Abstract
Hypoxia is a hallmark of cancer. Hypoxia inducible factor-1α (HIF-1α) is the key regulator of the hypoxia response. HIF-1α is overexpressed during sporadic breast carcinogenesis and correlated with poor prognosis. Little is known on the role of HIF-1α in hereditary breast carcinogenesis. A recent study suggests a role for BRCA1 in HIF-1α regulation. We therefore examined the expression of HIF-1α in BRCA1 related breast cancers. By immunohistochemistry we studied expression of HIF-1α and some of its downstream targets in 30 hereditary invasive breast cancers in comparison with 200 sporadic controls. HIF-1α overexpression was significantly more frequent in BRCA1 related breast cancers (27/30, 90%) than in sporadic controls (88/200, 44%) (P < 0.0001). 19/30 (63%) of BRCA1 tumors showed perinecrotic (hypoxia induced) and 8/30 (27%) a diffuse HIF-1α overexpression pattern, the latter more likely related to genetic alterations in oncogenes and tumor suppressor genes. In contrast, sporadic breast cancer HIF-1 expressing tumors showed an inverse ratio of perinecrotic/diffuse expression (31 vs. 69%, P = 0.0002). Glut-1 and CAIX, downstream HIF1 targets, were expressed in 27/30 (90%) and 15/21 (71%) of hereditary cases versus 54/183 (29%) and 24/183 (13%) in sporadic cases. p300 levels, necessary for HIF-1 downstream activation, were significantly higher in hereditary cases (20/21, 95%) compared to sporadic cases (91/183, 50%, P = 0.0001). In conclusion, in BRCA1 germline mutation related breast cancer, functional HIF-1α overexpression is seen at a much higher frequency than in sporadic breast cancer, mostly hypoxia induced. This points to an important role of hypoxia and its key regulator HIF-1α in hereditary breast carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carriers of germline mutations in BRCA1 or BRCA2 have a hereditary predisposition for developing breast and/or ovarian cancer. Several studies have indicated that the genetic makeup of BRCA1/2 related breast cancer is different from that of sporadic breast cancer. These differences comprise gains and losses of specific parts of chromosomes as well as differences in gene expression [1–6]. In line with this, the morphological and immunohistochemical phenotype of BRCA1 related breast cancer is also different [7, 8]. They often concern well demarcated medullary and poorly differentiated ductal cancers with conspicuous lymphocytoplasmic infiltrates [9, 10] that are of high-grade [11] and show high proliferation [12]. In addition, they do not express estrogen (ER), progesterone (PR) or HER-2/neu receptors [13], often lack p27Kip1 [14], but do accumulate p53 [15], and overexpress cyclin E [16], cytokeratins (CK) 5/6 and 14 [17, 18], and EGFR [19–21]. These observations point to a carcinogenetic pathway of BRCA1 related breast cancers different from that in sporadic cancers.
Hypoxia is a hallmark of many sporadic cancers [22]. Hypoxia inducible factor-1 (HIF-1) is the key regulator of the hypoxia response. HIF-1 consists of 2 subunits, HIF-1α and HIF-1β. While HIF-1β is constitutively expressed, the HIF-1α protein is continuously degraded under normoxia by the ubiquitin-proteasome pathway [23, 24]. Under hypoxia, HIF-1α protein degradation is inhibited resulting in its overexpression and subsequent binding to HIF-1β [24]. This HIF-1 complex then regulates the expression of its target genes through binding with hypoxia responsive elements in the promoter regions of these genes [25].
The overexpression of HIF-1α has been demonstrated in several types of cancer, with a negative impact on therapy response and prognosis [26–28]. In sporadic breast cancer, previous studies have demonstrated that HIF-1α overexpression plays a role in breast carcinogenesis [29–32] and is correlated with a poor prognosis in invasive breast cancer [31, 33, 34].
Little is known of the putative role of HIF-1α in hereditary breast carcinogenesis. A recent study suggested that BRCA1 plays a role in the hypoxic response by regulating HIF-1α stability and by modulating expression of vascular endothelial growth factor, a major downstream target of HIF-1α [35]. The aim of this study was therefore to examine the expression of HIF-1α in BRCA1 related breast cancer to find clues for its putative role in the BRCA1 carcinogenesis.
