Abstract
Multiscale entropy (MSE) model quantifies the complexity of brain functions by measuring the entropy across multiple time-scales. Although MSE model has been applied in children with Autism spectrum disorders (ASD) in previous studies, they were limited to distinguish children with ASD from those normally developed without corresponding severity level of their autistic features. Therefore, we aims to explore and to identify the MSE features and patterns in children with mild and severe ASD by using a high dense 64-channel array EEG system. This study is a cross-sectional study, where 36 children with ASD were recruited and classified into two groups: mild and severe ASD (18 children in each). Three calculated outcomes identified brain complexity of mild and severe ASD groups: averaged MSE values, MSE topographical cortical representation, and MSE curve plotting. Averaged MSE values of children with mild ASD were higher than averaged MSE value in children with severe ASD in right frontal (0.37 vs. 0.22, respectively, p = 0.022), right parietal (0.31 vs. 0.13, respectively, p = 0.017), left parietal (0.37 vs. 0.17, respectively, p = 0.018), and central cortical area (0.36 vs. 0.21, respectively, p = 0.026). In addition, children with mild ASD showed a clear and more increase in sample entropy values over increasing values of scale factors than children with severe ASD. Obtained data showed different brain complexity (MSE) features, values and topographical representations in children with mild ASD compared with those with severe ASD. As a result of this, MSE could serve as a sensitive method for identifying the severity level of ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASD) are a group of complex and heterogeneous developmental disorders involving multiple neural system dysfunctions that lead to social and communication impairments and restricted and stereotyped behaviors (Johnson and Myers 2007). Although electroencephalography (EEG) has been used for almost a century to identify neural activities in humans, traditional quantitative EEG methods analysis using interpretation of multichannel recordings, frequency analyses, and graphic representations were limited in their ability to analyze the complexity, wide range and multivariate ASD-EEG brain data, as well it can only analyze short-range of temporal EEG dynamics (Liu et al. 2017). This is because EEG signals exhibit complex temporal features that reflect non-linear dynamical processes (Abarbanel and Rabinovich 2001). The recent non-linear approaches to characterize the EEG complex temporal dynamics have provided new insights into EEG dynamical complexity in mental disorders including ASD (Takahashi 2013). One common EEG model for this purpose is the Multiscale entropy (MSE), which quantifies the complexity of a physiological signal by measuring the entropy across multiple time-scales, using a coarse-graining procedure (Costa et al. 2002, 2005).
Although previous studies (Bosl et al. 2011; Catarino et al. 2011; Jamal et al. 2014; Ghanbari et al. 2015; Liu et al. 2017) applied the MSE model in children with ASD, MSE features comparison and differences limited between children with ASD and normally developed children with no any attention for the severity of autistic features in their recruited children with ASD. This is because previous research identifies ASD based on ASD—Diagnostic and Statistical Manual (DSM-4 or DSM-5). Although DSM is a golden standard to distinguish children with autism from those normally developed, previous studies showed that DSM criteria under-identifies children with mild ASD and recommended using more socio-behavioral scales such as childhood autism rating scale (CARS) (Mayes et al. 2014) or ATEC (Magiati et al. 2011), which are considered sensitive to identify the severity of ASD.
On the other hand, there were only two studies have approached the aspect of severity degree in autism (Yousef et al. 2017; Chan et al. 2017). However, their results do not help in the understanding of the clinical differences of autistic features in terms of nonlinear dynamic analysis of the EEG signals. This is because their approach did not go beyond reporting EEG abnormalities without showing any specific EEG analysis approach or specific EEG outcome measures (Yousef et al. 2017), or the number of explored EEG channels was limited to three EEG channels only (Chan et al. 2017). Consequently, the nonlinear dynamic analysis of EEG signal in terms of MSE features and outcomes measures in accordance with autistic features severity in children with ASD is still unclear.
Therefore, this study aimed to explore the MSE patterns in EEG-resting state signals of children with ASD based on their autistic severity levels (mild and severe ASD), and to explore the sensitivity of the MSE analysis model to identify the ASD severity levels in children with ASD.
Methods
Study Design
The present work consists of a cross-sectional study identifying and examining the MSE patterns in children with mild and severe ASD. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee [Jordan University of Science and Technology/ Institutional Review Board (approval No.2015/8/5)] and with the 1964 Helsinki declaration and its later amendments. Written informed consent obtained from all children parents before participation in the study.
