Abstract
The combination of anticancer drugs used in the clinic has been based upon empiricism, and the potential permutations of currently available drugs overwhelm the clinical trials system. Recently, investigators have suggested that the combination of a blockade of vital signal transduction pathways in combination with more standard therapy might enhance anticancer effect. Using a panel of breast cancer cell lines and isobologram median effect analysis, a method of determining synergism or antagonism of drugs, we have investigated in vitro potentially clinically useful combinations of agents with the human cell lines MCF7/wt, MCF7/adr, BT474, and SK-BR-3 grown in log phase. Results were confirmed by curve shift analysis. Cells were exposed to the agent(s) for 72 h and then analyzed for cytotoxicity using a MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl-tetrazolium bromide) assay. Fluvastatin, an inhibitor of prenylation with excellent tolerability in man, was chosen to disrupt signal transduction pathways and thus potentially enhance the effect of more traditional anticancer agents. Anticancer agents tested were cytotoxics used in the treatment of breast cancer, trastuzumab, and rapamycin as an inhibitor of the AKT pathway. Fluvastatin combined with trastuzumab demonstrates global synergy of cytotoxic effect that is confirmed by apoptosis assay. These effects could only be partially reversed by adding farnesol or geranylgeraniol to restore prenylation. Epirubicin is also synergistic with fluvastatin in three of the four cell lines. Rapamycin, an inhibitor of MTOR, was synergistic with fluvastatin in two of the four cell lines and antagonistic in two other cell lines. The combination of fluvastatin or another inhibitor of prenylation and trastuzumab may be attractive for clinical development as the effect of trastuzumab in Her2/neu positive breast tumors is incomplete as a single agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of signal transduction in malignant growth has assumed greater importance with the realization of the limited clinical utility of the presently available cytotoxic agents and the potential to individualize therapy based upon the expression of both growth and anti-apoptotic signals in the tumor [1, 2]. This approach has been very successful with the use of trastuzumab to interfere with Her2/neu signaling in breast cancer [3], and targeted agents have enhanced therapeutic effect when combined with traditional agents in other malignancies [4]. Of the additional currently studied pathways that are amenable to clinical trials, interference with prenylation of essential cellular proteins such as RAS, RHO, PxF, lamins A and B, and other G-proteins is attractive because of the vital role of those proteins in cell growth and survival [5]. Inhibition of prenylation combined with either targeting agents causing a sequential blockade of a pathway or with a classical cytotoxic agent has been suggested as a mechanism to enhance antitumor effect [6, 7]. The HMG-CoA reductase inhibitors (statins) block the formation of mevalonic acid thus inhibiting the availability of precursors of prenylation [8, 9]. The HMG-CoA reductase inhibitors offer a potential advantage over a classical farnesyl transferase inhibitor as interference with the mevalonate pathway inhibits both farnesylation and geranylgeranylation of proteins. In addition, the statins have a well-known safety profile in man based upon their use in hyperlipidemic syndromes [10]. There are numerous statins in the clinic which vary in their efficacy to block HMG-CoA reductase and their toxicities in man [11]. Cerivastatin is the most potent inducer of apoptosis in tumor cells of the statins [12], but was associated with rhabdomyolysis and has been withdrawn from commercial use [13]. Fluvastatin has been suggested to have less significant toxicities in man [14, 15]. Fluvastatin was previously demonstrated to induce apoptosis in the human breast cancer cell line MCF7 [16]. Fluvastatin has also been shown to have potent antitumor activity in preclinical model systems, and in addition has antiangiogenic and antimetastatic effects [17]. Therefore, fluvastatin was chosen as our candidate statin.
We have previously emphasized our combination drug development studies in model systems of breast cancer. Therefore, our choices of preclinical combinations to evaluate with a statin were based upon our previously reported cytotoxic experience in breast cancer cell lines [18, 19], the availability of agents to block at different sites of the signal transduction pathways such as interfering with the AKT pathway [20], and an agent (trastuzumab) with clinical activity to interfere with the Her2/neu signal pathway. An additional reason to choose rapamycin to study was the finding that M-TOR inhibitors demonstrate activity in breast cancer cell lines by blocking estrogenic stimulation of MCF7 cells (estrogen receptor positive) and synergize with letrozole in inducing apoptosis in vitro [21].