Materials and methods
Patients
The study group comprised 30 invasive breast cancer cases from 17 patients with a proven BRCA1 germline mutation and 13 patients with invasive breast cancer who were not screened for mutations themselves, but were known to have a BRCA1 mutation in their family. All these patients were derived from the Familial Cancer Clinic of the VU University Medical Centre, Amsterdam. Use of anonymous or coded left over material for scientific purposes is part of the standard treatment contract with patients [36].
As sporadic controls, data from our previous study [33] on invasive breast cancers from patients unselected for family history were used.
Histopathology
Tumor size was measured in the fresh resection specimens, and tumor samples were subsequently fixed in neutral buffered formaldehyde, and processed to paraffin blocks according to standard procedures. A tissue array block was made as previously described [37].
About 4 μm thick sections were cut and stained with H&E for histopathology. Tumor type was assessed according to the WHO, and tumors were graded according to the Nottingham grading system. Mitoses counting was performed as previously described [38]. Presence of necrosis was noted. Scoring was performed by one observer (PJvD) who was blinded to the origin of the tumors.
Immunohistochemistry
After deparaffination and rehydration, target retrieval solution (DAKO) was used for antigen retrieval with all slides placed in a water bath for 45 min at 97°C. A cooling off period of 20 min preceded the incubation of the HIF-1α mouse monoclonal (BD Biosciences, Pharmingen, Lexington, USA), at a dilution of 1:500. The catalysed signal amplification system (DAKO) was used to detect HIF-1α as before [25]. For ER, PR, HER-2/neu, EGFR, Ki67, p53, p27 and p21 antigen retrieval was performed in an autoclave with the slides placed in a citrate buffer (pH 6). For Glut-1, CAIX and P300 antigen retrieval was performed in citrate buffer, pH = 6.0, for 20 min at 100°C and for CK5/6 and CK14 an EDTA buffer (pH 9) was used. A cooling off period of 30 min preceded the incubation (60 min at room temperature) with the primary antibodies. Mouse monoclonal antibodies used were: ER (1:50, DAKO), PR (1:50, Novocastra, Newcastle upon Tyne, United Kingdom), HER-2/neu (1:10,000, Prof. M. van der Vijver, Dutch Cancer Institute, Amsterdam, The Netherlands), EGFR (1:10, Novocastra), CK5/6 (1:3000, Chemicon, Temecula, USA), CK14 (1:400, Neomarkers, Lab Vision Corp, Fremont, CA, USA), Ki67 (1:40, MIB-1, Immunotech, Marseille Cedex, France), p53 (1:500, DAKO), p27 (1:1000, BD Biosciences Transduction laboratories, Lexington, USA), p21 (1:50, BD Biosciences, Pharmingen).
Polyclonal primary antibodies used were: Glut-1 (1:200, DAKO), CAIX (1:1000, Abcam, Cambridge Science Park, Cambridge, UK), P300 (1:200, clone N15, Santa Cruz, CA, USA) For detection of the primary antibodies against CK5/6, CK14, CAIX and p300, a poly HRP anti Mouse/Rabbit/Rat IgG (ready to use, ImmunoLogic, ImmunoVision Technologies, Brisbane, USA) was used for the other primary antibodies a biotinylated rabbit anti-mouse antibody (DAKO) or a biotinylated swine anti-rabbit antibody was used. The signal was amplified by avidin-biotin complex formation. All slides were developed with diaminobenzidine followed by haematoxylin counterstaining. Before the slides were mounted all sections were dehydrated in alcohol and xylene.
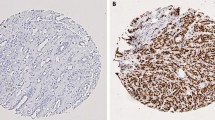
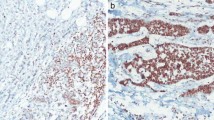
Scoring was performed by one observer (PJvD). HIF-1α, ER, PR, Ki67, p53, p27 and p21 staining was usually confined to the nucleus. Diffuse cytoplasmic staining was sometimes seen but ignored, estimating the percentage of positively stained nuclei. p300 nuclear staining intensity was in accordance with our previous study (39) scored as negative, 1+, 2+ or 3+ and for further statistical analysis grouped as negative (neg, 1+) or positive (2+, 3+). HIF-1α was regarded overexpressed when >1% of nuclei were positive as described before [27], and the expression pattern (perinecrotic or diffuse) was noted [32]. HER-2/neu, EGFR, and CAIX stainings were scored positive when a clear membrane staining pattern was seen. Glut-1 expression was scored positive if a clear membrane or a distinct cytoplasmic staining was seen, and Ck5/6, Ck14 were scored positive in case of cytoplasmic staining.
