Abstract
Antiestrogens used for breast cancer therapy can be categorized into two classes that differ in their effect on estrogen receptor (ER) alpha stability. The selective estrogen receptor modulators (SERMs) stabilize ER alpha and the selective estrogen receptor downregulators (SERDs) cause a decrease in cellular ER alpha levels. A clinically relevant antiestrogen, GW7604, appears to work through a SERD-like mechanism, despite sharing the same molecular scaffold as 4-hydroxytamoxifen, a SERM. In order to investigate potential structural features of GW7604 responsible for SERD activity, GW7604 and two analogs were synthesized using a new, improved synthetic route and tested for their effects on ER alpha function and cell proliferation. The two analogs, which have an acrylamide or a methyl vinyl ketone replacing the acrylic acid group of GW7604, display lower binding affinity for ER alpha than GW7604, but show similar antagonism of estradiol-induced activation of ER alpha-mediated transcription as GW7604 and inhibit estradiol-induced proliferation of the MCF-7 cell line with a similar potency as GW7604. Unlike GW7604, neither analog has a significant effect on cellular ER alpha levels, suggesting that the carboxylate is a key determinant in GW7604 action and, for the first time, showing that this group is responsible for inducing ER alpha degradation in breast cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
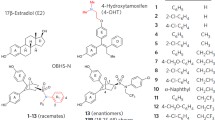
Tamoxifen (Fig. 1) antiestrogen therapy is one of the first and most effective treatments for the treatment and prevention of estrogen receptor (ER) positive breast cancer. Another antiestrogen, fulvestrant, has recently entered the clinic in the United States (Fig. 1). Dramatic differences between tamoxifen and fulvestrant at both the cellular and structural level have been demonstrated [1]. Tamoxifen, which belongs to a class of compounds known as selective estrogen receptor modulators (SERMs), stabilizes ER alpha and causes a slight increase in receptor levels; in contrast, fulvestrant causes rapid ER alpha degradation, leading some to classify compounds such as fulvestrant as selective estrogen receptor downregulators (SERDs) [2]. These differences in mechanism of action of SERMs and SERDs appear to extend to the mechanisms of resistance to these compounds [3]. Many tumors that acquire tamoxifen resistance but remain ER positive are still sensitive to fulvestrant. As a result, there is much interest in finding other compounds with SERD-like mechanisms and understanding how those compounds cause estrogen receptor degradation.
Two antiestrogens under clinical investigation, GW5638 and its hydroxylated metabolite GW7604 (Fig. 1), have been identified to possess SERD activity similar to fulvestrant and the ability to inhibit the growth of tamoxifen-resistant breast tumors [4, 5]. In contrast to fulvestrant, GW7604 possesses a nonsteroidal structure with a triphenylethylethylene core similar to 4-hydroxytamoxifen. However, GW7604 contains an acrylic acid side chain extending from the triphenylethylethylene core, instead of the basic amine-containing side chain of 4-hydroxytamoxifen (Fig. 1). Exploring the relative importance of the acrylic acid side chain in the overall SERD profile of the GW7604 compound could give insight into the structural determinants for distinguishing SERM and SERD mechanisms and lead to the design of improved antiestrogen therapies for tamoxifen-resistant tumors. In this report, we describe the synthesis and characterization of two new GW7604 analogs and demonstrate that although the carboxylate of GW7604 is essential for eliciting the degradation of ER alpha, this group is not essential for inhibiting the proliferation of breast cancer cells.
Methods
Synthesis of 7604 analogs
The detailed synthetic procedures and characterization for the compounds used in this work can be found in the supplementary material.