Methods
Reagents
Docetaxel, reagent grade, was a gift of Sanofi-Aventis Pharmaceuticals (Bridgewater, NJ). Vinorelbine was a gift of Glaxo SmithKline (Research Triangle, NC). Fluvastatin, was obtained from Novartis Pharma AG (Basel, Switzerland). The following agents were obtained from Sigma-Aldrich (St. Louis, Mo): carboplatin, cisplatin, 5’DFUR, farnesyl pyrophosphate, geranylgeranyl pyrophosphate, paclitaxel, and rapamycin. Epirubicin was obtained from Calbiochem (San Diego, CA). Trastuzumab was obtained from commercial stock. A variety of human breast cancer cell lines with differing phenotypic properties were evaluated in order to look for global effects. The following cell lines were obtained from the American Tissue Culture Collection (ATCC, Rockville, MD): breast lines: MCF7/wt, BT474, and SK-BR-3. MCF7/adr, a multiply resistant cell line, was a gift of Dr. Kenneth Cowan (University of Nebraska Medical Center, Omaha, Nebraska). The phenotypes of these cell lines have been previously described [22–25]. MCF 7 and BT 474 both express the estrogen receptor. BT 474 has loss of normal P53 as does MCF7/adr [26]. MCF7/adr and SK-BR-3 are receptor negative and over express Her2/neu [22, 23, 27]. MCF7/adr is known to over express MDR-1 (multiple drug resistance protein) and pi-GST (pi-glutathione transferase). MCF7/adr is grown in minimal essential media with 10% fetal calf serum, supplemented with glutamine, antibiotics and antimycotics in the presence of 10 μM doxorubicin. For experiments with this cell line, the doxorubicin was removed from the media one week prior to the studies.
Assessment of MTT cytotoxicity produced by therapeutic agents
Determination of cytotoxicity of the various drugs and median effect analysis was done by previous methods [28–30]. In brief, the cells were grown to confluence in T 150 tissue culture flasks (Corning Glass Works, Corning, NY) using RPMI 1640 (Invitrogen, Carlsbad, CA) with 5% CO2 and 10% heat inactivated fetal calf serum. All other reagents were obtained from Invitrogen. All cultures contained penicillin (100 μg /ml), streptomycin (0.25 μg/ml), and glutamine to a final concentration of 2 mM. All cell lines were repeatedly tested for mycoplasma (Invitrogen kit) and had viabilities by Trypan blue exclusion greater than 95%. Harvested cells were aliquoted into 96 well dishes (Falcon 3072) at concentrations of 5000–8000 cells per well. The cells were then cultured for 24 h, cytotoxic agents or solvent controls were introduced for a 72-h incubation, and cell growth evaluated by a MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl-tetrazolium bromide) assay [31] using a BioRad 3550 Micro plate Reader (BioRad, Hercules, CA) [32]. IC50 (the dose of drug needed to cause inhibition of growth in 50% of the cells) concentration was determined by the EZ-ED50 Program (Perrella Scientific, Conyers, CA). All reported values are the means of at least three experiments with each study having four wells per dose level. In addition, the inhibitory activity of selected combinations were measured serially over 72 hours using the MTT assay at concentrations which are known to be achieved clinically. For the initial studies of fluvastatin, we fixed the dose of fluvastatin in cell culture to be the highest accepted chronic therapeutic drug level (0.125 μM) based upon pharmacokinetic studies in man and also evaluated 50% of that level [33]. Results are shown for the highest concentration.
Measurement of synergy
The median effect model allows one agent to be fixed in concentration while the other agent’s concentration can be varied to obtain a dose–response curve of the combination. The experimental conditions were previously reported in detail and are similar to the methods used to determine the IC50 of individual agents [18, 19, 28–30, 32, 34–36]. All reported values are the means of at least three experiments with 72 h incubations in each study having four wells per dose level.