Results
High levels of HIF-1α expression were detectable in 27/30 (90%) of the hereditary breast cancer cases, compared to 88/200 (44%) of the sporadic controls (P < 0.0001) (Table 1). Necrosis was present in 19/30 (63%) of the hereditary cases compared to 38/200 (19%) of controls (P < 0.0001). In 19/27 of the hereditary cases that showed HIF-1α expression, a perinecrotic staining pattern was observed and in 8/27 a diffuse pattern was seen, compared to 27/88 (31%) and 61/88 (69%) of sporadic cases, respectively (P = 0.0002). Glut-1 expression was detected in 27/30 hereditary cases and CAIX in 15/21 cases, and both were correlated with HIF-1α overexpression (P-value < 0.001 for both). In the sporadic cases the expression of Glut-1 and CAIX was 29% (54/183) and 13% (24/183) respectively. p300 levels were significantly higher in hereditary cases 95% (20/21) compared to hereditary cases 50% (91/183), (P = 0.0001). Furthermore, high levels of HIF-1α expression in these hereditary breast cancers were associated with a poor histological grade (P = 0.061) and EGFR expression (P = 0.099) (Table 2). HIF-1α correlated significantly negatively with the presence of ER (P = 0.033), PR (P = 0.001), and HER-2/neu (P = 0.001). For the remaining markers no significant correlations with HIF-1α expression were found.
About 21/30 (70%) cases were both HIF-1α and EGFR positive. All of these HIF-1α and EGFR positive cases were Glut-1 positive, 14/21 of these cases showed a perinecrotic HIF-1α expression pattern and the remaining cases showed a diffuse HIF-1α expression pattern. In nine of the perinecrotic HIF-1α cases CAIX staining was also present. In the seven diffuse HIF-1α cases four cases were CAIX positive.
Discussion
The aim of this study was to examine the expression of HIF-1α in BRCA1 related breast cancers to establish whether the HIF-1α pathway plays a role in the BRCA-1 carcinogenesis and progression. About 90% of BRCA1 related breast cancers showed expression of HIF-1α, a percentage significantly higher than in sporadic controls. Mostly, this concerned the perinecrotic type of HIF-1α expression. Necrosis, likely caused by the well known rapid tumor cell proliferation of these hereditary cancers while the vasculature is lagging behind, was clearly more present than in the sporadic cancers. Likewise, a perinecrotic pattern of overexpression of HIF-1α was more frequent in BRCA1-related than in sporadic breast cancers. The perinecrotic type of HIF-1α expression was accompanied by overexpression of the HIF-1α downstream genes Glut-1 and CAIX, pointing towards functional HIF-1α. This type of HIF-1α overexpression is thought to be caused by (severe) hypoxia, whereas diffuse HIF-1α overexpression at (relative) normoxia is thought to be induced by growth factors like HER2 [39], HIF-1α gene amplifications [40] or mutations [41], or by other oncogenes or loss of tumour suppressor genes. We have previously shown that, compared to a diffuse staining pattern, perinecrotic HIF-1α overexpression is associated with the worst survival of sporadic breast cancer patients [33].
The present results are in contrast with a recent in vitro study where increased levels of BRCA1 were seen to increase the response of the VEGF promoter to hypoxia in a HIF-1α dependent fashion [35]. In that study, reduced levels of BRCA1 protein reduced the ability of hypoxia to induce VEGF. We, however, observed marked upregulation of HIF-1α in human BRCA1 related breast cancers. In view of the frequent presence of necrosis and perinecrotic HIF-1α expression, we hypothesize that in breast cancers in BRCA1 germline mutation carriers, hypoxia overrides the potential negative effect of BRCA1 expression loss on HIF-1α expression, yet leading to frequent perinecrotic HIF-1α expression and subsequently to activation of HIF-1 downstream genes. In line with this, p300 expression levels, a prerequisite for HIF-1 downstream activation, were high in hereditary cancers.