ER alpha binding assay
Commercially available fluorescent polarization based competition binding assays (Invitrogen) were used to determine the relative affinity of the GW7604 analogs. Briefly, serial dilutions of the different compounds were prepared in ES2 screening buffer (100 mM potassium phosphate, pH7.4, 100 μg/ml bovine gamma globulin) and 50 μl of each concentration was aliquoted into three wells of a black 96 well assay plate. Fifty microliters of a solution containing 20 nM recombinant ER alpha and 2 nM of a proprietary fluorescent ER ligand (Fluormone-ES2) were added to each well. The plate was incubated for 2 h at room temperature (in the dark with shaking). Fluorescence polarization signals were then measured using a Packard Fusion fluorimeter. The data were fit to a single binding site competition curve by nonlinear regression analysis (Prism 4 software package, Graphpad software). K i values were determined from the average of 3 different experiments and calculated using a K D = 4 nM for Fluormone binding to ER alpha.
Transcriptional reporter assays
MCF7/ERE-Luc cells, derived from MCF7 cells stably transfected with a luciferase report construct driven by the estrogen responsive element in pS2 promoter (ERE-pS2-Luc) [6], were seeded in steroid-free medium for 3 days prior to drug treatment. Cell lysates were prepared with passive lysis buffer (Promega Corp., Madison, WI) and luciferase activity determined using the Luciferase Assay System (Promega). Luciferase activity was normalized against total cellular protein and expressed as the mean unit/mg protein ± SE of three independent experiments.
MCF7 proliferation assays
MCF7 cells (2000/well) were plated in 96-well dishes in steroid-free medium and treated with various doses of drugs. Cell numbers were determined by MTT assay after 3, 6, 9, and 12 days of drug treatment.
ER alpha stability assays
MCF7 cells (5 × 105/dish) were plated in 60-mm dishes in steroid-free medium for 3 days prior to drug exposure. Whole cell extracts were prepared by suspending cells in 0.1 ml of lysis buffer (62 mM Tris, pH 6.8, 2% sodium dodecyl sulfate; 10% glycerol; 10 μl protease inhibitor cocktail set III). After sonication (3 × 10 sec), insoluble material was removed by centrifugation (15 min at 12,000 g), and protein concentration in the supernatant was determined using the Bio-Rad Laboratories, Inc. protein assay kit. The protein extracts were mixed with 1/4 vol of 5× electrophoresis sample buffer and boiled for 5 min at 90 C. Protein extract (50 μg per lane) was then fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and probed with antibodies. Primary antibody was detected by horseradish peroxidase-conjugated second antibody and visualized using enhanced SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical Co., Rockford, IL). The band density of exposed films was evaluated with ImageJ software (http://rsb.info.nih.gov/ij/).
Results
Design and Synthesis of GW7604 Analogs
Although GW5638 and its 4-hydroxylated analog GW7604 share many structural similarities with tamoxifen and 4-hydroxytamoxifen, they appear to modulate ER alpha activity by different mechanisms. Structural information garnered from a crystallographic study with GW5638 bound to the ligand binding domain (LBD) of ER alpha suggests that the acrylic acid side chain of GW5638 induces helix 12 of the LBD to adopt a conformation distinct from the conformation induced by 4-hydroxytamoxifen [7]. The carboxylic acid of the acrylic acid side chain of GW5638 appears to be involved in hydrogen bonds with a bound water molecule and the side chain of aspartate 351 and the backbone amide of leucine 536. The acrylic acid side chain of GW5638 has been shown previously to be important in the overall function of the compound––GW5638 analogs possessing an acrylamide side chain showed equivalent uterotrophic activity as tamoxifen in immature rats compared to the non-uterotrophic activity of 5638 [8]. Furthermore, modification of the acrylic acid side chain to either an acrylamide or a vinyl methyl ketone altered the activity of ER alpha at a specific AP-1 regulated promoter [9].