Median effect analysis, based upon the Hill equation, allowed the determination of synergistic, additive, or antagonistic effects when up to three agents were combined together. This effect was determined by the method of Chou [37, 38] using their computer program [39]. The resulting CI (combination index) which reflects synergy when less than 1, additive effects when equal to 1, and antagonism when greater than 1 was calculated for varying levels of drug effect (Fa). We have previously defined additive effects to be all values within one standard deviation of unity. Statistical differences were confirmed using the curve shift analysis of Zhao et al. [40]. Ten fixed drug ratios above and below the IC50 with a range of 0.0156N to 8N where N is a value near the IC50 of an individual drug were explored by incubating the drug combinations with cells for 72 h and then determining the degree of cytotoxic effect by the MTT assay. Fa is defined as the fraction of cells affected, and a plot of log dose versus log \( (\frac{{{\hbox{Fa}}}} {{1 - {\hbox{Fa}}}}) \) gives parallel slopes if no biologic interaction is present (mutually exclusive) or converge if there is an interaction between the drugs (mutually nonexclusive) thus suggesting the appropriate model to determine the CI [37]. Fa50 is defined at that point where 50% of the cells are affected. The results of the drug interactions are shown in tabular form at the Fa50 as the median effect equation is a linear approximation of a higher order equation and most accurate at the Fa50. These results were confirmed by curve shift analysis. Curve shift analysis was performed with the program ACT Analysis (Optimum Therapeutics LLC., Columbus, Ohio) using non-linear regression of the concentration–effect data. The results were then normalized to IC50 equivalents. A shift of the survival versus IC50 equivalent curve to the left for the combination treatment is an indication of synergy [40].
Reversal of prenylation
The method of Wong et al. [12] was used at the IC50 (fluvastatin 0.125 μM; trastuzumab 1.55 μg/ml) and the IC70 (fluvastatin 0.125 μM; trastuzumab 8.5 μg/ml) determined by a 72 h co-incubation of agents with cells. Cell lines growing in exponential phase had either farnesyl pyrophosphate or geranylgeranyl pyrophosphate added to the culture at time 0 in increasing concentrations (0 μM–10 μM) with media used for control samples as previously described [12]. Cell death was measured by MTT.
Evaluation of apoptosis
The commercially available Cell Death Detection ELISAplus kit (Roche Applied Science, Penzberg, Germany) was used to detect DNA fragmentation by an ELISA assay as previously described [41]. This assay exploits the amount of cytoplasmic histone-associated DNA fragments produced upon cell death. Cells, after the appropriate time of drug(s) exposure and after centrifugation, were lysed in 96-well plates. About 20 μl of the supernatant was transferred to a streptavidin-coated plate that was supplied with the kit. This supernatant was incubated for 2 h in the presence of the immune reagent containing the antibodies against the histone proteins and DNA fragments. The complex was then simultaneously conjugated to form an immunocomplex on the plate, which then was subsequently read for optical density at 405 nm with a reference wavelength at 490 nm. Samples were measured in duplicate and a positive control was provided with the kit. The result is described as an enrichment factor, which was thus a relative indicator of the number of cells undergoing apoptosis as calculated by the following formula:
where mU = absorbance (405 nm–490 nm) and reflects the amount of histones and DNA fragments released into the cytoplasm from the apoptotic cells. Statistical differences were determined by paired t-tests.
Cell counts were performed using Trypan blue exclusion. Cells are exposure to drug(s) for the indicated time period, typsinized, concentrated via centrifugation and washed twice in phosphate buffered saline. Cells that did not take up the dye were counted in triplicate on a hemocytometer.
Results
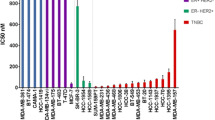
The IC50 concentrations as determined by a 72-h drug exposure with the various cell lines are shown in Table 1. The activities of the various drugs varied in a three-log range between cell lines with the MCF7/wt being the most resistant cell line.
Median effect analysis of the doublet combinations is shown in Table 2. Global synergy was noted for the combination of fluvastatin with trastuzumab (CI 0.4–0.7 for all cell lines examined). Fluvastatin was studied at the clinically achievable plasma level. This synergism was confirmed by using curve shift analysis and is shown for the SK-BR-3 cell line (Fig. 1) but was also confirmed in other cell lines. Synergy with fluvastatin was also noted at one-half the maximal concentration of fluvastatin (data not shown). Epirubicin was synergistic with fluvastatin in three of the four cell lines with only the multiply resistant, MDR expressing MCF7/adr demonstrating absence of synergistic effect (CI = 1.5 ± 0.4). This synergy as measured by MTT assay was time dependent with the most profound effects seen after 72 h (data not shown). Even though the expression of Her2/neu on MCF7 cells is low [22] and undetectable by Western blot in our hands (data not shown), the induction of apoptosis in this cell line with concurrent 48 or 72 h incubations with trastuzumab was also demonstrated (Fig. 2). At both time points, there is a low but significant induction of apoptosis in this cell line by trastuzumab which is further enhanced by the addition of fluvastatin. Fluvastatin by itself also demonstrates the induction of apoptosis in this assay system (Fig. 2). Cell death was also confirmed by cell counts over the 72-h incubation (data not shown). The effect of the combination of fluvastatin and trastuzumab were also examined in the SK-BR-3 cell line which highly expresses Her2/neu [22]. As seen in Fig. 3, both trastuzumab and fluvastatin demonstrate significant induction of apoptosis with the combination being more effective. These findings were also confirmed by cell count (data not shown).
