Abstract
Motor symptoms such as neurological soft signs (NSS) are characteristic phenomena of schizophrenia at any stage of the illness. Neuroimaging studies in schizophrenia patients have shown regional thinning of the cortical mantle, but it is unknown at present whether NSS are related to cortical thickness changes. Whole brain high-resolution magnetic resonance imaging at 3 Tesla was used to investigate cortical thickness in 28 patients with recent-onset schizophrenia. Cortical reconstruction was performed with the Freesurfer image analysis suite. NSS were examined on the Heidelberg Scale and related to cortical thickness. Age, education, and medication were considered as potential confounders. Higher NSS scores were associated with morphological changes of cortical thickness in multiple areas comprising paracentral gyrus, postcentral lobule, precuneus, inferior parietal lobule and temporal lobe. Our results confirm the hypothesis of a significant relationship between cortical thickness changes and the extent of NSS in schizophrenia. Investigation of cortical thickness may help to explain subtle motor symptoms such as NSS in schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with schizophrenia frequently experience genuine, not drug induced, motor symptoms such as catatonia, parkinsonism, abnormal involuntary movement, psychomotor slowing, or neurological soft signs (NSS) (Walther and Strik 2012). NSS include a variety of subtle abnormalities in sensory integration, motor coordination and sequencing of complex motor acts (Heinrichs and Buchanan 1988; Buchanan and Heinrichs 1989; Schroder et al. 1991). In the last two decades, several studies observed NSS not only in patients with chronic schizophrenia but also in young, antipsychotic-naive patients at the time of the first psychotic episode (Chan et al. 2010; Chen et al. 2000). Interestingly, NSS have been discussed as potential indicators of vulnerability to psychotic disorders and as endophenotypes for schizophrenia (Chan and Gottesman 2008; Peng et al. 2012).
At present, the putative neurobiological basis of NSS is intensively discussed in neuroimaging research. Structural magnetic resonance imaging (sMRI) studies demonstrated that increased NSS scores are related to aberrant morphology of both cortical and subcortical structures (Thomann et al. 2009a, b; Venkatasubramanian et al. 2008; Hirjak et al. 2012). Previous functional MRI (fMRI) investigations reported abnormal BOLD response in the pre- and postcentral gyrus, the premotor area, as well as in the middle and inferior frontal gyri in subjects with increased NSS scores (Schroder et al. 1995; Schroder et al. 1999). However, the aforementioned studies only examined atrophy or other abnormalities of circumscribed brain regions and disregarded the fact that NSS might be based on a sequel of gray matter reductions in movement circuits (Wen et al. 2011; Bassett et al. 2008).
In the last decade, voxel based morphometry (VBM) became the most commonly used approach to study gray matter abnormalities in schizophrenia (Ashburner and Friston 2000). Furthermore, the development of powerful 3D-based image-processing techniques based on T1-weighted structural MRI scans has made it possible to not only estimate the brain tissue volume and surface (Patenaude et al. 2011), but also to compute the surface and thickness of the cortex automatically (Fischl 2012). In particular, cortical thickness is defined as the distance between the gray-white matter boundary and the gray matter-cerebrospinal fluid boundary. Measurements of cortical thickness using MRI might reflect the cytoarchitecture of the cerebral cortex and are useful parameters to assess neuroanatomical patterns associated with cognitive performance, clinical symptoms and vulnerability to psychosis (Shaw et al. 2008; van Swam et al. 2012). Recent studies have proposed cortical thickness as an important indicator of brain degeneration in schizophrenia spectrum disorders (Schultz et al. 2010; He et al. 2007; Crespo-Facorro et al. 2011).
Using surface based morphometric (SBM) methods, sMRI studies have consistently demonstrated widespread cortical thinning in frontal and temporal regions (Goldman et al. 2009; Kuperberg et al. 2003; Nesvag et al. 2008; Rimol et al. 2010; Crespo-Facorro et al. 2011) and circumscribed cortical thinning in occipital and parietal areas (Rimol et al. 2010) among patients with schizophrenia spectrum disorders (Rimol et al. 2012). In addition, the aforementioned results provide a valuable complemental description of disease-related pathogenetic mechanisms such as brain growth and maturation (Oertel-Knochel et al. 2012; Kong et al. 2012b). Of these studies, many correlated cortical thickness with various psychometric measurements and found significant associations between particular psychopathological symptoms and cortical thinning in schizophrenia (Oertel-Knochel et al. 2012).
However, most of the brain mapping studies investigated cortical thickness across inhomogenuous patient cohorts with potential confounding factors such as longterm antipsychotic treatment, age, and a long duration of illness, respectively. In addition, it is still a matter of debate whether NSS are associated with changes of the cortical mantle in schizophrenia.