The unique effects of the acrylamide and methyl vinyl ketone analogs of GW5638, combined with the fact that the 4-hydroxylated compound GW7604 showed significantly more potent activity than GW5638, led to the design of a new synthesis to make a novel acrylamide derivative and remake the methyl vinyl ketone derivative of GW7604. The previously reported synthesis of GW7604 and its methyl vinyl ketone derivative was found to be inadequate for the needs of this study due to two very poor yielding steps that were intractable to optimization––the protection of the phenol as a tetrahydropyran acetal and the formation of a vinyl bromide intermediate. As a result, a new synthesis was designed that relied on a high yielding Friedel–Crafts acylation and Grignard coupling reaction to generate the triphenylethylene core (Fig. 2) [10, 11]. The dehydration generated both stereoisomers of the double bond, but after deprotection of the phenol, the double bond of the triphenylethylene interconverted readily at room temperature, as had been shown previously [9]. That work also showed that only one isomer of GW5638 had biological activity, so it is highly likely that ER alpha only bound to the E isomer of these GW7604 analogs. The remainder of the synthesis followed previously reported work to readily generate GW7604 and 7604-ket and a novel analog, 7604-NH2.
Synthetic scheme for the preparation of 7604 analogs. (a) 2-phenylbutyric acid, trifluoroacetic acid anhydride, phosphoric acid, anisole, 10 °C, 100% yield, (b) (i)., THF, magnesium, 4-bromobenzaldehyde diethyl acetal; H3O+(ii). HCl, ethanol, reflux, 76% yield. (c) (i). diethyl (2-oxopropyl)phosphonate, potassium bis(trimethylsilyl)amide, THF, −78 °C to room temp. (ii). BBr3, CH2Cl2, 0 °C, 54% yield. (d) (i).trimethlyphosphonoacetate, potassium bis(trimethylsilyl)amide, THF, −78 °C to room temp. (ii). KOH, EtOH/THF, reflux (iii). BBr3, CH2Cl2, 0 °C, 37% yield. (e) EDC, HOBT, Et3N, NH4OH, DMF, 80% yield
Estrogen receptor binding assays
After synthesizing the compounds, we first determined whether the modifications altered the binding affinity to ER alpha. Using a fluorescence polarization-based competition assay with purified full-length ER alpha, the K i values were determined to be 27 ± 10 nM for GW7604, 240 ± 35 nM for 7604-NH2 and 210 ± 30 nM for 7604-ket (Fig. 3). The K i determined for GW7604 and 7604-ket are consistent with previous studies [9]. The binding data suggest that although altering the carboxylic acid to either a carboxamide or a methyl ketone reduces the affinity of the ligand for ER alpha significantly (P < 0.01, one-way ANOVA test with Dunnett’s post-test), the compounds possess sufficient receptor affinity to perform cell-based experiments.
Estrogen receptor transcriptional activity
After testing the binding affinity, we examined the ability of these compounds to modulate ER alpha transcriptional activity inside cells by using MCF7 breast cancer cells stably transfected with an ERE-pS2-Luc construct [6]. All three GW7604 compounds acted as antagonists but showed different potencies, depending on whether hormone was present or absent. In the absence of E2, inhibition of basal reporter gene activity by 7604-NH2 was greater than GW7604 or 7604-ket. However, GW7604 displayed greater inhibition of E2-induced reporter gene activity than 7604-NH2 and 7604-ket (Fig. 4). Consistent with the ER alpha receptor binding data, both 7604-NH2 and 7604-ket were significantly less potent than GW7604 at antagonizing E2-induced transcription of the stably integrated ERE-pS2-Luc reporter.
Effect of 7604 analogs on ER alpha transcription activity. MCF7/ERE-Luc cells were seeded in hormone-free medium for three days, then treated with 7604 analogs as indicated, in the absence or presence of 1 nM E2. Luciferase activity was examined at 24 h after drug treatment. Luciferase activities are normalized against total cellular protein and expressed as the mean units/mg protein ± SE of three independent experiments
Receptor stability
One of the most interesting properties of GW7604 is its ability to induce ER alpha degradation after binding to the receptor [12]. In order to determine whether the carboxylic acid group was important in inducing degradation, ER alpha levels were measured in MCF7 cells after treatment with the various analogs. As shown in Fig. 5, GW7604 induced ERα degradation in a dose dependent manner, but the acrylamide and methyl vinyl ketone analogs did not induce degradation to nearly the same extent. Even with extended incubation times, the extent of ER alpha degradation induced by the acrylamide and the methyl vinyl ketone was much less than the degradation induced by GW7604. Taken together, these observations indicate that the carboxylate moiety of GW7604 is essential for its selective estrogen receptor degradation properties.