To determine whether or not the synergistic effects demonstrated were due to inhibition of prenylation, increasing concentrations of either farnesyl pyrophosphate or geranylgeranyl pyrophosphate were added back to the combination of fluvastatin with trastuzumab at the IC50 and IC70 concentrations previously indicated and incubated for 72 h prior to MTT assay. Both prenylation agents partially reversed the cytotoxic effect at the IC50 (Fig. 4) compared to control but did not reverse the cytotoxic effect at the IC70 (data not shown) even though the concentration of fluvastatin was held constant in both studies.
Incomplete reversal of cytotoxic effect by reversal of prenylation at the IC50 of the combination of fluvastatin and trastuzumab in SK-BR-3 cells. Addition of farnesyl pyrophosphate (F-PP) (●) or geranylgeranyl pyrophosphate (GG-PP) (□) did not totally prevent cell death. Cytotoxic effect as measured by MTT assay is normalized to control cells. Values are shown with SD
Fluvastatin was synergistic with rapamycin in only two of the cell lines, MCF7/wt and MCF7/adr. The apoptotic assay results for the MCF7/adr, the highly resistant cell line, as shown in Fig. 5 demonstrate enhancement of fluvastatin and rapamycin cytotoxic effect.
Conclusion
The plethora of anticancer agents in development and in the clinic has led to permutations of combinations of agents that cannot be clinically tested. Most of the present chemotherapeutic combinations used for the treatment of human solid tumors have been developed on an empiric basis by adding active agents together. However, this approach may result in drug interactions in which the combination may demonstrate additive, synergistic, or antagonistic cytotoxic effects in vitro thus questioning the validity of at least some combination therapies in the clinical setting [30]. In the majority of cases, drug combinations have been studied in small phase II trials or the nihilistic approach in metastatic breast cancer has been the use of single sequential agents to minimize toxicity [42]. As a result, there is an acute need for more relevant preclinical model systems and various proposals of cell line, xenograft, and mouse allograft models have been suggested [43]. However, all models remain inexact with limited predictive value. We have pursued a semi automated screening methodology to identify in vitro combinations of drugs which either suggest synergistic or antagonistic cytotoxic effects in the hope that a global effect in a variety of cell lines may suggest a potentially attractive combination to pursue or identify an antagonistic combination which is not worthy of further examination [30, 32]. The potential deficiencies of the assay are that it does not reflect therapeutic index or the heterogeneity of human tumors.
To accomplish this aim, we have used defined cell lines growing in exponential phase with isobologram analysis which has been defined as the standard [44] and modified by several investigators [35, 40, 44–46]. The use of median effect analysis which is based upon the Hill equation allows less experimental points per sample and the ability to study up to three different agents in combination [37–39, 47, 48]. In addition, the shape of the synergy curve can be examined. This method also has been criticized as it is a linear approximation of a higher order equation [49] and thus is most accurate at the point of 50% cytotoxicity. We therefore have defined synergistic effect using the Fa50 (50% cytotoxicity) as measured by a 72-h incubation of drugs and cells in a 96 well micro titer dish. A recent modification using curve shift analysis has also statistically strengthened the methodology [40].
Most of the studies of putative targeted agents in malignancy have been disappointing clinically which may be a reflection of the marked redundancy of signaling networks and the marked cross talk between networks [50]. The statins offer an advantage that by interfering with the mevalonate pathway, they down regulate the production of both farnesyl and geranylgeranyl moieties with an acceptable therapeutic index. Numerous proteins are affected by this loss of prenylation including up regulation by fluvastatin of p21 and p53 in the murine renal cancer cell Renca [17]. These agents are undergoing a reevaluation as an adjunctive therapy [51, 52]. As monotherapy, statins have a marked antitumor effect in vitro [8, 9, 51, 53–71]. Part of this antitumor effect is through geranylgeranylation [68, 72–74]. These agents also have diverse biological effects including abrogation of the stimulatory effect of insulin-like growth factor I (IGF-I) in 3T3-L1 cells[75], inhibition of the P-glycoprotein MDR transporter by lovastatin and simvastatin[76], disturbance of the cell membrane through functional inhibition of the Rho family G-proteins[77], down regulation of Bcl-2 in breast cancer MCF-7 cells without up regulation of p53 [16], translocation of BAX to the mitochondria with activation of the apoptosis pathway [78], and blocking adaptive cholesterol responses to against oxidant injury in leukemia cells [66]. Most recently, lovastatin has also been demonstrated to up regulate PTEN expression in MCF7 cells [79], and the magnitude of PTEN expression has been correlated with trastuzumab cytotoxicity in SK-BR-3 cells [80].