Participants
A sample size of 36 children, with a formal medical diagnosis with ASD, have been recruited in this study from the local special education centers (aged between 3 and 13 years old). Recruited children had mild and severe ASD (the two groups, eighteen for each, matched in age and gender). CARS and ATEC scales had been used to determine the severity level of autistic features of the participated children. Both scales were carried out by licensed medical practitioners who were blinded about the purpose of this study (Table 1). All children were in stable medical condition for at least 3 weeks before entering the study and had no seizure or epilepsy.
EEG Recording
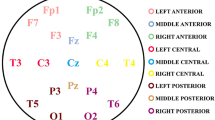
EEG data recorded at resting state, where all the children have been asked to sit quietly on a chair, using the 64 standard scalp electrodes placed following the 10–10 International EEG System (64 EEGo Sport system, ANT Neuro). A reference electrode was the CPz with ground electrode at the GND. The EEG recording session lasted 25 min under the supervision of the EEG technician, who made events marker for any disturbance actions or artifacts made by the child such as head movement, eye blinking, body movement, and crying. to be considered later in EEG analysis process. In addition, eye-movements were monitored using bi-polar channels with electrodes above and below the left eye (vertical electro-oculogram) and 1 cm from the outer canthus of each eye (horizontal electro-oculogram). Impedances at all sites maintained below 5 ohms. EEG data obtained at a sampling frequency of 500 Hz, with a 0.3–200 Hz online band-pass filter and 50 Hz notch filter.
MSE Analysis
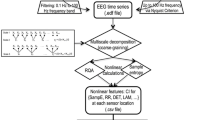
Consistent with previous MSE studies (Escudero et al. 2006; Takahashi et al. 2010), the data was not subjected to other pre-processing steps (i.e., filtering, artifact removal or data reconstruction algorithms) since this could distort the data and influence the MSE analysis results. Instead, 30 s artifact-free segments of data were chosen for analysis. MSE analysis merely is done by computing the sample entropy (SE) at multiple time scales based on coarse-graining procedure (Costa et al. 2005), as explained below. The SE of raw EEG time series is defined as the negative of the logarithmic probability of two similar sequences of m consecutive data points with the condition that the point remains similar at the next point (m + 1) as given by the following equation:
where \({\text{C}}_{\text{m}} \left( {\text{r}} \right) = \left( {{\text{N}} - {\text{m}}} \right)^{ - 1} \sum\nolimits_{\text{i}}^{{{\text{N}} - {\text{m}}}} {{\text{C}}_{\text{i}}^{\text{m}} ({\text{r}})}\), and r is the coefficient of tolerance.
Given that \({\text{d}} = \left| {{\text{x}}_{\text{i}}^{\text{m}} - {\text{x}}_{\text{j}}^{\text{m}} } \right|\) is the distance between points \({\text{x}}_{\text{i}}^{\text{m}}\) and \({\text{x}}_{\text{j}}^{\text{m}}\) in the m space of dimensions, \({\text{C}}_{\text{i}}^{\text{m}} \left( {\text{r}} \right)\) is the number of all probable pairs (i,j) with d < r times the time-series standard deviation. In general, the SE provides a measure of the self-similarity of the time series (Richman and Moorman 2000). Whereas, the MSE analysis provides a measure of the time-series complexity, in which the complex dynamics reveal different structures over multiple time scales. In our case, we applied the MSE analysis to find out differences between the EEG in the autism population groups (mild vs., severe).
In MSE analysis, given an EEG time-series of length N, [x1,x2,…,xN], the signal was constructed into consecutive coarse-grained time series, yτ, associated with the scale factor, τ. First, the original time-series was divided into non-overlapping windows of length τ; second, for each window, the average of the data points inside it was computed. Generally, each element of a coarse-grained time-series is calculated based on the following equation:
The coarse-graining procedure provides consecutive time-series, each of length equal to the length of the original time series (N) divided by the scale factor (τ). Thus, for scale one; the time series represents the original EEG signal. Second, sample entropy value for each coarse-grained time-series was computed producing sample entropy values plot as a function of scale factors. Previous literature has proved that the optimal parameter values for sample entropy to get a good statistical validity are m = 1 or 2, and 0.15 ≤ r ≤ 2.0 times of standard deviation of the time series signal (Richman et al. 2004). In this study, the m value set to 2; r was 0.15 times of the signal standard deviation; N = 15,000 data points; and 15 scale factors (SFs, from 1 to 15) have been explored. The shortest coarse-grained time-series segment was, therefore, N/s = 15,000/15 = 1000 data points, which is reported previously to be enough to get a reliable estimation of the sample entropy value (Richman and Moorman 2000).