Effects of 7604 analogs on ER alpha stability. MCF7 cells were seeded in hormone-free medium for three days, then treated with 7604 analogs for various times as indicated. ER alpha levels in whole cell extracts were determined by immunoblotting with anti-ERα antibody. GAPDH or tubulin was used as the loading control. Representative results of experiments performed in duplicate are shown. Relative ER alpha levels (versus untreated cells) are shown in the corresponding histogram
Proliferation assays
Because the extent of ER alpha degradation induced by the two GW7604 analogs was not significant, it was unclear whether these compounds would still inhibit estrogen-induced proliferation of breast cancer cells. A standard MTT cell proliferation was performed using MCF-7 cells grown in hormone free media (Fig. 6). In the absence of estradiol, GW7604 and 7604-ket, but not 7604-NH2, significantly inhibited basal cell growth at high doses (10−7–10−6 M, P < 0.05 versus vehicle, student’s t-test). In the presence of 1 nM estradiol, however, inhibition of cell growth was observed for all three compounds at approximately the same concentrations, suggesting that the two 7604 analogs act as antiestrogens in the breast, even though they do not induce ER alpha degradation in a fashion similar to GW7604.
Discussion
Selective estrogen receptor degradation represents an emerging, clinically validated paradigm in designing antiestrogen treatments for breast cancer. One major benefit to using a SERD such as fulvestrant compared to using a SERM such as tamoxifen is that SERDs have been found to still effectively treat some ER alpha-positive, tamoxifen-resistant breast cancers [13]. Thus, compounds that induce ER alpha degradation may be used to extend the period of time that breast cancer patients can be treated successfully with antiestrogen therapies, presumably by using different SERMs, aromatase inhibitors and SERDs in succession [14].
While fulvestrant is considered an effective therapeutic agent for treatment of advanced breast cancer [1, 13], a major problem at the current time is poor bioavailability, thereby requiring monthly intramuscular injections for drug delivery. In addition, the synthesis of fulvestrant is lengthy and difficult to modify in order to study structure-activity relationships related to the ability of the drug to induce ER alpha degradation. Due to the difficulty of working with fulvestrant, the finding that GW7604 induced ER alpha degradation provided an excellent opportunity to study the molecular mechanisms of SERD activity.
Even though both fulvestrant and GW7604 induce ER alpha degradation, these compounds are significantly different molecules. Fulvestrant is a steroidal compound with an extremely long, flexible extending side chain, whereas GW7604 has a rigid, nonsteroidal structure and an extending side chain that terminates in a carboxylic acid––a rarity in compounds that target the ER alpha. The fact that both of these compounds could induce ER alpha degradation was initially puzzling. However, the crystal structures of GW5638 and fulvestrant bound to the ER alpha ligand binding domain (LBD) were recently reported [7, 15], revealing that receptor conformations induced by both compounds exposed hydrophobic residues, which are normally “packed” inside the LBD, to the surrounding solvent. Exposed hydrophobic patches on the protein surface are known targeting signals for protein degradation [16], and fulvestrant and GW5638 induce this repositioning of hydrophobic residues through different mechanisms. The long side chain of fulvestrant blocks any interaction of helix 12 with the rest of the LBD, resulting in exposure of the hydrophobic core of the receptor binding pocket to solvent. In contrast, GW5638 causes less disruption of helix 12 than fulvestrant, but the carboxylic acid of GW5638 forms hydrogen bonds with the amide backbone of Leu536 and Tyr537, tethering that region of helix 12 closer to the ligand binding pocket and distorting the positioning of the other hydrophobic residues of helix 12 (Fig. 7). This key interaction between the carboxylic acid and the residues of helix 12 led us to explore the effect of changing that carboxylic acid on the function of GW7604.
