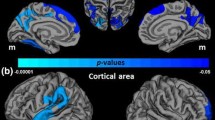
Abstract
Neurological soft signs (NSS) are frequently found in psychiatric disorders of significant neurodevelopmental origin. Previous MRI studies in schizophrenia have shown that NSS are associated with abnormal cortical, thalamic and cerebellar structure and function. So far, however, no neuroimaging studies investigated brain correlates of NSS in individuals with Asperger-Syndrome (AS) and the question whether the two disorders exhibit common or disease-specific cortical correlates of NSS remains unresolved. High-resolution MRI data at 3 T were obtained from 48 demographically matched individuals (16 schizophrenia patients, 16 subjects with AS and 16 healthy individuals). The surface-based analysis via Freesurfer enabled calculation of cortical thickness, area and folding (local gyrification index, LGI). NSS were examined on the Heidelberg Scale and related to cortical measures. In schizophrenia, higher NSS were associated with reduced cortical thickness and LGI in fronto-temporo-parietal brain areas. In AS, higher NSS were associated with increased frontotemporal cortical thickness. This study lends further support to the hypothesis that disorder-specific mechanisms contribute to NSS expression in schizophrenia and AS. Pointing towards dissociable neural patterns may help deconstruct the complex processes underlying NSS in these neurodevelopmental disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “Neurological soft signs” (NSS) refers to a broad range of subtle neurological deficits such as discrete impairments in sensory integration, motor coordination, sequencing of complex motor movements, and occasionally clumsiness and occurrence of primitive reflexes (Schroder et al. 1991; Bombin et al. 2005). From a clinical perspective, NSS belong to a group of genuine motor abnormalities that consist of spontaneous and medication-independent motor phenomena (Hirjak et al. 2015b). A higher prevalence of NSS is frequently found in patients with schizophrenia (Bombin et al. 2005; Chan and Gottesman 2008). Recent neuroimaging studies of NSS in schizophrenia have revealed structural and functional alterations in cortical and subcortical brain areas (see meta-analysis Zhao et al. 2014 and systematic review Hirjak et al. 2015b for details). These findings strongly support the hypothesis that NSS may be related to a disrupted cortico-cerebellar-thalamic-cortical circuit as conceptualized in the model of “cognitive dysmetria” (Andreasen et al. 1998).
From a clinical perspective, schizophrenia and Asperger-Syndrome (AS) show a substantial overlap across a broad range of motor abnormalities. In particular, motor symptoms and signs in these clinical groups may reflect a neurodevelopmental signature of motor function (Bombin et al. 2005) inviting the suggestion of a crucial role of the sensorimotor system in disease pathophysiology (Esposito and Pasca 2013; Qiu et al. 2010; Rinehart et al. 2006; Whyatt and Craig 2013). Along these lines, some authors do consider sensorimotor symptoms as a putative endophenotype for schizophrenia (Chan and Gottesman 2008) and AS (Esposito and Pasca 2013). More recently, clinical research interest in AS has extended to the investigation of NSS (Hirjak et al. 2014a; Mayoral et al. 2010; Tani et al. 2006). However, no neuroimaging studies investigated brain correlates of NSS in AS and it is still unknown which brain regions might contribute to NSS expression in these individuals. Furthermore, the question whether schizophrenia and AS share a common or a disease-specific pattern of brain changes underlying NSS is unresolved at present.
The purpose of the present structural MRI (sMRI) study was to investigate the structural neuroanatomy of NSS in recent-onset schizophrenia patients and individuals with AS. The current paper argues that in both disorders NSS levels would be higher compared to healthy individuals. On the neural level, a direct comparison of NSS expression in schizophrenia and AS could reveal disorder-specific and transdiagnostic cortical correlates of NSS. Given that NSS can be considered as part of a broader “motor phenotype” (Hirjak et al. 2015b), the current transdiagnostic study may also illustrate the utility of a “motor domain” (Bernard and Mittal 2015) in characterizing mental disorders of presumed neurodevelopmental origin.
In this study, we used high-resolution sMRI data acquired at 3 T together with a fully automatic data analysis method. We used Freesurfer (Fischl et al. 2002) for cortical reconstruction and employed a hierarchical statistical approach to detect and characterize the relationship between NSS and the three cortical measures (cortical thickness, area and folding). Based on results of previous studies on NSS (Hirjak et al. 2014a; Tani et al. 2006), we predicted that schizophrenia patients and subjects with AS will be characterized by different quantitative and qualitative NSS expression. Further, we expected that different qualitative NSS expression in schizophrenia and AS would be related to changes of cortical thickness, cortical area and local gyrification index (LGI). Eventually, we investigated whether the combination of behavioral data and cortical measurements might be useful to predict the diagnosis of schizophrenia and AS.
