure) but would have dramatic effect preventing tumor recurrence. On the other hand, an agent that targets the bulk of tumor cells but not CSC self-renewal will initially show good clinical response but will not prevent recurrence. Such a trial may result in a failure because of evidence of tumor progression. Therefore, innovative clinical trial designs are required to assess efficacy of these drugs with appropriate biological and clinical endpoints. For example, 80% of breast cancer patients show good clinical outcome in five years, therefore, a clinical trial designed against breast cancer stem cells would be directed to patients that have failed a second or third line of treatment (usually chemotherapy) where they are less likely to respond to any treatment. It will be lengthy process and involve a significant number of patients, thus the cost would be huge. It is necessary to introduce recurrence in the adjuvant setting to identify effective CSC targeting agents. For new agents that are tested against CSCs, in order to expedite their approval by the regulatory authorities, it might be desirable to seek niche indications where rapid clinical endpoints can be assessed. For example, small-cell–lung-carcinoma typically responds well to first-line chemotherapy , however, most patients relapse within 12 months. Therefore, a valid indication would be to treat with anti-CSCs agents just after the first line of chemotherapy, where the endpoint would be to look for relapse-free survival. Once a novel agent is approved, its transition to other indications is faster. Another possibility would be to combine current chemotherapeutic treatment with anti-CSC phytochemicals, however, this scenario would complicate the design of clinical trials. Only if such a phytochemical is proved to lower the resistance threshold of a known chemotherapeutic would it be advisable to use them in combination.
Dietary phytochemicals are considered attractive alternatives for development in cancer chemoprevention . As outlined before, resveratrol , piperine, genistein, or curcumin have undergone extensive mechanistic and preclinical efficacy investigation, although their clinical use is still very scarce. As opposed to chemical anticancer drugs, that are designed to act on specific targets, dietary agents exert a plethora of actions with an unknown hierarchy of biological importance, lacking a clear correlation between effect and mechanistic information. Moreover, as chemopreventive agents, clinical trials involve lengthy periods of time to assess efficacy, as well as a significant number of patients. Dose determination is also tricky, as effective doses used in vitro usually are several orders of magnitude above the typical dose of the phytochemical found in the ordinary diet, with potential for appearance of toxic effects in vivo. Of course, any toxic effect for chemopreventive agents is unacceptable. Altogether, these caveats make clinical trials with dietary compounds unattractive to trial sponsors, which explains the lack of funding.
Nevertheless, it would be very promising to study dietary compounds’ efficacy against CSCs. Given that these diet-based compounds are usually multitargeted, they may mediate other cellular events, for example, induction of CSC differentiation and sensitization of CSCs to chemotherapeutic agents, in addition to their potential impact on self-renewal signaling. No specific clinical trial has been designed thus far to assess phytochemicals effect on CSCs, although numerous trials are actively seeking to investigate their use as more effective strategies for cancer treatment, and to reduce cancer resistance and recurrence, thus improving patient survival.
References
Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, Barbachano A, Lopez deSI, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, Gonzalez-Sancho JM, Munoz A (2007) The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha, 25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 28:1877–1884
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988
Balasubramanian S, Adhikary G, Eckert RL (2010) The Bmi-1 polycomb protein antagonizes the (-)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis 31:496–503
Beachy PA, Karhadkar SS, Berman DM (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432:324–331
Bertrand J, Begaud-Grimaud G, Bessette B, Verdier M, Battu S, Jauberteau MO (2009) Cancer stem cells from human glioma cell line are resistant to Fas-induced apoptosis. Int J Oncol 34:717–727
Bijlsma MF, Spek CA, Zivkovic D, Water S van de, Rezaee F, Peppelenbosch MP (2006) Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol 4:e232
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737
Bose M, Hao X, Ju J, Husain A, Park S, Lambert JD, Yang CS (2007) Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (-)-epigallocatechin-3-gallate and fish oil. J Agric Food Chem 55:7695–7700
Bradlow HL, Telang NT, Sepkovic DW, Osborne MP (1999) Phytochemicals as modulators of cancer risk. Adv Exp Med Biol 472:207–221
Bruggemann LW, Queiroz KC, Zamani K, Straaten A van, Spek CA, Bijlsma MF (2010) Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biol Ther 10:79–88
Charafe-Jauffret E, Ginestier C, Birnbaum D (2009) Breast cancer stem cells: tools and models to rely on. BMC Cancer 9:202
Cirenajwis H, Smiljanic S, Honeth G, Hegardt C, Marton LJ, Oredsson SM (2010) Reduction of the putative CD44+ CD24– breast cancer stem cell population by targeting the polyamine metabolic pathway with PG11047. Anticancer Drugs 21:897–906
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC (2009) Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 106:13820–13825
Das A, Banik NL, Ray SK (2010) Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 116:164–176
De Amicis F, Giordano F, Vivacqua A, Pellegrino M, Panno ML, Tramontano D, Fuqua SA, Ando S (2011) Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor alpha gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J 25:3695–3707
Lemos ML de (2001) Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Ann Pharmacother 35:1118–1121
Deleyrolle LP, Ericksson G, Morrison BJ, Lopez JA, Burrage K, Burrage P, Vescovi A, Rietze RL, Reynolds BA (2011) Determination of somatic and cancer stem cell self-renewing symmetric division rate using sphere assays. PLoS One 6:e15844
Dontu G, Wicha MS (2005) Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia 10:75–86
Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, Clarke MF, Hoey T, Lewicki J, Gurney AL (2008) Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One 3:e2428
Eguiara A, Holgado O, Beloqui I, Abalde L, Sanchez Y, Callol C, Martin AG (2011) Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle 10:3751–3757
Epstein J, Sanderson IR, Macdonald TT (2010) Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 103:1545–1557
Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM (2010) Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett 293:65–72
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567
Hadnagy A, Gaboury L, Beaulieu R, Balicki D (2006) SP analysis may be used to identify cancer stem cell populations. Exp Cell Res 312:3701–3710
Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS (2007) Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res 143:58–65
Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM (2009) Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer 61:598–606
Iliopoulos D, Hirsch HA, Wang G, Struhl K (2011) Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A 108:1397–1402
Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS (2010) Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122:777–785
Karmakar S, Banik NL, Patel SJ, Ray SK (2006) Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci Lett 407:53–58
Kemper K, Rodermond H, Colak S, Grandela C, Medema JP (2012) Targeting colorectal cancer stem cells with inducible caspase-9. Apoptosis 17:528–537
Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS (2006) Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem 281:10865–10875
Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP (2007) A novel prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res 67:4303–4310
Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG (2012) Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene 31:1354–1365
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D (2010) Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 16:2580–2590
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5:67
Lou H, Dean M (2007) Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene 26:1357–1360
Madjd Z, Mehrjerdi AZ, Sharifi AM, Molanaei S, Shahzadi SZ, Asadi-Lari M (2009) CD44+ cancer cells express higher levels of the anti-apoptotic protein Bcl-2 in breast tumours. Cancer Immun 9:4
Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, Yu Y, Elliott AA, Levi E, Sarkar FH (2009) Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr Cancer 61:544–553
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Messina MJ, Persky V, Setchell KD, Barnes S (1994) Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer 21:113–131
Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y (2001) Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 24:351–356
Morton CL, Houghton PJ (2007) Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2:247–250
Nakamura Y, Yogosawa S, Izutani Y, Watanabe H, Otsuji E, Sakai T (2009) A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer 8:100
Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, Herreros AG de, Lafarga M, Munoz A (2001) Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 154:369–387
Pendas-Franco N, Garcia JM, Pena C, Valle N, Palmer HG, Heinaniemi M, Carlberg C, Jimenez B, Bonilla F, Munoz A, Gonzalez-Sancho JM (2008) DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene 27:4467–4477
Pradeep CR, Kuttan G (2002) Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis 19:703–708
Qiu B, Wang Y, Tao J, Wang Y (2012) Expression and correlation of Bcl-2 with pathological grades in human glioma stem cells. Oncol Rep 28:155–160
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115
Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, S Oh (2008) Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun 377:1304–1308
Sakamoto T, Horiguchi H, Oguma E, Kayama F (2010) Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem 21:856–864
Selvendiran K, Banu SM, Sakthisekaran D (2004) Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta 350:73–78
Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, Stanbridge EJ, Lee EY (2008) Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res 68:3243–3250
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Sladek NE, Kollander R, Sreerama L, Kiang DT (2002) Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: a retrospective study. Rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer Chemother Pharmacol 49:309–321
Su Y, Simmen FA, Xiao R, Simmen RC (2007) Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics 30:8–16
Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, Mori M, Hirata K, Imai K, Shinomura Y, Baylin SB, Tokino T (2008) Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer 98:1147–1156
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15:4234–4241
Wilson A, Laurenti E, Oser G, Wath RC van der, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135:1118–1129
Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD (2005) Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther 4:233–241
Yang R, Zhang S, Kong D, Gao X, Zhao Y, Wang Z (2012) Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res 29:3512–3525
Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen YC, Kliethermes B, Sauter ER (2012) Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer 64:393–400
Zobalova R, McDermott L, Stantic M, Prokopova K, Dong LF, Neuzil J (2008) CD133-positive cells are resistant to TRAIL due to up-regulation of FLIP. Biochem Biophys Res Commun 373:567–571
Acknowledgments
The Regulation of Cell Growth Laboratory is supported by grants from Obra Social Kutxa, Gobierno Vasco (Saiotek program, Departamento de Educación PI2010-25 and Departamento de Sanidad 2011111141), Diputación Foral de Gipuzkoa OF 53/2011 and Instituto de Salud Carlos III Acción Estratégica en Salud PI2010-01035.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Leis, O., Gumuzio, J., Martin, A. (2013). Therapeutic Action of Phytochemicals on Cancer Stem Cells. In: Chandra, D. (eds) Mitochondria as Targets for Phytochemicals in Cancer Prevention and Therapy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9326-6_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9326-6_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9325-9
Online ISBN: 978-1-4614-9326-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)