As single agents, the activity of statins against established tumors in the clinical setting has been negligible [52, 81]. Hence, the degree of inhibition of this pathway may be critical for cell survival with incomplete inhibition of prenylation as seen with statins not being able to demonstrate in man significant cytotoxic effect by itself, while highly efficient inhibitory agents of the farnesyl and geranylgeranyl pathways are toxic [82]. In our studies, reversal of inhibition of prenylation by the addition of either farnesyl pyrophosphate or geranylgeranyl pyrophosphate was incomplete at a dosage of drugs which caused a moderate cell kill (IC50) but was unable to reverse the cytotoxicity at high cell kill (IC70) implying that the combination effect observed may be due to more than one mechanism. The value of combining an optimal dose of a statin with more established anticancer agents or an additional signal transduction drug in the clinic remains unexplored.
The EGFR superfamily is now under intense evaluation as a target for pharmacologic manipulation in the treatment of breast cancer. Clinically, the management of Her2/neu positive breast cancer has been revolutionized by the introduction of trastuzumab to block this signal transduction pathway [83]. Efforts are underway to identify additive and synergistic combinations of trastuzumab with more conventional agents in clinical trials [3]. These results have led to a major pharmaceutical effort to target growth factor signaling pathways [84]. The RAS pathway and its interaction with AKT were therefore chosen for in vitro study as pharmacologic agents now exist which allow perturbation of more than one site in the PI3 kinase/AKT pathway and thus may enhance antitumor effect as previously suggested.
The results reported here have not been described previously except for the ability of statins to synergize with doxorubicin or interferon in some model systems [85–87]. In the present in vitro studies, we have demonstrated that the combination of fluvastatin with trastuzumab displays global synergy in our breast cancer cell lines which vary in their expression of Her2/neu, and that this effect is not due to a cytostatic effect but rather reflects enhanced apoptosis. As trastuzumab is not of value in almost half the Her2/neu over expressing patients with breast tumors and also is of no value in patients with absent over expression of Her2/neu [88, 89], methods to enhance this drug’s activity are of potential great value. The global nature of the interaction suggests that perhaps the inhibition of prenylation and potential other mechanisms of statin action such as PTEN expression might also broaden the spectrum of trastuzumab and may also heighten the degree of anticancer activity in Her2/neu positive patients.
In a similar manner, epirubicin and fluvastatin are synergistic in three of the four cell lines and this effect is time dependent. The anthracyclines are some of the most important agents in the treatment of breast cancer [90]. Whether or not the enhancement of cytotoxic effect seen with an anthracycline in our studies can be extended to an improved therapeutic index in man awaits further study. In patients with the AKT transduction pathway adding to the tumor’s survival and proliferation, the use of a blocker of prenylation combined with an inhibitor of this pathway (in our studies an M-TOR inhibitor) may also be of benefit. Our results with rapamycin indicate that antagonism may occur in some cell lines (both with over expression of the EGFR superfamily), but the mechanism of antagonism remains unknown at present.
The mechanism of global synergy between fluvastatin and trastuzumab noted in the current studies is under evaluation. In Toto, inhibition of prenylation and perhaps additional pathways may have a useful role in future management of breast cancer when combined with additional signal transduction inhibitors such as trastuzumab or classical cytotoxic agents such as anthracyclines.