Whilst we previously reported frequent overexpression of EGFR in hereditary breast cancers [19, 42], we now find concomitant expression of HIF-1α and EGFR in 70% of BRCA1 related breast cancers. Furthermore, all of these cases had evidence of HIF1 downstream activation, suggesting that EGFR enhances the hypoxic response.
Several previous studies have elucidated in vitro the role of both the PI3K and the MAPK pathway in the induction of HIF-1α, including its upregulation by HER-2/neu. In addition, the upregulation of EGFR has been related to elevated levels of downstream targets of HIF-1α, like VEGF and survivin [43]. This suggests a role for specific oncogenes in the (normoxic) induction of HIF-1α. The association between HIF-1α and EGFR might be explained by the EGFR induced activation of the PI3K/PTEN/AKT/FRAP pathway, through which HER-2/neu also acts on HIF-1α [44, 45]. Further studies will have to elucidate the role of EGFR in the carcinogenesis of BRCA1 related breast cancer. Recent in vitro studies on breast basal-like cell lines showed that these cell lines are more sensitive for EGFR inhibitors and for carboplatin with a synergistic effect when these are combined [46]. This might lead to new therapy strategies for BRCA1 related breast cancer patients.
In contrast to EGFR, an association between HIF-1α and HER-2/neu as observed in previous studies [27, 39] could not be confirmed in this study. 26/27 (96%) of HIF-1α positive cases were HER-2/neu negative, in which the usual HER-2/neu negativity of hereditary breast cancers likely plays a role.
We conclude that the BRCA1 germline mutation related breast cancers show a high frequency of HIF-1α overexpression. In view of the predominantly perinecrotic staining pattern, overexpression of HIF-1α in hereditary breast cancer seems to be caused by hypoxia rather than by activation of oncogenes or inactivation of tumor suppressor genes. However, the frequent overexpression of EGFR and concomitant expression of EGFR and HIF-1α may open up news ways of treatment of BRCA1 related breast cancer by targeting EGFR.
References
Jonsson G, Naylor TL, Vallon-Christersson J et al (2005) Distinct genomic profiles in hereditary breast tumors identified by array-based comparative genomic hybridization. Cancer Res 65:7612–7621
van Beers EH, van Welsem T, Wessels LFA, Li Y, Oldenburg RA, Devilee P, Cornelisse CJ, Verhoef S, Hogervorst FBL, van’t Veer LJ, Nederlof PM (2005) Comparative genomic hybridization profiles in human BRCA1 and BRCA2 breast tumors highlight differential sets of genomic aberrations. Cancer Res 65:822–827
Tirkkonen M, Johannsson O, Agnarsson BA, Olsson H, Ingvarsson S, Karhu R, Tanner M, Isola J, Barkardottir RB, Borg A, Kaffioniemi P (1997) Distinct somatic genetic changes associated with tumor progression in carriers of BRCA1 and BRCA2 germ-line mutations. Cancer Res 57(7):1222–1227
Alvarez S, Diaz-Uriarte R, Osorio A, Barroso A, Melchor L, Paz MF, Honrado E, Rodríguez R, Urioste M, Valle L, Díez O, Cigudosa JC, Dopazo J, Esteller M, Benitez J (2005) A predictor based on the somatic genomic changes of the BRCA1/BRCA2 breast cancer tumors identifies the non-BRCA1/BRCA2 tumors with BRCA1 promoter hypermethylation. Clin Cancer Res 11:1146–1153
Hedenfalk I, Ringner M, Ben-Dor A, Yakhini Z, Chen Y, Chebil G, Ach R, Loman N, Olsson H, Meltzer P, Borg A, Trent J (2003) Molecular classification of familial non-BRCA1/BRCA2 breast cancer. Proc Natl Acad Sci USA 100(5):2532–2537
Wessels LF, van Welsem T, Hart AA, van’t Veer LJ, Reinders MJ, Nederlof PM (2002) Molecular classification of breast carcinomas by comparative genomic hybridization: a specific somatic genetic profile for BRCA1 tumors. Cancer Res 62:7110–7117
Armes JE, Venter DJ (2002) The pathology of inherited breast cancer. Pathology 34:309–314
Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF (2002) The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol 20(9):2310–2318