Methods
Subjects
The study sample consisted of 16 clinically stable patients with recent-onset schizophrenia, 16 individuals with AS, and 16 healthy controls. The study sample was consecutively recruited between 2010 and 2013 from the Department of General Psychiatry in Heidelberg, Germany and from SALO GmbH in Ludwigshafen, Germany, a professional rehabilitation institution of education for individuals with autism. All participants were Caucasians. Study participants were excluded if: (1) they were aged <18 or >35 years, (2) they had a history of brain trauma or neurological disease, (3) they had a comorbid Axis-I- or Axis-II-Disorder according to ICD-10 or DSM-IV, (4) they had shown alcohol/substance abuse or dependence within 24 months prior to participation, or (5) they had an IQ < 70. Diagnoses of schizophrenia and AS were made by specialized clinicians (DH and PAT) corresponding to ICD-10 criteria and supplemented by an extensive neuropsychological assessment. Patients with schizophrenia had an initial onset of psychosis within 2 years prior to study entry. At the time of inclusion, all schizophrenia patients were treated with a single second-generation antipsychotic agent and consistent medication doses for 4 weeks or longer. Individuals with AS and healthy controls did not take any antipsychotic, mood stabilizing, anti-cholinergic or antidepressive medications. All study participants gave informed consent to participation, and the study has been approved by the local ethics committee of the Medical Faculty, University of Heidelberg, Germany.
Clinical Assessments
Clinical symptom determinations and structured clinical diagnostic interviews were conducted by trained clinical raters (DH, IP and KMK). In particular, all study individuals were assessed for lifetime psychiatric diagnoses by trained psychiatrists (DH and PAT) via the German version of the Structured Clinical Interview for DSM-IV (SCID-DSM-IV) (Wittchen et al. 1997b). Demographics and psychiatric history of the two clinical samples were retrieved from medical records. Schizophrenia patients in our study were recruited and examined as soon as possible after symptom remission; at least partial remission is necessary for the assessment of NSS, since florid psychotic symptoms, agitation and severe formal thought disorders considerably influence the patients’ cooperation (both with respect to clinical investigations and to MRI scanning) as well as their ability to understand the instructions.
All participants in the AS group had previously received a clinical diagnosis of AS (F84.5) from an independent clinician according to standard criteria (a valid diagnosis of autism is an admission criterion for SALO GmbH). In addition, diagnoses of the participants with AS were confirmed with the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 1989) administered by a trained and clinically experienced psychiatrist (DH) and Autism Quotient (AQ) (Baron-Cohen et al. 2006). All AS individuals exceeded a threshold score of 26/50 proposed by Woodbury-Smith et al. (2005). None of the participants with AS had to be excluded due to mental disorders such as major depressive disorder, current or chronic substance abuse, schizophrenia spectrum disorder or personality disorder, as assessed with the SCID-DSM-IV (Wittchen et al. 1997a). In addition, IQ of individuals with AS has been systematically assessed with the german version of the Culture Fair Intelligence Test (CFT-20-R) (Weiß 2006). Level of IQ among individuals with AS ranged from 86 to 120 (mean IQ: 105.43 ± 6.47) according to CFT-20-R (Weiß 2006). We chose a CFT-20-R threshold of ≥80 to ensure normal intelligence and minimize the effect of IQ on motor assessment. IQ in schizophrenia patients and healthy controls was not explicitly assessed, but clinically judged to be average or above average. Both patients with schizophrenia and healthy controls were required to have a leaving certificate from one of the secondary schools (“Hautpschule, 9 years; “Realschule”, 10 years) or Gymnasium (13 years) in order to participate in our study. To examine the possible effect of medications on NSS, we standardized the dosage of antipsychotic medications chlorpromazine equivalents (CPZ).
The severity of psychopathological symptoms was assessed with the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham 1962), the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen 1984) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1983). Predictors of outcome were rated on the Strauss–Carpenter Scale (SCS) (Strauss and Carpenter 1974). The social, occupational and psychological functioning in individuals with AS was assessed with the Global Assessment of Functioning (GAF) scale (Hall 1995). Potential extrapyramidal side effects, parkinsonian signs and abnormal involuntary movement according to Abnormal involuntary movement scale (AIMS) (Guy, 1976) were excluded before study entry by an experienced psychiatrist who was not directly involved in the study. In the control group, the Structured Clinical Interview for Axis I and Axis II DSM-IV Disorders (SCID-VI) (Wittchen et al. 1997a) was administered to rule out Axis I and Axis II disorders. In addition, we used the PRIME early psychosis screening test (Prevention through Risk Identification, Management, and Education—PRIME) to screen for the presence of early psychotic symptoms, including information on any contact or treatment for any mental or psychological disorder including autism (Miller et al. 2003).