MSE Features
Two main features of MSE have been utilized to differentiate between children with mild ASD and those with severe ASD: (1) regional averaged MSE and (2) profiles of the MSE curves.
To start with, regional averaged MSE values were calculated by averaging the sample entropy values over the 15 SFs for five electrodes’ clusters that represent five interested cortical regions; right frontal (Fp2, F4, F8, F2, F6), left frontal (Fp1, F3, F1, F5, F7), right parietal (P2, P6, P4, P8, PO4, PO6, PO8), left parietal (P1, P3, P5, P7, PO3, PO5, PO7), and central (FC5, FC1, FC2, FC6, C3, Cz, C4, CP5, CP1, CP2, CP6, C5, C1, C2, C6, CP3, CP4, FC3, FCz, FC4). This choice was to reduce the number of statistical comparisons to facilitate viewing the group differences in terms of cortical regions and in accordance with a previous study (Coben et al. 2008).
Finally, the profile of MSE curves plot, which is the curve resulting from plotting the sample entropy value of each scale factor, ranging from 1 to 15 scale factors. In addition, the difference in curve behavior serves as an index for measuring signal complexity as systems with higher complexity will show a curve of higher values of sample entropy sustained over increasing values of the scale factor (Costa et al. 2005), for overall MSE calculations procedures, an explanation figure is presented (Fig. 1).
Statistical Analysis
The statistical analyses were carried out using SPSS Statistics (v20.0 software) for windows on core i5 Laptop. A Kolmogorov—Smirnov normality test was used to confirm the normal distribution of the calculated MSE values of each scale factors (totally 15 scale factors) at each EEG electrodes (totally 64 channels) for each child in both ASD groups (mild ASD and severe ASD, totally 36 children).
Once normal distribution was confirmed, a repeated-measure ANOVA was applied to identify any potential differences between the two groups as between subject-factors (mild ASD vs. severe ASD) in terms of the MSE values of 15 scale factors, MSE values of 64 EEG channels, averaged MSE values of interested cortical regions (totally 5 regions; left/right frontal, left/right parietal, and central). Bonferroni test for all post-hoc analysis, and Greenhouse–Geisser adjustment was applied to the degrees of freedom for all of the analyses. Values of P less than 0.05 were considered indicative of significant differences. This statistical process comes with previous MSE studies (Catarino et al. 2011; Liu et al. 2017).
Results
Normal distribution of MSE outcomes measure was confirmed using the Kolmogorov–Smirnov normality test as well as by examination of skewness and kurtosis values, for each electrode of the 64 EEG channels of each group of mild and severe ASD.
Statistical analysis showed significant difference in MSE value based on severity groups, F (65, 474) = 84.186; p < .0005; partial η2 = .135).
In terms of average MSE values, there were significant differences in averaged MSE values based on severity of ASD, in which averaged MSE values of children with mild ASD were higher than averaged MSE values in children with severe ASD in right frontal (0.37 vs. 0.22, respectively, p = 0.022), right parietal (0.31 vs. 0.13, respectively, p = 0.017), left parietal (0.37 vs. 0.17, respectively, p = 0.018), and central cortical area (0.36 vs. 0.21, respectively, p = 0.026). However, although averaged MSE values of left frontal in children with mild ASD was slightly higher than those with severe, it showed no statistical difference (p = 0.138) (Figs. 2, 3).
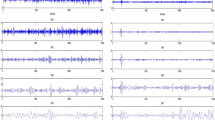
In term of MSE curve plot, MSE curve plot data showed asymmetrical increasing patterns between children with mild ASD and those with severe ASD, where children with mild ASD showed a remarkable and noticeable increase in sample entropy values sustained over increasing values of scale factors, compared with children with severe ASD (Fig. 4).
MSE curve plot. EEG Schematic with sample entropy by scale factor (SF) graphs for representing channels, where sample entropy values in mild ASD increase remarkably over increasing in scale factors compared with sample entropy value increases over increases scale in factors in severe ASD. Full line—children with mild ASD, dashed line—children with severe ASD
Discussion
The primary aim of this work was to explore the MSE patterns in EEG-resting state signals of children with ASD based on their autistic severity levels (mild and severe ASD), and to examine the sensitivity MSE analysis approach to identify the ASD severity levels in children with ASD.