Marcus JN, Watson P, Page DL, Narod SA, Lenoir GM, Tonin P, Linder-Stephenson L, Salerno G, Conway TA, Lynch HT (1996) Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer 77(4):697–709
Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases (1997) Breast cancer linkage consortium. Lancet 349(9064):1505–1510
Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, Begin LR, Hamel N, Goffin JR, Wong N, Trudel M, Kapusta L, Porter P, Akslen LA (2004) The prognostic implication of the basal-like (Cyclin Ehigh/P27low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res 64:830–835
Eisinger F, Stoppa-Lyonnet D, Longy M, Kerangueven F, Noguchi T, Bailly C, Vincent-Salmon A, Jacquernier J, Birnbaum D, Sobol H (1996) Germ line mutation at BRCA1 affects the histoprognostic grade in hereditary breast cancer. Cancer Res 56(3):471–474
Chappuis PO, Nethercot V, Foulkes WD (2000) Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol 18:287–295
Chappuis PO, Kapusta L, Bégin LR, Wong N, Brunet JS, Narod SA, Slingerland J, Foulkes WD (2000) Germline BRCA1/2 mutations and p27(Kip1) protein levels independently predict outcome after breast cancer. J Clin Oncol 18:4045–4052
Greenblatt MS, Chappuis PO, Bond JP, Hamel N, Foulkes WD (2001) TP53 mutations in breast cancer associated with BRCA1 or BRCA2 germ-line mutations: distinctive spectrum and structural distribution. Cancer Res 61:4092–4097
Straume O, Chappuis PO, Salvesen HB, Halvorsen OJ, Haukaas SA, Goffin JR, Begin LR, Foulkes WD, Akslen LA (2002) Prognostic importance of glomeruloid microvascular proliferation indicates an aggressive angiogenic phenotype in human cancers. Cancer Res 62:6808–6811
Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423
Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA (2003) Germline BRCA1 mutations and a basal epithelialphenotype in breast cancer. J Natl Cancer Inst 95:1482–1485
Van der Groep P, Bouter A, van der Zanden R, Menko FH, Buerger H, Verheijen RH, van der Wall E, van Diest PJ (2004) Re: germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 96(9):712–713
Van der Groep P, Bouter A, van der Zanden R, Siccema I, Menko FH, Gille JJ, van Kalken C, van der Wall E, Verheijen RH, van Diest PJ (2006) Distinction between hereditary and sporadic breast cancer on the basis of clinicopathological data. J Clin Pathol 59(6):611–617
Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, Chang-Claude J, Hamann U, Cornelisse CJ, Devilee P, Beckmann MW, Nestle-Kramling C, Daly PA, Haites N, Varley J, Lalloo F, Evans G, Maugard C, Meijers-Heijboer H, Klijn JG, Olah E, Gusterson BA, Pilotti S, Radice P, Scherneck S, Sobol H, Jacquemier J, Wagner T, Peto J, Stratton MR, McGuffog L, Easton DF (2005) Breast cancer linkage consortium. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res 11(14):5175–5180
Vaupel P, Thews O, Hoeckel M (2001) Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 18:243–259
Salceda S, Caro J (1997) Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272:22642–22647
Huang LE, Gu J, Schau M, Bunn HF (1998) Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependert degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci 95:7987–7992
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo synthesis binds to the human erythropoeitin gene enhancer at a site required for transcriptational activation. Mol Cell Biol 12:5447–5454
Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E (2003) Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast cancer. Cancer 97:1573–1581
Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E (2001) Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst 93:309–314
Horrée N, Van Diest PJ, Van der Groep P, Sie-Go DMDS, Heintz APM (2007) Hypoxia and angiogenesis in endometrioid endometrial carcinogenesis. Cell Oncol 29:219–227
Bos R, van der Hoeven JJM, van der Wall E, van der Groep P, van Diest PJ, Comans EFI, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, Molthoff CFM (2002) Biologic correlates of 18fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 20:379–387