In the current study, we performed cortical surface reconstruction on 3 Tesla MRI data of 28 patients with recent onset schizophrenia using a SBM method (http://surfer.nmr.mgh.harvard.edu/) with well-established topologic accuracy in order to explore the relationship between cortical thickness and the severity of NSS. Since VBM is sensitive to changes in gray matter thickness, intensity, cortical surface area and cortical folding and prone to smoothing across neighbouring gyri (Kuhn et al. 2012), we sought to investigate—independently of regional surface area—whether cortical thickness changes match the topography of structural alterations previously reported in VBM studies on NSS and schizophrenia. We used a correlative approach to provide a quantitative assessment with regard to the relationship between cortical thickness and NSS. Specifically, we expected that patients with higher NSS will exhibit significant cortical thickness changes in cortical regions essential for regulating motor activity such as precentral and paracentral gyrus, postcentral lobule, prefrontal cortex and inferior parietal lobule.
Methods and Data Analysis
Subjects
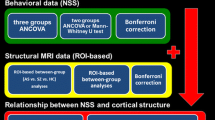
A sample of 28 patients was consecutively recruited from the Department of General Psychiatry in Heidelberg, Germany between 2009 and 2012. Our sample consisted of 7 women and 21 men, all right-handed Caucasians with a mean age of 23.21 ± 4.33 and a mean of 12.14 ± 2.42 years of education. 11 subjects have already been included in a previous study of our group (Hirjak et al. 2012). Patients were excluded if: (i) they were aged <18 or >35 years, (ii) they had a history of brain trauma or neurological disease, or (iii) they had shown alcohol/substance abuse or dependence within 12 months prior to participation. Diagnoses were made by staff psychiatrists and confirmed using a clinical interview (Wittchen et al. 1997) and examination of the case notes by two experienced psychiatrists (P.A.T. and U.S.). All patients fulfilled the DSM-IV criteria (Wittchen et al. 1997) for schizophrenia and had an initial onset of psychosis within two years prior to study entry with a mean duration of illness of 8.45 ± 3.71 months (range 6–24 months). At the time of inclusion, all patients were receiving treatment with a single antipsychotic agent according to their psychiatrists’ choice. The antipsychotic treatment included clozapine (n = 8), olanzapin (n = 6), aripiprazole (n = 5), quetiapine (n = 5), ziprasidone (n = 1) or risperidone (n = 3), respectively. The average dose in chlorpromazine (CPZ) equivalents was 505.75 ± 316.02 mg (range: 133–1,100) (Woods 2003). The mean duration of neuroleptic treatment was 2.13 ± 3.31 months (range 5–18 months). All patients gave informed consent to participation, and the study has been approved by the local ethics committee of the Medical Faculty, University of Heidelberg, Germany.
Clinical Assessments
NSS were assessed using the Heidelberg Scale (Schroder et al. 1991) that consists of five items assessing motor coordination (Ozeretski’s test, diadochokinesia, pronation/supination, finger-to-thumb opposition, speech articulation), three items assessing integrative functions (station and gait, tandem walking, two-point discrimination), two items assessing complex motor tasks (finger-to-nose test, fist-edge-palm test), four items assessing right/left and spatial orientation (right/left orientation, graphesthesia, face-hand test, stereognosis), and two items assessing hard signs (arm holding test, mirror movements). Items were rated on a 0 (no prevalence) to 3 (marked prevalence) point scale. A sufficient internal reliability and test–retest reliability have been established previously (Schroder et al. 1991; Bachmann et al. 2005). Handedness was assessed on the Edinburgh Inventory (Oldfield 1971). The severity of psychopathological symptoms were assessed with the Brief Psychiatric Rating Scale (Overall and Gorham 1962), the Scale for the Assessment of Positive Symptoms (Andreasen 1984) and the Scale for the Assessment of Negative Symptoms (Andreasen 1983). Predictors of outcome were rated on the Strauss-Carpenter Scale (Strauss and Carpenter 1974). Potential extrapyramidal side effects were excluded before study entry by an experienced psychiatrist who was not directly involved in the study.
MR Imaging Data Acquisition
Subjects underwent structural scanning at the German Cancer Research Center (DKFZ), Heidelberg, Germany, on a 3 Tesla Magnetom TIM Trio MR scanner (Siemens Medical Solutions, Erlangen, Germany) using a T1-weighted 3D magnetization prepared rapid gradient echo sequence (MP-RAGE, 160 sagittal slices, image matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3, TR = 2,300 ms, TE = 2.98 ms, TI = 900 ms, flip angle = 9°). An experienced neuroradiologist (B.S.) reviewed all MRI brain scans; no gross abnormalities (e.g., tumour, space-occupying cystic lesion greater 3 mm, signs of bleeding, contusion, infarction, major grey or white matter lesions) were found.