References
Adjei AA (2001) Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst 93:1062–1074
Elsayed YA, Sausville EA (2001) Selected novel anticancer treatments targeting cell signaling proteins. Oncologist 6:517–537
Pegram MD, Konecny GE, O’Callaghan C et al (2004) Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96:739–749
Blay JY, Le Cesne A, Alberti L et al (2005) Targeted cancer therapies. Bull Cancer 92:E13–18
Gelb MH, Scholten JD, Sebolt-Leopold JS (1998) Protein prenylation: from discovery to prospects for cancer treatment. Curr Opin Chem Biol 2:40–48
Russo P, Loprevite M, Cesario A et al (2004) Farnesylated proteins as anticancer drug targets: from laboratory to the clinic. Curr Med Chem Anti-Canc Agents 4:123–38
Graaf MR, Richel DJ, van Noorden CJ et al (2004) Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev 30:609–641
Bouterfa HL, Sattelmeyer V, Czub S et al (2000) Inhibition of Ras farnesylation by lovastatin leads to downregulation of proliferation and migration in primary cultured human glioblastoma cells. Anticancer Res 20:2761–2771
Cave WT Jr (1994) Isoprenoids and neoplastic growth. World Rev Nutr Diet 76:70–73
Bottorff M, Hansten P (2000) Long-term safety of hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors. Arch Intern Med 160:2273–2280
Mason RP, Walter MF, Day CA et al (2005) Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am J Cardiol 96:11F-23F
Wong WW, Tan MM, Xia Z et al (2001) Cerivastatin triggers tumor-specific apoptosis with higher efficacy than lovastatin. Clin Cancer Res 7:2067–2075
Staffa JA, Chang J, Green L (2002) Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 346:539–540
Scripture CD, Pieper JA (2001) Clinical pharmacokinetics of fluvastatin. Clin Pharmacokinet 40:263–281
De Angelis G (2004) The influence of statin characteristics on their safety and tolerability. Int J Clin Pract 58:945–955
Muck AO, Seeger H, Wallwiener D (2004) Inhibitory effect of statins on the proliferation of human breast cancer cells. Int J Clin Pharmacol Ther 42:695–700
Horiguchi A, Sumitomo M, Asakuma J et al (2004) 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin Cancer Res 10:8648–8655
Budman DR, Calabro A, Wang LG et al (2000) Synergism of cytotoxic effects of vinorelbine and paclitaxel in vitro. Cancer Invest 18:695–701
Budman DR, Calabro A (2002) In vitro search for synergy and antagonism: evaluation of docetaxel combinations in breast cancer cell lines. Breast Cancer Res Treat 74:41–46
Vignot S, Faivre S, Aguirre D et al (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16:525–537
Boulay A, Rudloff J, Ye J et al (2005) Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res 11:5319–5328
Konecny G, Pauletti G, Pegram M et al (2003) Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95:142–153
deFazio A, Chiew YE, Sini RL et al (2000) Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer 87:487–498
Lasfargues EY, Coutinho WG, Redfield ES (1978) Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst 61:967–978
Love-Schimenti CD, Gibson DF, Ratnam AV et al (1996) Antiestrogen potentiation of antiproliferative effects of vitamin D3 analogues in breast cancer cells. Cancer Res 56:2789–2794
Cai Z, Capoulade C, Moyret-Lalle C et al (1997) Resistance of MCF7 human breast carcinoma cells to TNF-induced cell death is associated with loss of p53 function. Oncogene 15:2817–2826
Budman DR, Soong R, Calabro A et al (2006) Identification of potentially useful combinations of epidermal growth factor receptor tyrosine linase antagonists with conventional agents using median effect analysis. Anticancer Drugs 17:921–928
Budman DR, Calabro A (2004) Studies of synergistic and antagonistic combinations of conventional cytotoxic agents with the multiple eicosanoid pathway modulator LY 293111. Anticancer Drugs 15:877–881
Budman DR, Calabro A, Kreis W (2001) In vitro effects of dexrazoxane (Zinecard) and classical acute leukemia therapy: time to consider expanded clinical trials? Leukemia 15:1517–1520
Budman DR, Calabro A, Kreis W (1998) In vitro evaluation of synergism or antagonism with combinations of new cytotoxic agents. Anticancer Drugs 9:697–702
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to profileration and cytotoxicity assays. J Immunol Methods 65:55–63
Kreis W, Budman DR, Calabro A (1997) Unique synergism or antagonism of combinations of chemotherapeutic and hormonal agents in human prostate cancer cell lines. Br J Urol 79:196–202