Binding of GW5638 and 4-hydroxytamoxifen side chains. Cartoon schematic of the interactions between the side chains of the ER alpha ligand binding domain with the side chain extension of (A) GW5638 and (B) 4-hydroxytamoxifen. Triphenylethylethylene core and side chain residues of Leu537 and Tyr537 are omitted for clarity. Dashed lines represent hydrogen bonds
The analysis of the GW5638-ER alpha LBD structure suggests that the acrylic acid group on GW5638 is protonated. If this is true, then converting the carboxylic acid of GW7604 to a carboxamide is a fairly conservative change. The carboxamide is not exactly isosteric with the carboxylic acid and the protons on the carboxamide are much less acidic, but the carboxamide is still capable of hydrogen bonding and could potentially hold the helix 12 backbone in the same degradation-inducing conformation when bound in the binding site. Converting the carboxylic acid to a methyl ketone would generate a compound capable of fitting into the binding pocket but unable to engage in the same number of hydrogen bonds as the carboxylic acid of GW7604. The ketone would likely not be able to maintain the necessary contacts with backbone amide hydrogens in helix 12 to induce degradation.
Making conservative changes in the carboxylic acid moiety proved to be deleterious when the ER alpha binding affinity of the two analogs was measured. Both analogs bound to the receptor with lower affinity but the equilibrium dissociation constants were still in the nanomolar range, suggesting that the modifications were still mostly compatible with the binding pocket. Both analogs also inhibited ER alpha mediated transcription from an ERE-controlled promoter, another indication that the compounds were able to disrupt the normal packing of helix 12 to form the coactivator binding pocket. Even though the two analogs do show some differences with GW7604 from the viewpoint of binding and transcriptional regulation, the two analogs differed significantly from GW7604 in terms of effects on ER alpha stability. GW7604 induced ER alpha degradation in a dose dependent and time dependent manner, whereas the two analogs had minimal effects on ER alpha levels. Overall, this difference did not have a significant effect on the ability of the two analogs to inhibit estradiol-induced MCF7 proliferation, as both GW7604-ket and 7604-NH2 inhibited cell growth to nearly the same extent as GW7604. For both the ERE transcriptional assays and the cell proliferation assays, the different effects seen for the 3 compounds in the absence of estradiol are not easily rationalized, but we speculate that these differences reflect the ability of the compounds to induce distinctive conformational changes in ER alpha that affect basal levels of activity.
Ultimately, these results suggest that modification of the carboxylate moiety of GW7604 converts the mechanism of action from a SERD-like mechanism found with fulvestrant to a SERM-like mechanism found with tamoxifen and raloxifene. Comparing the binding modes of the side chain extension of GW5638 and 4-hydroxtamoxifen with ER alpha (Fig. 7) shows that GW5638 is able to make hydrogen bond contacts with the helix 12 backbone protons whereas 4-hydroxytamoxifen does not. It is likely that the acrylamide and methyl vinyl ketone analogs are also unable to make the necessary number of hydrogen bonds to the helix 12 backbone, either due to steric effects or lack of appropriate hydrogen bond donor or acceptor groups. Because GW7604-ket and 7604-NH2 likely interact with Asp351, helix 12 can still be displaced and antagonize transcription in a manner similar to 4-hydroxytamoxifen, i.e., a more “SERM-like” mechanism of action. The analogs do not induce ER alpha degradation, indicating that repositioning of helix 12 into a conformation that exposes hydrophobic residues does not occur.