MR Image Processing
The Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) was used for cortical surface reconstruction followed by a process to obtain a representation of gray/white matter boundary and the pial surface (Fischl and Dale 2000; Fischl et al. 1999; Dale et al. 1999). Freesurfer is a freely available image analysis suite that can be used for both cortical reconstruction and volumetric segmentation. The technical details of these procedures are described in prior publications (Fischl et al. 1999; Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 2004). Briefly, the stream consists of multiple stages such as removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al. 2004); affine registration with Talairach space specifically designed to be insensitive to abnormalities and to maximize the accuracy of the final segmentation; tissue classification and correction of the variation in intensity resulting from the B1 bias field (Sled et al. 1998); tessellation of the gray matter white matter boundary; automated topology correction, and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al. 1999). Cortical thickness was calculated as the shortest distance between the previous surfaces at each vertex across the cortical mantle. After the automatic processing, the entire cortex of each patient was visually inspected and if necessary manually edited. After creation of cortical masks the cerebral cortex has been parcellated into anatomical structures and the cortical thickness was computed.
Statistical Analyses
Using a general linear model (GLM) approach provided by Query Design Estimate Contrast (QDEC) interface of Freesurfer, we performed vertex-wise analysis to explore significant correlations between cortical thickness and NSS scores on all subscales in patients with recent onset schizophrenia. Importantly, age, education and CPZ equivalents were included as nuisance covariates in these analyses. The effect of gender was regressed out in our model. Cortical thickness maps were smoothed using a Gaussian kernel with a full-width-at-half-maximum (FWHM) of 15 mm, because of our rather modest sample size. It is well known that the extent of the smoothing filter has a strong impact on the sample size needed for a well-powered cross-sectional analysis (Pardoe et al. 2012). That is, lower sample size requires a higher surface smoothing filter to detect a given thickness difference (for example 0.25 mm) across the cortical surface (Pardoe et al. 2012). All of these analyses were performed on the right and left hemisphere separately. Significant correlations between cortical thickness and NSS scores will be reported when their area exceeded 100 mm2. The cluster size of 100 mm² was chosen in accordance with the ‘matched filter theorem’ (Chung et al. 2003; Chung et al. 2005; Jung et al. 2011). In order to account for false positive results (correction for multiple comparisons) we used the false discovery rate (FDR) at p < 0.05 (Genovese et al. 2002). Here, only those correlations that remained significant after FDR-correction will be discussed in detail. Further, p values of the identified regions (peak maxima) were corrected for the number of tested NSS subscales and for the factor hemisphere by using the Bonferroni method.
Results
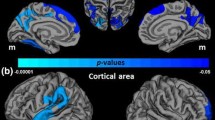
Demographic and clinical data are shown in Table 1. We identified a total of 48 regions (25 in the left and 23 in the right hemisphere) with a relationship between cortical thickness and NSS, at a significance level of p < 0.001, varying in size between 103.26 and 2,685.06 mm2 (Table 2). After FDR-correction 11 of 48 regions (Table 2) remained significant. Negative correlations between cortical thickness and higher NSS total scores (Fig. 1) were found in the left paracentral lobule and precuneus. Positive correlations between cortical thickness and higher NSS total scores (Fig. 1) were found in the middle temporal gyrus bilaterally. Negative correlations between cortical thickness and higher NSS scores on the subscales “hard signs” were identified in the left postcentral gyrus. Positive correlations between cortical thickness and higher NSS scores on the subscale “complex motor tasks” were found in the middle temporal gyrus bilaterally. Further, positive correlation was found between cortical thickness in the left postcentral gyrus and higher NSS scores on the subscale “left/right and spatial orientation”. Higher NSS scores on the subscale “integrative functions” were positively correlated with cortical thickness (Fig. 1) in the left middle temporal and supramarginal gyrus. Finally, we observed negative correlations between higher NSS scores on the subscale “integrative functions” and cortical thickness (Fig. 1) in the right paracentral lobule, inferior parietal lobe (IPL) and precuneus. Further, p values of the 48 identified regions (peak maxima; Table 2) were corrected for the number of tested NSS subscales in our main analysis using the Bonferroni method. To this end, α was set to p < 0.05/N, where N is the number of correlations (classical Bonferroni correction). N equalled 12. The significance in Qdec (FreeSurfer) is displayed as −log10 of the p value, and not as a straight p value. For this reason, the corrected threshold was set to p = 0.00416 (alpha level 0.05/12 tests [number of NSS scales × two hemispheres] or −log10 0.00461 = −2.386). Since the correlation analyses via Freesurfer were performed on right-handed schizophrenia patients, we did not correct for handedness. All but 3 regions hold Bonferroni correction for multiple testing.
Significant associations between NSS and cortical thickness for the NSS total score (upper panel) and for the NSS subscale “integrative function” (lower panel). For illustrational purposes, statistical maps were thresholded at p < 0.001, uncorrected. The scatter-plots show significant associations between NSS scores and cortical thickness at peak maxima after FDR-correction at p < 0.05. SPM supramarginal gyrus, PRE precentral gyrus, INS insula, LO occipital lobule, LOFG lateral orbitofrontal gyrus, FUS fusiform gyrus
No significant correlation of cortical thickness with age, gender, educational level, CPZ equivalents, and duration of treatment or severity of psychopathological symptoms was found. Moreover, NSS total scores (r: 0.205; p = 0.296) and scores on the subscales motor coordination (r: 0.153; p = 0.438), complex motor tasks (r: 0.188; p = 0.337), hard signs (r: 0.384; p = 0.044), integrative functions (r: 0.026; p = 0.894) and right/left and spatial orientation (r: 0.017; p = 0.930) were not significantly associated with CPZ equivalents.