Barilla D, Prasad P, Hubert M et al (2004) Steady-state pharmacokinetics of fluvastatin in healthy subjects following a new extended release fluvastatin tablet, Lescol XL. Biopharm Drug Dispos 25:51–59
Budman DR, Calabro A, Kreis W (2002) Synergistic and antagonistic combinations of drugs in human prostate cancer cell lines in vitro. Anticancer Drugs 13:1011–1016
Konecny GE, Pegram MD (2004) Gemcitabine in combination with trastuzumab and/or platinum salts in breast cancer cells with HER2 overexpression. Oncology (Huntingt) 18:32–36
Kreis W, Budman DR, Calabro A (2001) A reexamination of PSC 833 (Valspodar) as a cytotoxic agent and in combination with anticancer agents. Cancer Chemother Pharmacol 47:78–82
Chou TC, Talalay P (1981) Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem 115:207–216
Chou TC (1994) Assessment of synergistic and antagonistic effects of chemotherapeutic agents in vitro. Contrib Gynecol Obstet 19:91–107
Chou TC (1998) Drug combinations: from laboratory to practice. J Lab Clin Med 132:6–8
Zhao L, Wientjes MG, Au JL (2004) Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res 10:7994–8004
Liu X, Yue P, Zhou Z et al (2004) Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst 96:1769–1780
Seidman A (2003) Introduction. Single-agent or combination chemotherapy in metastatic breast cancer. Oncology (Huntingt) 17:9–14
Voskoglou-Nomikos T, Baral S, Seymour L (2003) The role of in vitro cell line, human xenograft, and mouse allograft models in cancer drug development. In: Budman D et al (Eds) Handbook of anticancer drug development. Lippincott, Williams & Wilkins, Baltimore, pp 129–148
Gessner PK (1995) Isobolographic analysis of interactions: an update on applications and utility. Toxicology 105:161–179
Grabovsky Y, Tallarida RJ (2004) Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther 310:981–986
Tallarida RJ (2001) Drug synergism: its detection and applications. J Pharmacol Exp Ther 298:865–872
Chakrabarti D, Azam T, DelVecchio C et al (1998) Protein prenyl transferase activities of Plasmodium falciparum. Mol Biochem Parasitol 94:175–184
Chou TC (2002) Synergy determination issues. J Virol 76:10577 author reply 10578
Greco WR, Bravo G, Parsons JC (1995) The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385
Sachs K, Perez O, Pe’er D et al (2005) Causal protein-signaling networks derived from multiparameter single-cell data. Science 308:523–529
Cohen LH, Pieterman E, van Leeuwen RE et al (2000) Inhibitors of prenylation of Ras and other G-proteins and their application as therapeutics. Biochem Pharmacol 60:1061–1068
Stamm J, Ornstein D (2005) The role of statins in cancer prevention and treatment. Oncology 19:739–750
Ayral-Kaloustian S, Salaski EJ (2002) Protein farnesyltransferase inhibitors. Curr Med Chem 9:1003–1032
Blume E (1993) Drug designers target Ras for cancer treatment. J Natl Cancer Inst 85:1542–1544
Bredel M, Pollack IF, Freund JM et al (1998) Inhibition of Ras and related G-proteins as a therapeutic strategy for blocking malignant glioma growth. Neurosurgery 43:124–131; discussion 131–132
Canevari S, Biocca S, Figini M (2002) Re: blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst 94:1031–1032 (author reply 1032)
Collisson EA, Carranza DC, Chen IY et al (2002) Isoprenylation is necessary for the full invasive potential of RhoA overexpression in human melanoma cells. J Invest Dermatol 119:1172–1176
Cortes J (2003) Farnesyltransferase inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Clin Lymphoma 1(4 Suppl):S30–S35
Crick DC, Andres DA, Danesi R et al (1998) Geranylgeraniol overcomes the block of cell proliferation by lovastatin in C6 glioma cells. J Neurochem 70:2397–2405
Di Paolo A, Danesi R, Caputo S et al (2001) Inhibition of protein farnesylation enhances the chemotherapeutic efficacy of the novel geranylgeranyltransferase inhibitor BAL9611 in human colon cancer cells. Br J Cancer 84:1535–1543
Dimster-Denk D, Schafer WR, Rine J (1995) Control of RAS mRNA level by the mevalonate pathway. Mol Biol Cell 6:59–70
Furst J, Haller T, Chwatal S et al (2002) Simvastatin inhibits malignant transformation following expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Cell Physiol Biochem 12:19–30
Jones KD, Couldwell WT, Hinton DR et al (1994) Lovastatin induces growth inhibition and apoptosis in human malignant glioma cells. Biochem Biophys Res Commun 205:1681–1687
Khosravi-Far R, Cox AD, Kato K et al (1992) Protein prenylation: key to ras function and cancer intervention? Cell Growth Differ 3:461–469