Previous works that applied the MSE and nonlinear dynamic approaches in their EEG analysis tended to avoid the high complexity and heterogeneity of ASD, and limited to predict the existing of the autistic feature compared with normally developed children, or limited to study only the differences in MSE patterns in response to different cognitive tasks, and ended with discrepant findings (Bosl et al. 2011; Catarino et al. 2011; Jamal et al. 2014; Ghanbari et al. 2015; Liu et al. 2017). For example, Bosl et al. (2011) reported a reduced complexity in infants with high risk of autism, particularly in the frontal regions of the brain. Catarino et al. (2011) then further reported a reduction of EEG signal complexity within the temporo-parietal and occipital regions in an autistic group compared with the normal group. Ghanbari et al. (2015) then showed lower complexity in ASD in the frontal areas and the occipital-parietal regions. On the other hand, Liu et al. (2017) found notably descended EEG complexity in individuals with autism in the right cortical area during observation task.
This discrepancy is expected because previous studies (Liu et al. 2017; Bosl et al. 2011; Catarino et al. 2011; Ghanbari et al. 2015) gave no attention to the severity of the autistic features of their participant children with ASD, as children recruitment based on the ASD—Diagnostic and Statistical Manual, Fourth or Fifth Edition (DSM-4 or DSM-5, respectively), and explored/described the MSE pattern in response to a particular task. However, ASD consists of a group of complex and heterogeneous developmental disorders involving multiple neural system dysfunctions with significant variability in the severity of core deficits (Johnson and Myers 2007). In addition, a recent study showed that although DSM is the golden standard to diagnosis the autistic child or distinguish the autistic child from the normal child, the DSM under-identifies children with mild ASD (Mayes et al. 2014). Moreover, resting-state EEG studies are used to monitor brain activity in the absence of overt task performance or sensory stimulation. These measurements can, therefore, identify abnormalities for which evoked potential MSE studies are not well-suited (Fox and Greicius 2010; Wang et al. 2013). This is because task-dependent changes in brain complexity (MSE) are difficult to interpret without fundamental knowledge of functional differences in individuals with ASD at resting state (Wang et al. 2013), as well in task-based evoked potential studies, only time-locked neural responses to events of interest are studied, whereas all other spontaneous activity is typically considered background noise (Fox and Greicius 2010).
On the other hand, there are very few number of studies that have approached the aspect of severity degree in autism, for example, Yousef et al. (2017) have analyzed the EEG abnormalities in relation to the level of severity, and they reported a statistically significant association between the abnormalities in EEG results and the severity of autism. However, their approach did not go beyond saying EEG abnormalities without showing any specific EEG analysis approach or specific EEG outcome measures hereby they reach to no complete or distinct classification parameters. In addition, Chan et al. (2017) have used the short windowed fourier transform and the genetic algorithm to classify and predict the different severity categories. However, the number of explored channels is so small and limited to three EEG channels (C3, C4, Cz), hereby their results do not help in the understanding of the clinical differences or the distributions of the features.
Therefore, this study is innovative for three reasons; first, it is the first to explore the MSE patterns in EEG-resting state in children with ASD in terms of mild ASD and severe ASD using the highly dense array of 64 EEG system. Second, it identified more MSE outcome measures and patterns such as the MSE topographical cortical representation in 3D brain map besides the averaged MSE values and MSE curve plotting that reported in previous MSE works (Liu et al. 2017; Bosl et al. 2011; Catarino et al. 2011; Ghanbari et al. 2015). Third, it would provide a clear understanding of ASD and determine the sensitivity of EEG complexity (MSE) to identify the ASD severity besides its reported sensitivity to distinguish autistic child from the normal child in previous studies (Liu et al. 2017; Bosl et al. 2011; Catarino et al. 2011; Ghanbari et al. 2015).