Discussion
The present study aimed at exploring the relationship between NSS and cortical thickness in schizophrenia by using a rater-independent automatic approach for the reconstruction of the cerebral cortex. Two main findings emerged: First, higher NSS scores are negatively associated with cortical thickness. In particular, the left postcentral gyrus, the paracentral lobule bilaterally, the right IPL, and the left precuneus are cortical regions exhibiting a highly significant relationship between thickness and NSS. Second, higher NSS scores are positively correlated to cortical thickness in the temporal lobe bilaterally, the left supramarginal and left postcentral gyrus.
In line with our predictions, there were significant negative correlations between NSS total scores and cortical thickness mainly in the left postcentral gyrus (primary somatosensory cortex—S1) and the paracentral lobule (supplementary motor area—SMA) bilaterally. The postcentral gyrus is part of the parietal lobe and plays a crucial role in language processing, attention, spatial working memory and sense of touch (Shenton et al. 2001). The pyramidal neurons in S1 (Zhou et al. 2005) are connected to subcortical areas and hence, they are important for planning, controlling and executing of body movement (DeLong 2000; Dazzan et al. 2004). The SMA is located in the junction of the precentral gyrus and S1 and is primarily involved in complex finger movements and motor function of hand and arm (Strother et al. 2012). Our findings are generally in line with those of Exner et al. (Exner et al. 2006), who found reduced volume of the SMA to be related to impaired learning of motor sequences in young patients with schizophrenia. Our findings are further consistent with those of Schröder et al. (Schroder et al. 1995; Schroder et al. 1999) who described a relationship between reduced activation of the sensorimotor cortex and SMA and motor NSS such as finger-to-thumb opposition and pronation-supination. In addition, previous VBM studies of our own group in two independent patient samples found subjects high for NSS scores to be characterized by morphological changes of gray matter density in the postcentral gyrus bilaterally (Thomann et al. 2009b; Heuser et al. 2011).
The findings of the present study may further help to enhance our understanding on the function of the precuneus in the anatomical network underlying motor symptoms in schizophrenia. The precuneus is an area located in the posterior region of the medial parietal cortex. It has its major connections to the prefrontal and cingulate cortex, and plays a crucial role in visuospatial tasks (Corbetta et al. 1993), integration of stimuli, language comprehension and experience of agency (Dean et al. 2006; Binder 1997). However, to date, only one sMRI study found an association between motor symptoms such as “minor physical anomalies” (MPA) and bihemispheric gray matter volume reduction in the precuneus (Dean et al. 2006). In our study, we found elevated NSS total scores and higher NSS scores on the subscales “motor coordination” and “integrative functions” to be negatively associated with cortical thickness in the precuneus bilaterally. Since the precuneus is involved in limb position imagery and language, we speculate that its contribution to NSS might explain elevated scores on the NSS subscale “motor coordination”, which includes finger-to-thumb opposition and speech. In fact, the aforementioned results of Dean et al. (2006), and the associations reported in our study may provide further support for structural deficits in neural circuits normally responsible for the fluid execution of motor processes, comprising the precuneus, the precentral gyrus and the SMA. Both findings endorse the importance of the exchange of information between motor and parietal cortical areas when processing visuo-spatial stimuli with or without the execution of body action (Cavanna and Trimble 2006; Stephan et al. 1995). Moreover, they support the hypothesis that structural changes in the precuneus may be involved in the development of impaired motor functions such as Ozeretski’s test, diadochokinesia, pronation/supination, finger-to-thumb opposition and speech articulation, as indicated by the above mentioned neuroimaging studies.
We also found significant negative correlations between NSS scores on the subscale “integrative function” and “complex motor tasks” and cortical thickness in the right IPL. The IPL consists of the supramarginal and the angular gyrus, and is responsible for several functions such as social cognition, working memory, sustained attention and task control, amongst others (Palaniyappan and Liddle 2012). Additionally, the IPL has been described as an essential cerebral site for NSS in schizophrenia (Torrey 2007). This finding corresponds well with the requirement on the “complex motor tasks” subscale items, since the IPL is directly connected to the dorsolateral prefrontal cortex, the cingulate gyrus (CG) and the pre-motor areas. The crucial role of the IPL for NSS in schizophrenia has also been emphasized by a previous VBM study of our group, where increased NSS scores on the subscale “motor coordination” were associated with a reduced gray matter density in the left IPL (Heuser et al. 2011).