Kusama T, Mukai M, Tatsuta M et al (2003) Selective inhibition of cancer cell invasion by a geranylgeranyltransferase-I inhibitor. Clin Exp Metastasis 20:561–567
Li HY, Appelbaum FR, Willman CL et al (2003) Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood 101:3628–3634
Morgan MA, Ganser A, Reuter CW (2003) Therapeutic efficacy of prenylation inhibitors in the treatment of myeloid leukemia. Leukemia 17:1482–1498
Osman H, Mazet JL, Maume G et al (1997) Geranylgeranyl as well as farnesyl moiety is transferred to Ras p21 overproduced in adrenocortical cells transformed by c-Ha-rasEJ oncogene. Biochem Biophys Res Commun 231:789–792
Rubins JB, Greatens T, Kratzke RA et al (1998) Lovastatin induces apoptosis in malignant mesothelioma cells. Am J Respir Crit Care Med 157:1616–1622
Wang CY, Zhong WB, Chang TC et al (2003) Lovastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, induces apoptosis and differentiation in human anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab 88:3021–3026
Zhong WB, Wang CY, Chang TC et al (2003) Lovastatin induces apoptosis of anaplastic thyroid cancer cells via inhibition of protein geranylgeranylation and de novo protein synthesis. Endocrinology 144:3852–3859
Vogt A, Sun J, Qian Y et al (1997) The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces p21(WAF1/CIP1/SDI1) in a p53-independent manner. J Biol Chem 272:27224–27229
Xia Z, Tan MM, Wong WW et al (2001) Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells. Leukemia 15:1398–1407
van de Donk NW, Kamphuis MM, van Kessel B et al (2003) Inhibition of protein geranylgeranylation induces apoptosis in myeloma plasma cells by reducing Mcl-1 protein levels. Blood 102:3354–3362
Siddals KW, Marshman E, Westwood M et al (2004) Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem 279:38353–38359
Wang E, Casciano CN, Clement RP et al (2001) HMG-CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharm Res 18:800–806
Cordle A, Koenigsknecht-Talboo J, Wilkinson B et al (2005) Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem 280:34202–34209
Werner M, Sacher J, Hohenegger M (2004) Mutual amplification of apoptosis by statin-induced mitochondrial stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br J Pharmacol 143:715–724
Teresi RE, Shaiu CW, Chen CS et al (2006) Increased PTEN expression due to transcriptional activation of PPARgamma by Lovastatin and Rosiglitazone. Int J Cancer 118:2390–2398
Fujita T, Doihara H, Kawasaki K et al (2006) PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer 94:247–252
Bonovas S, Filioussi K, Tsavaris N et al (2005) Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 23:8606–8612
deSolms SJ, Ciccarone TM, MacTough SC et al (2003) Dual protein farnesyltransferase-geranylgeranyltransferase-I inhibitors as potential cancer chemotherapeutic agents. J Med Chem 46:2973–2984
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Wakeling AE (2005) Inhibitors of growth factor signalling. Endocr Relat Cancer 1(12 Suppl):S183–187
Feleszko W, Mlynarczuk I, Olszewska D et al. (2002) Lovastatin potentiates antitumor activity of doxorubicin in murine melanoma via an apoptosis-dependent mechanism. Int J Cancer 100:111–8
Holstein SA, Hohl RJ (2001) Synergistic interaction of lovastatin and paclitaxel in human cancer cells. Mol Cancer Ther 1:141–9
Muller-Tidow C, Kiehl M, Sindermann JR et al (2003) Synergistic growth inhibitory effects of interferon-alpha and lovastatin on bcr-abl positive leukemic cells. Int J Oncol 23:151–158
Jones RL, Smith IE (2004) Efficacy and safety of trastuzumab. Expert Opin Drug Saf 3:317–327
Gasparini G, Longo R, Torino F et al (2005) Therapy of breast cancer with molecular targeting agents. Ann Oncol 4(16 Suppl):iv28–iv36
Tack DK, Palmieri FM, Perez EA (2004) Anthracycline vs nonanthracycline adjuvant therapy for breast cancer. Oncology (Huntingt) 18:1367–1376 (discussion 1378, 1381)
Acknowledgements
The authors wish to thank Dr. Merrill Egorin (University of Pittsburgh) for his intellectual support of this study. Supported in part by NCI CA-88104-02, NCI CA-35279, and a grant from the Don Monti Foundation. Presented in part at the San Antonio Breast Cancer Symposium 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Budman, D.R., Tai, J. & Calabro, A. Fluvastatin enhancement of trastuzumab and classical cytotoxic agents in defined breast cancer cell lines in vitro. Breast Cancer Res Treat 104, 93–101 (2007). https://doi.org/10.1007/s10549-006-9395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9395-5