In conclusion, we have characterized the activity of two new antiestrogens and demonstrated, for the first time using very slight chemical changes, the conversion of an antiestrogenic compound and “ER downregulator” into a SERM and “receptor stabilizer”. The implications of our findings may have clinical significance. Breast tumors that become resistant to one antiestrogen class often maintain sensitivity to another class of antiestrogens. Based on our observations, we suggest that two distinct classes of therapeutics can be derived from one tight binding lead structure. Modifications that allow for additional interactions between the ligand and receptor appear to be key determinants for designing new ER downregulators (i.e. SERDs) with potential clinical use. Such interactions, which also cause a slight unfolding of the LBD, expose hydrophobic residues to solvent. Unfortunately, at this time, there are no general rules for eliciting such unfolding, and further study into the mechanistic differences between different types of antiestrogens is needed in order to extend the usefulness of high affinity pharmacophores.
References
Robertson JF (2004) Selective oestrogen receptor modulators/new antioestrogens: a clinical perspective. Cancer Treat Rev 30:695-706
Osborne CK, Wakeling A, Nicholson RI (2004) Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 90(Suppl 1):S2–6
Normanno N, Di Maio M, De Maio E et al (2005) Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer 12:721–747
Willson TM, Norris JD, Wagner BL et al (1997) Dissection of the molecular mechanism of action of GW5638, a novel estrogen receptor ligand, provides insights into the role of estrogen receptor in bone. Endocrinology 138:3901–3911
Wijayaratne AL, Nagel SC, Paige LA et al (1999) Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140:5828–5840
Fan M, Long X, Bailey JA et al (2002) The activating enzyme of NEDD8 inhibits steroid receptor function. Mol Endocrinol 16:315–330
Wu YL, Yang X, Ren Z et al (2005) Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell 18:413–424
Willson TM, Henke BR, Momtahen TM et al (1994) 3-[4-(1,2-Diphenylbut-1-enyl)phenyl]acrylic acid: a non-steroidal estrogen with functional selectivity for bone over uterus in rats. J Med Chem 37:1550–1552
Weatherman RV, Clegg NJ, Scanlan TS (2001) Differential SERM activation of the estrogen receptors (ERalpha and ERbeta) at AP-1 sites. Chem Biol 8:427–436
Eaddy III, JF, Heyer D, Katamreddy SR et al (2005) Preparation of acyloxydiphenylbutenylcinnamates as estrogen receptor modulator prodrugs PCT application WO2005033056
Smyth TP, Corby BW (1998) Toward a clean alternative to Friedel-Crafts acylation: In situ formation, observation, and reaction of an acyl bis(trifluoroacetyl)phosphate and related structures. J Org Chem 63:8946–8951
Wijayaratne AL, McDonnell DP (2001) The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem 276:35684–35692
Robertson JF, Come SE, Jones SE et al (2005) Endocrine treatment options for advanced breast cancer–the role of fulvestrant. Eur J Cancer 41:346–356
Shao W, Brown M (2004) Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res 6:39–52
Pike AC, Brzozowski AM, Walton J et al (2001) Structural insights into the mode of action of a pure antiestrogen. Structure (Camb) 9:145–153
Bohley P (1996) Surface hydrophobicity and intracellular degradation of proteins. Biol Chem 377:425–435
Acknowledgements
RVW acknowledges the Purdue Cancer Center, the Indiana Elks Charities and the Army Breast Cancer Research Program (BC030507) for supporting this work. KPN gratefully acknowledges the following agencies for supporting this work: the Walther Cancer Institute, U.S. Army Medical Research Acquisition Activity, Award Numbers DAMD 17-02-1-0418 and DAMD17-02-1-0419; American Cancer Society Research and Alaska Run for Woman Grant TBE-104125
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fan, M., Rickert, E.L., Chen, L. et al. Characterization of molecular and structural determinants of selective estrogen receptor downregulators. Breast Cancer Res Treat 103, 37–44 (2007). https://doi.org/10.1007/s10549-006-9353-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9353-2