In addition to the aforementioned negative associations we also identified—contrary to our predictions—positive correlations between NSS scores and cortical thickness. These changes were evident in the superior, middle and inferior temporal gyrus, the supramarginal gyrus, and the postcentral gyrus, respectively. The temporal lobe and the adjacent supramarginal gyrus are involved in auditory and language perception and processing, visual information, visual recognition and identification, and complex processes of audiovisual integration. Especially within the initial clinical disease states of schizophrenia, a reorganisation of brain structures by means of neuroplasticity such as shrinkage of the neuropil and increased neuronal density has been suggested (Olabi et al. 2011). Given that our sample consisted of relatively young individuals with a rather short duration of illness, it is possible that our findings in the aforementioned regions could reflect compensatory mechanisms to maintain proper sensorimotor and language functions, perhaps due to a structural decline of primary regions such as the S1, the SMA, the precuneus and the IPL, respectively (Cobia et al. 2012).
Taken together, our findings are mostly consistent with those of other neuroimaging studies on NSS. However, direct comparison of our and previous results is difficult, especially since the majority of the previous investigations analyzed their data using VBM and none of the aforementioned studies investigated the relationship between NSS and cortical thickness. For instance, previous MRI studies indicated cortical thickness analysis as being more sensitive to subtle brain morphometric changes than the VBM approach (Corbo et al. 2005; Liu et al. 2012). Technically, this might be due to the fact that the maps produced in FreeSurfer are not restricted to the voxel resolution of the original data and thus are capable of detecting morphological submillimeter alterations (Fischl and Dale 2000). Subsequently, some slight discrepancies between our study and previous studies on NSS in schizophrenia need to be highlighted: Our findings differ from those previously presented by Venkatasubramanian et al. (2008) and Mouchet-Mages et al. (2007, 2011) who did not find any significant correlations between NSS levels and gray matter morphology in the S1, the SMA and the precuneus. Further, in contrast to our present results and those of Heuser et al. (2011), none of the previous studies found any associations between NSS levels and morphological abnormalities in the IPL. The latter discrepancy might be caused by the fact that the IPL is one of the last brain regions to mature and therefore having rather unstable anatomical landmarks (Torrey 2007; Geschwind 1965), a fact that might be especially sensitive to differences in MRI analysis techniques. In addition, discrepancies between studies might be at least partly explained by the MRI data acquisition at different field strengths, i.e. 3 Tesla in our present study compared to 1.5 Tesla in the NSS studies mentioned above. Correspondingly, a previous study reported MRI at 3 Tesla as being more sensitive to signal changes than MRI at 1.5 Tesla (Phal et al. 2008).
In general, it is important to bear in mind that the demarcation of gray matter boundaries from MRI data depends on grey-white matter contrast which is very sensitive to motion artefacts. Due to subject motion or other imaging artifacts, gray–white matter boundaries might be of reduced quality leading to spatial inaccuracies. Consequently, the spatial distribution of cortical thickness might not correspond to the factual anatomical boundaries of brain regions and thus, the data analysis could possibly produce a plenty of false positive results. This statistical problem can be minimized by correcting for multiple comparisons and increasing the cluster-size threshold. In the present study, the cluster size of 100 mm² was chosen in accordance with the ‘matched filter theorem’ (Chung et al. 2003; Chung et al. 2005; Jung et al. 2011). Of note, there was a considerable number of smaller cortical regions that showed associations with NSS; however, none of these associations survived FDR-correction.
Apart from MRI related issues, aspects of sample recruitment, patients’ disease state, mode of NSS rating, and antipsychotic medication need to be considered as potential confounding factors. In accordance with the majority of the previous studies on NSS and brain morphology in schizophrenia, patients in the present study were recruited consecutively among referrals from a larger university department in an urban area. All patients were examined after remission of acute psychotic symptoms before discharge. NSS were assessed on the Heidelberg Scale, for which sufficient measures of internal, inter-rater and test–retest reliability have been established previously (Schroder et al. 1991; Bachmann et al. 2005). However, for the interpretation of the results it is important to bear in mind that there are several other NSS scoring methods that might be differentially related to brain morphometric alterations. In order to partial out a putative dose-dependent effect of antipsychotics on brain structure, patients CPZ equivalents were considered as potential confounders in our morphometric analyses. In addition, no significant correlations between NSS scores and CPZ equivalents emerged.