In terms of averaged MSE, there was a significant decrease in averaged MSE values in children with severe ASD compared with children with mild ASD. Brain complexity (MSE) measures the extent to which interactions produce patterns of temporal correlations or functional connectivity both within and across cortical areas, and this temporal correlation combines the dual requirements of functional segregation and integration (Sporns et al. 2000). In addition, it suggested that dysfunction or impaired temporal integration of information from spatially discrete brain areas would be the main root and the core of autistic deficits in social interaction, language, and repetitive and restrictive behaviors. In another word, changes in brain complexity indicate to some extent changes in the neural connectivity (Takahashi et al. 2010), and this associated with an atypical pattern of cortical functional connectivity (Ghanbari et al. 2015), and such atypical patterns are related to autistic deficits (Wass 2011). Another interpretation of biological complexity is that it reflects a system’ s ability to adapt quickly and function in a changing environment (Costa et al. 2005). Brain complexity was found in one study (Kulisek et al. 2008) to be associated with the ability to attend to a task and to adapt to new cognitive tasks as well it reflects the synchronization functioning between the different cortical regions, and all of these functions were found to be involved in children with ASD.
On the other hand, based on CARS scale, children with mild ASD manifested less deficits in social interaction, language and repetitive and restrictive behavior than those with severe ASD (Magiati et al. 2011). Therefore, we assumed that our children with severe ASD had more behavioral, language and social deficits, hereby had less temporal integration and correlation of discrete brain areas. As a result of this, they had less brain complexity and averaged MSE values than children with mild ASD in most of the cortical regions. This assumption is supported by the findings of MSE curves plot profile that serves as an index for brain complexity (Costa et al. 2005), and previous study (Bosl et al. 2011) reported that normally developed children showed increasing in the sample entropy values as the scale factors increase comparing with children with ASD, hereby normally developed child had more brain complexity than those with ASD. In the current study, MSE curve plot data showed asymmetrical increases patterns between children with mild ASD and those with severe ASD, where children with mild ASD showed remarkable and noticeable increasing in sample entropy values of sample entropy sustained over increasing values of scale factor, compared with children with severe ASD.
In terms of cortical regions and hemisphere lateralization, children with mild ASD demonstrated higher averaged MSE values in right frontal, right parietal, central and left parietal cortical areas compared with those of severe ASD. The dominancy of right hemisphere over left hemisphere in this study could be explained by the findings of a resting-state fMRI that conducted a comprehensive investigation of hemispheric asymmetry in adolescents with ASD, and reported atypical rightward asymmetry that spreading out through brain organization in ASD, and such asymmetry affecting sensorimotor, visual and audio processing, as well as higher cognitive functions (Cardinale et al. 2013).
On the other hand, available researches have suggested that dysfunctional mirror neurons may explain and contribute to the major deficits characteristics and features observed in ASD including impaired social interaction and restricted behavior (Iacoboni and Dapretto 2006; Oberman and Ramachandran 2007; Rizzolatti and Fabbri-Destro 2010). A single cortical area does not represent the mirror neurons, instead, mirror neurons is a neural network that includes dorsolateral prefrontal cortex, premotor, supplementary motor, primary motor and primary somatosensory areas (Oberman et al. 2005), which interestingly all represented by the central and parietal EEG electrodes in our studies. Therefore, this study data would infer that differences in averaged MSE values that were dominant in central, left and right parietal areas would be an indication of a possible dysfunction in the mirror neurons in children with ASD. However, although this assumption would come with other studies findings (Liu et al. 2017; Cardinale et al. 2013), further studies are needed.
Limitation, this study showed the differences in MSE outcome measures and features between the two autistic groups (mild ASD vs. severe ASD), which helps to fill in the gaps of the existing MSE literature that lacks the complete EEG tool and analysis approach to classify the different ASD severity degrees. However, a further study that includes MSE outcome measures comparisons between children with different autistic levels with normally developed children is needed.
Conclusion
This study introduced the new application of MSE-EEG analysis methods to characterize and identify the differences in the brain complexity features in children with ASD in accordance to their autistic feature severity levels. Our data showed that children with mild ASD had more brain complexity patterns compared with children with severe ASD, and there was rightward lateralization dominancy in central, and parietal areas. This study finding would support the theory of dysfunctional mirror neurons in children with ASD, as well as the sensitivity of MSE-EEG analysis method as a predictor EEG analysis tools to identify the autistic features severity levels.