Bos R, Van Diest PJ, van der Groep P, Greijer AE, Hermsen MAJA, Heijnen I, Meijer GA, Baak JPA, Pinedo HM, van der Wall E, Shvarts A (2003) Protein expression of B-cell lymphoma gene 6 (BCL-6) in invasive breast cancer is associated with cyclin D1 and hypoxia-inducible factor-1alpha (HIF-1alpha). Oncogene 22:8948–8951
Bos R, van Diest PJ, van der Groep P, Shvarts A, Greijer AE, van der Wall E (2004) Expression of hypoxia-inducible factor-1a and cell cycle proteins in invasive breast cancer are estrogen receptor related. Breast Cancer Res 6:R450–R459
Bos R, Van Diest PJ, De Jong JS, Van der Groep P, Van der Valk P, Van der Wall E (2005) Hypoxia-inducible factor-1a is associated with angiogenesis and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology 46:31–36
Vleugel MM, Greijer AE, Shvarts A, van der Groep P, van Berkel M, Aardbodem Y, van Tinteren H, van Diest PJ, van der Wall E (2005) Differential prognostic impact of hypoxia induced and diffuse HIF-1α expression in invasive breast cancer. J Clin Pathol 58(2):172–177
Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouysségur J, Berra E (2007) HIF-1α and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer 120:1451–1458
Kang HJ, Kim HJ, Rih JK, Mattson TL, Kim KW, Cho CH, Isaacs JS, Bae I (2006) BRCA1 plays a role in the hypoxic response by regulating HIF-1α stability and by modulating Vascular endothelial growth factor expression. J Biol Chem 281(19):13047–13056
van Diest PJ (2002) No consent should be needed for using leftover body material for scientific purposes. BMJ 325:648–651
van der Groep P, Bouter A, van der Zanden R et al (2004) Re: germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 96:712–713
Van Diest PJ, Baak JP, Matze-Cok P, Wisse-Brekelmans EC, van Galen CM, Kurver PH, Bellot SM, Fijnheer J, van Gorp LH, Kwee WS et al (1992) Reproducibility of mitosis counting in 2469 breast cancer specimens: results from the multicenter morphometric mammary carcinoma project. Hum Pathol 23:603–607
Laughner E, Taghari P, Chiles K, Mahon PC, Semenza GL (2001) Her2(neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1 mediated vascular endothelial growth factor expression. Mol Cell Biol 21(12):3995–4004
Vleugel MM, Greijer AE, van der Wall E, van Diest PJ (2005) Mutation analysis of the HIF-1α oxygen dependent degradation domain in invasive breast cancer. Cancer Genet Cytogenet 163(2):168–172
Vleugel MM, Bos R, Buerger H, van der Groep P, Saramäki OR, Visakorpi T, van der Wall E, van Diest PJ (2004) No amplifications of hypoxia-inducible factor-1α gene in invasive breast cancer: a tissue microarray study. Cell Oncol 26(5–6):347–351
Van Diest PJ, van der Groep P, van der Wall E (2006) EGFR expression predicts BRCA1 status in patients with breast cancer. Clin Cancer Res 12(2):670
Peng XH, Kama P, Cao Z, Jiang BH, Zhou M, Yang L (2006) Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by upregulating survivin gene expression. J Biol Chem 281(36):25903–25914
Vleugel MM, Shvarts D, van der Wall E, van Diest PJ (2006) p300 and p53 levels determine activation of HIF-1 downstream targets in invasive breast cancer. Hum Pathol 37(8):1085–1092
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL (2000) Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogensis and therapeutics. Cancer Res 60:1541–1545
Hoadley KA, Weigman VJ, Fan C, Sawyer LR, He X, Troester MA, Sartor CI, Rieger-House T, Bernard PS, Carey LA, Perou CM (2007) EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics 8(1):258
Acknowledgement
Supported by an unrestricted educational grant from Aegon Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Groep, P., Bouter, A., Menko, F.H. et al. High frequency of HIF-1α overexpression in BRCA1 related breast cancer. Breast Cancer Res Treat 111, 475–480 (2008). https://doi.org/10.1007/s10549-007-9817-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9817-z