Major limitations of our study are the cross-sectional design and a relatively modest sample size. Further, although we controlled for medication dosage, we are not able to fully exclude the possibility that antipsychotics might have influenced the present findings. However, previous studies examining the effect of neuroleptic treatment on brain structure have shown that predominantly subcortical gray matter structures, mainly with regard to typical antipsychotics, are subjected to morphological changes (Scherk and Falkai 2006) with neocortical regions being rather spared (Kuperberg et al. 2003; Nesvag et al. 2008). Eventually, we did not include a healthy control group in these analyses. In the present study, we were particularly interested in the within-group associations between cortical thickness and NSS in recent-onset schizophrenia. While the approach of not including a control group in our analysis might be considered as a limitation under some circumstances (e.g. type I error), it is important to emphasize that we were not primarily interested in detecting group differences between schizophrenia patients and healthy individuals. We considered our approach as being less prone to type II errors, since even in the case of significant between-group differences, which one might be inclined to label as “abnormal”, we may have to take into account the high likelihood that structural differences between patients with schizophrenia and controls may not be exclusively related to NSS levels. Depending on the study population, NSS prevalence rates in healthy individuals vary between 0 and 50 % (Cox and Ludwig 1979; Hertzig and Birch 1968; Rochford et al. 1970; Kennard 1960; Heinrichs and Buchanan 1988; Gay et al. 2012). This inconsistency may result from differences in gender, age, IQ, years of education and ethnic composition between the study samples. Over the last two decades, at least seven MRI studies investigated the relationship between NSS and brain morphology in healthy controls (Thomann et al. 2009a; Venkatasubramanian et al. 2008; Thomann et al. 2009b; Kong et al. 2012a; Bottmer et al. 2005; Keshavan et al. 2003; Dazzan et al. 2006). However, only two studies found an association between NSS levels and volume reduction in particular cortical areas (Dazzan et al. 2006; Thomann et al. 2009b). Apart from the limitations already mentioned above, aspects of MRI data acquisition and analysis might also provide an explanation for the discrepancies between the discussed studies. Unfortunately, given the availability of only few MRI studies, it is difficult to draw a firm conclusion on structural correlates in healthy individuals in whom NSS might be rather related to peristatic aspects such as less developed skills, factors occurring at random in each subject (Thomann et al. 2009a; Buchanan and Heinrichs 1989). This assumption corresponds well with an earlier study from North Finland: Ridler (Ridler et al. 2006) and colleagues investigated the infant motor development at age 1 year. Cerebral changes of the same individuals were assessed by MRI at age of 33–35 years. Interestingly, the authors found a relationship between abnormal infant motor development and cerebral changes in the healthy adult sample, but not in patients with schizophrenia. In fact, some other authors suggest that morphological changes underlying NSS are not entirely preformatted, but may increase with psychosis onset (Thomann et al. 2009a; Pantelis et al. 2003).
Last but not least, we are fully aware of the fact that our results might not be specific to a diagnosis of schizophrenia in contrast to a healthy condition, but are confident that the present findings at least depict cortical contributions to NSS in a patient population diagnosed with schizophrenia.
Conclusion
In summary, we found significant quantitative relationships between NSS and regional cortical thickness in the network of anatomical connections responsible for bodily movement that comprise the S1, the SMA, the IPL and the precuneus. The majority of the aforementioned cortical thickness changes match the topography of alterations previously reported in neuroimaging studies on NSS and schizophrenia. In addition, the present findings clearly emphasize that cortical thickness might not only serve as a highly sensitive metric but also as a potential indicator of a distinct process in schizophrenia patients with NSS. However, further multimodal structural and functional imaging investigations incorporating larger cohorts are needed to replicate these morphological correlates and to examine more precisely how abnormalities in the integrity of cortical network nodes affect sensory integration, motor coordination and sequencing of complex motor acts.
References
Andreasen NC (1983) The scale for the assessment of negative symptoms (SANS). University of Iowa, Iowa City
Andreasen NC (1984) The scale for the assessment of positive symptoms (SAPS). University of Iowa, Iowa City
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. NeuroImage 11(6 Pt 1):805–821
Bachmann S, Bottmer C, Schroder J (2005) Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry 162(12):2337–2343
Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A (2008) Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28(37):9239–9248. doi:10.1523/JNEUROSCI.1929-08.2008
Binder J (1997) Functional magnetic resonance imaging. Language mapping. Neurosurg Clin N Am 8(3):383–392
Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad LR, Magnotta V, Schroder J (2005) Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res 140(3):239–250
Buchanan RW, Heinrichs DW (1989) The neurological evaluation scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 27(3):335–350. doi:0165-1781(89)90148-0