References
Abarbanel HD, Rabinovich MI (2001) Neurodynamics: nonlinear dynamics and neurobiology. Curr Opin Neurobiol 11(4):423–430
Bosl W, Tierney A, Tager-Flusberg H, Nelson C (2011) EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med 9(1):18
Cardinale RC, Shih P, Fishman I, Ford LM, Müller RA (2013) Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry 70(9):975–982
Catarino A, Churches O, Baron-Cohen S, Andrade A, Ring H (2011) Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol 122(12):2375–2383
Chan ZH, Sudirman R, Omar C (2017) Autistic spectrum disorder: EEG analysis and classification. J Telecommun Electron Comput Eng 9(3–9):53–57
Coben R, Clarke AR, Hudspeth W, Barry RJ (2008) EEG power and coherence in autistic spectrum disorder. Clin Neurophysiol 119(5):1002–1009
Costa M, Goldberger AL, Peng CK (2002) Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 89(6):068102
Costa M, Goldberger AL, Peng CK (2005) Multiscale entropy analysis of biological signals. Phys Rev E 71(2):021906
Escudero J, Abásolo D, Hornero R, Espino P, López M (2006) Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol Meas 27(11):1091
Fox MD, Greicius M (2010) Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19
Ghanbari Y, Bloy L, Edgar JC, Blaskey L, Verma R, Roberts TP (2015) Joint analysis of band-specific functional connectivity and signal complexity in autism. J Autism Dev Disord 45(2):444–460
Iacoboni M, Dapretto M (2006) The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 7(12):942
Jamal W, Das S, Oprescu IA, Maharatna K, Apicella F, Sicca F (2014) Classification of autism spectrum disorder using supervised learning of brain connectivity measures extracted from synchrostates. J Neural Eng 11(4):046019
Johnson CP, Myers SM (2007) Identification and evaluation of children with autism spectrum disorders. Pediatrics 120(5):1183–1215
Kulisek R, Hrncir Z, Hrdlicka M, Faladova L, Sterbova K, Krsek P et al (2008) Nonlinear analysis of the sleep EEG in children with pervasive developmental disorder. Neuro Endocrinol Lett 29(4):512–517
Liu T, Chen Y, Chen D, Li C, Qiu Y, Wang J (2017) Altered electroencephalogram complexity in autistic children shown by the multiscale entropy approach. NeuroReport 28(3):169
Magiati I, Moss J, Yates R, Charman T, Howlin P (2011) Is the Autism Treatment Evaluation Checklist a useful tool for monitoring progress in children with autism spectrum disorders? J Intellect Disabil Res 55(3):302–312
Mayes SD, Calhoun SL, Murray MJ, Pearl A, Black A, Tierney CD (2014) Final DSM-5 under-identifies mild autism spectrum disorder: agreement between the DSM-5, CARS, CASD, and clinical diagnoses. Res Autism Spectr Disord 8(2):68–73
Oberman LM, Ramachandran VS (2007) The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull 133(2):310
Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA (2005) EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn Brain Res 24(2):190–198
Richman JS, Moorman JR (2000) Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278(6):H2039–H2049
Richman JS, Lake DE, Moorman JR (2004) Sample entropy. In: Methods in enzymology, vol 384. Academic Press, San Diego, pp. 172–184
Rizzolatti G, Fabbri-Destro M (2010) Mirror neurons: from discovery to autism. Exp Brain Res 200(3–4):223–237
Sporns O, Tononi G, Edelman GM (2000) Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Netw 13(8–9):909–922
Takahashi T (2013) Complexity of spontaneous brain activity in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry 45:258–266
Takahashi T, Cho RY, Mizuno T, Kikuchi M, Murata T, Takahashi K, Wada Y (2010) Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage 51(1):173–182
Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, Sweeney JA (2013) Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord 5(1):24
Wass S (2011) Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn 75(1):18–28
Yousef A, Youssef U, El-Shabrawy A, Fattah NA, Khedr H (2017) EEG abnormalities and severity of symptoms in non-epileptic autistic children. Egypt J Psychiatry 38(2):59–59
Acknowledgements
This work is supported by the grant from Scientific Research Support Fund – Jordanian Ministry of Higher Education (MPH/1/20/2014) under number of 275/2015 at deanship of research of Jordan University of Science &Technology. In addition, thanks go to Dr. Muhamed Nazzal, Dr. Hanan Khalil, and Dr. Nihad Almasri for their logistics help of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Additional information
Handling Editor: Armida Mucci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hadoush, H., Alafeef, M. & Abdulhay, E. Brain Complexity in Children with Mild and Severe Autism Spectrum Disorders: Analysis of Multiscale Entropy in EEG. Brain Topogr 32, 914–921 (2019). https://doi.org/10.1007/s10548-019-00711-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-019-00711-1