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(Pt 3):564–583
Chan RC, Gottesman II (2008) Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a northern star? Neurosci Biobehav Rev 32(5):957–971
Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y (2010) Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull 36(6):1089–1104
Chen YL, Chen YH, Mak FL (2000) Soft neurological signs in schizophrenic patients and their nonpsychotic siblings. J Nerv Ment Dis 188(2):84–89
Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, Rapoport JL, Evans AC (2003) Deformation-based surface morphometry applied to gray matter deformation. Neuroimage 18(2):198–213
Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC (2005) Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage 25(4):1256–1265
Cobia DJ, Smith MJ, Wang L, Csernansky JG (2012) Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr Res 139(1–3):1–6
Corbetta M, Miezin FM, Shulman GL, Petersen SE (1993) A PET study of visuospatial attention. J Neurosci 13(3):1202–1226
Corbo V, Clement MH, Armony JL, Pruessner JC, Brunet A (2005) Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry 58(2):119–124
Cox SM, Ludwig AM (1979) Neurological soft signs and psychopathology. I. Findings in schizophrenia. J Nerv Ment Dis 167(3):161–165
Crespo-Facorro B, Roiz-Santianez R, Perez-Iglesias R, Rodriguez-Sanchez JM, Mata I, Tordesillas-Gutierrez D, Sanchez E, Tabares-Seisdedos R, Andreasen N, Magnotta V, Vazquez-Barquero JL (2011) Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med 41(7):1449–1460. doi:10.1017/S003329171000200X
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9(2):179–194
Dazzan P, Morgan KD, Orr KG, Hutchinson G, Chitnis X, Suckling J, Fearon P, Salvo J, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM (2004) The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain 127(Pt 1):143–153
Dazzan P, Morgan KD, Chitnis X, Suckling J, Morgan C, Fearon P, McGuire PK, Jones PB, Leff J, Murray RM (2006) The structural brain correlates of neurological soft signs in healthy individuals. Cereb Cortex 16(8):1225–1231
Dean K, Fearon P, Morgan K, Hutchinson G, Orr K, Chitnis X, Suckling J, Mallet R, Leff J, Jones PB, Murray RM, Dazzan P (2006) Grey matter correlates of minor physical anomalies in the AeSOP first-episode psychosis study. Br J Psychiatry 189:221–228. doi:10.1192/bjp.bp.105.016337
DeLong MR (2000) The basal ganglia. In: Kandel ER, Schwartz JH, Jessell TM (eds) Principles of neural science, vol 4. McGraw-Hill, New York, pp 647–659
Exner C, Weniger G, Schmidt-Samoa C, Irle E (2006) Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr Res 84(2–3):386–396
Fischl B (2012) FreeSurfer. NeuroImage 62(2):774–781
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97(20):11050–11055
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. NeuroImage 9(2):195–207
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14(1):11–22
Gay O, Plaze M, Oppenheim C, Mouchet-Mages S, Gaillard R, Olie JP, Krebs MO, Cachia A (2012) Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. doi:10.1093/schbul/sbs083
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15(4):870–878
Geschwind N (1965) Disconnexion syndromes in animals and man: I. Brain 88(2):237–294
Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer-Lindenberg A (2009) Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry 66(5):467–477
He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17(10):2407–2419. doi:10.1093/cercor/bhl149
Heinrichs DW, Buchanan RW (1988) Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 145(1):11–18
Hertzig ME, Birch HG (1968) Neurologic organization in psychiatrically disturbed adolescents. A comparative consideration of sex differences. Arch Gen Psychiatry 19(5):528–537
Heuser M, Thomann PA, Essig M, Bachmann S, Schroder J (2011) Neurological signs and morphological cerebral changes in schizophrenia: an analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res 192(2):69–76
Hirjak D, Wolf RC, Stieltjes B, Seidl U, Schroder J, Thomann PA (2012) Neurological soft signs and subcortical brain morphology in recent onset schizophrenia. J Psychiatr Res 46(4):533–539
Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, Han JY, Choi CH, Kang DH, Chung CK, Kwon JS (2011) Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull 37(4):839–849
Kennard MA (1960) Value of equivocal signs in neurologic diagnosis. Neurology 10:753–764
Keshavan MS, Sanders RD, Sweeney JA, Diwadkar VA, Goldstein G, Pettegrew JW, Schooler NR (2003) Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am J Psychiatry 160(7):1298–1304
Kong L, Bachmann S, Thomann PA, Essig M, Schroder J (2012a) Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res 134(1):27–32
Kong L, Herold C, Stieltjes B, Essig M, Seidl U, Wolf RC, Wustenberg T, Lasser MM, Schmid LA, Schnell K, Hirjak D, Thomann PA (2012b) Reduced gray to white matter tissue intensity contrast in schizophrenia. PLoS ONE 7(5):e37016
Kuhn S, Kaufmann C, Simon D, Endrass T, Gallinat J, Kathmann N (2012) Reduced thickness of anterior cingulate cortex in obsessive–compulsive disorder. Cortex. doi:10.1016/j.cortex.2012.09.001
Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60(9):878–888
Liu Y, Li YJ, Luo EP, Lu HB, Yin H (2012) Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS ONE 7(6):e39025
Mouchet-Mages S, Canceil O, Willard D, Krebs MO, Cachia A, Martinot JL, Rodrigo S, Oppenheim C, Meder JF (2007) Sensory dysfunction is correlated to cerebellar volume reduction in early schizophrenia. Schizophr Res 91(1–3):266–269
Mouchet-Mages S, Rodrigo S, Cachia A, Mouaffak F, Olie JP, Meder JF, Oppenheim C, Krebs MO (2011) Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand 123(6):451–458. doi:10.1111/j.1600-0447.2010.01667.x
Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, Agartz I (2008) Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res 98(1–3):16–28
Oertel-Knochel V, Knochel C, Rotarska-Jagiela A, Reinke B, Prvulovic D, Haenschel C, Hampel H, Linden DE (2012) Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb Cortex. doi:10.1093/cercor/bhr380
Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM (2011) Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry 70(1):88–96
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Palaniyappan L, Liddle PF (2012) Dissociable morphometric differences of the inferior parietal lobule in schizophrenia. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-012-0314-y
Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK (2003) Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361(9354):281–288
Pardoe HR, Abbott DF, Jackson GD (2012) Sample size estimates for well-powered cross-sectional cortical thickness studies. Hum Brain Mapp. doi:10.1002/hbm.22120
Patenaude B, Smith S, Kennedy D, Jenkinson M (2011) A bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. doi:10.1016/j.neuroimage.2011.02.046
Peng ZW, Xu T, Miao GD, He QH, Zhao Q, Dazzan P, Chan RC (2012) Neurological soft signs in obsessive–compulsive disorder: the effect of co-morbid psychosis and evidence for familiality. Prog Neuropsychopharmacol Biol Psychiatry. doi:10.1016/j.pnpbp.2012.06.015
Phal PM, Usmanov A, Nesbit GM, Anderson JC, Spencer D, Wang P, Helwig JA, Roberts C, Hamilton BE (2008) Qualitative comparison of 3-T and 1.5-T MRI in the evaluation of epilepsy. AJR Am J Roentgenol 191(3):890–895
Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, Murray GK, Haapea M, Jones PB, Isohanni MK, Bullmore ET (2006) Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci USA 103(42):15651–15656
Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ Jr, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I (2010) Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 68(1):41–50
Rimol LM, Nesvag R, Hagler DJ Jr, Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, Melle I, Andreassen OA, Agartz I, Dale AM (2012) Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. doi:10.1016/j.biopsych.2011.11.026
Rochford JM, Detre T, Tucker GJ, Harrow M (1970) Neuropsychological impairments in functional psychiatric diseases. Arch Gen Psychiatry 22(2):114–119
Scherk H, Falkai P (2006) Effects of antipsychotics on brain structure. Curr Opin Psychiatry 19(2):145–150
Schroder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M, Sauer H (1991) Neurological soft signs in schizophrenia. Schizophr Res 6(1):25–30
Schroder J, Wenz F, Schad LR, Baudendistel K, Knopp MV (1995) Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry 167(2):197–201
Schroder J, Essig M, Baudendistel K, Jahn T, Gerdsen I, Stockert A, Schad LR, Knopp MV (1999) Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. NeuroImage 9(1):81–87
Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlosser RG (2010) Reduced cortical thickness in first episode schizophrenia. Schizophr Res 116(2–3):204–209
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in MRI. NeuroImage 22(3):1060–1075
Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP (2008) Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28(14):3586–3594
Shenton ME, Dickey CC, Frumin M, McCarley RW (2001) A review of MRI findings in schizophrenia. Schizophr Res 49(1–2):1–52
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17(1):87–97
Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS (1995) Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73(1):373–386
Strauss JS, Carpenter WT Jr (1974) The prediction of outcome in schizophrenia. II. Relationships between predictor and outcome variables: a report from the WHO international pilot study of schizophrenia. Arch Gen Psychiatry 31(1):37–42
Strother L, Medendorp WP, Coros AM, Vilis T (2012) Double representation of the wrist and elbow in human motor cortex. Eur J Neurosci 36(9):3291–3298
Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schroder J (2009a) Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res 173(2):83–87
Thomann PA, Wustenberg T, Santos VD, Bachmann S, Essig M, Schroder J (2009b) Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med 39(3):371–379
Torrey EF (2007) Schizophrenia and the inferior parietal lobule. Schizophr Res 97(1–3):215–225
van Swam C, Federspiel A, Hubl D, Wiest R, Boesch C, Vermathen P, Kreis R, Strik W, Dierks T (2012) Possible dysregulation of cortical plasticity in auditory verbal hallucinations—a cortical thickness study in schizophrenia. J Psychiatr Res 46(8):1015–1023
Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS (2008) Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Res 164(3):215–222
Walther S, Strik W (2012) Motor symptoms and schizophrenia. Neuropsychobiology 66(2):77–92
Wen W, He Y, Sachdev P (2011) Structural brain networks and neuropsychiatric disorders. Curr Opin Psychiatry 24(3):219–225
Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M (1997) SKID-I: strukturiertes klinisches interview für DSM-IV. Hogrefe, Göttingen
Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64(6):663–667
Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Matsui M, Seto H, Kurachi M (2005) Volumetric analysis of sulci/gyri-defined in vivo frontal lobe regions in schizophrenia: precentral gyrus, cingulate gyrus, and prefrontal region. Psychiatry Res 139(2):127–139
Acknowledgments
The authors cordially thank all patients for participating in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirjak, D., Wolf, R.C., Stieltjes, B. et al. Cortical Signature of Neurological Soft Signs in Recent Onset Schizophrenia. Brain Topogr 27, 296–306 (2014). https://doi.org/10.1007/s10548-013-0292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-013-0292-z