NSS were assessed using the Heidelberg Scale (Schroder et al. 1991) that consists of five items assessing motor coordination (MOCO) (Ozeretski’s test, diadochokinesia, pronation/supination, finger-to-thumb opposition, speech articulation), three items assessing integrative functions (IF) (station and gait, tandem walking, two-point discrimination), two items assessing complex motor tasks (COMT) (finger-to-nose test, fist-edge-palm test), four items assessing right/left and spatial orientation (RLSPO) (right/left orientation, graphesthesia, face-hand test, stereognosis), and two items assessing hard signs (HS) (arm holding test, mirror movements). Items were rated on a 0 (no prevalence) to 3 (marked prevalence) point scale. A sufficient internal reliability and test–retest reliability have been established previously (Bachmann et al. 2005; Schroder et al. 1991). The NSS assessment has been conducted by one rater. Handedness was assessed on the Edinburgh Inventory (Oldfield 1971).
MR Imaging Data Acquisition
Participants underwent structural scanning at the German Cancer Research Center (DKFZ), Heidelberg, Germany, on a 3 T Magnetom TIM Trio MR scanner (Siemens Medical Solutions, Erlangen, Germany) using a T1-weighted 3D magnetization prepared rapid gradient echo sequence (MP-RAGE, 160 sagittal slices, image matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3, TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, flip angle = 9°). An experienced neuroradiologist reviewed all MRI brain scans; no gross abnormalities (e.g., tumour, space-occupying cystic lesion greater 3 mm, signs of bleeding, contusion, infarction, major gray or white matter lesions) were found.
Behavioral and MRI Data Processing and Analysis (Statistical analysis flowcharts are depicted in Fig. 1)
Cortical Reconstruction
Freesurfer (for detailed description of the method see (http://surfer.nmr.mgh.harvard.edu/) (Khan et al. 2008) was used for cortical surface reconstruction (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999). Briefly, the stream consists of multiple stages such as removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al. 2004); affine registration with Talairach space specifically designed to be insensitive to abnormalities and to maximize the accuracy of the final segmentation; tissue classification and correction of the variation in intensity resulting from the B1 bias field (Sled et al. 1998); tessellation of the gray matter white matter boundary; automated topology correction, and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al. 1999). These surface representations enabled calculations of regional grey matter surface and thickness across the cortical mantle. After the automatic processing, the entire cortex of each patient was visually inspected and if necessary manually edited by a single rater as recommended by the FreeSurfer tutorial (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial). After creation of cortical masks the cerebral cortex has been parcellated into units based on gyral and sulcal structure, resulting in values for cortical thickness and surface area (Desikan et al. 2006; Fischl et al. 2004). Cortical thickness was calculated as the shortest distance between the previous surfaces at each vertex across the cortical mantle. Surface area was calculated by taking the sum of the area of the vertices in each parcellation (Bhojraj et al. 2011). The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. In addition, the maps produced are not restricted to the voxel resolution of the original data and thus are capable of detecting submillimeter differences within diagnostic groups. This means that if the pial boundary is between a grey matter voxel and a cerebrospinal fluid voxel, some grey matter change might cause the boundary to move into the voxel (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999). On a T1-MRI-image, the voxel intensity declines and provides the information we need to detect this type of effect. Furthermore, because of smoothness constraints implemented in Freesurfer, the surface placement and the thickness estimation might reach subvoxel accuracy (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999). This automated approach and the subvoxel accuracy of cortical thickness and cortical area measurements were validated against manual measurements (Kuperberg et al. 2003; Rosas et al. 2002): High-resolution MRI images of 11 patients with Huntington’s disease, 13 age-matched healthy controls and two postmortem brains were analyzed using Freesurfer to obtain cortical thickness measurements. To validate this procedure, cortical thickness of the two autopsy brains were also analyzed using traditional neuropathologic methods (i.e. microscope ex vivo). Cortical thickness measurements obtained from these two methods (MRI images vs. neuropathology) agreed within 0.2 mm, with a mean difference of 0.077 mm (Rosas et al. 2002).
Cortical Gyrification
Based on the pial surface reconstruction, an algorithm for measuring 3D LGI at each vertex across each hemisphere, including the default smoothing of individual LGI maps at a full width at half maximum (FWHM) kernel of 25 mm, was performed. Details of the LGI computation process can be found in the validation paper (Schaer et al. 2012), previous studies in psychiatric patients (Hirjak et al. 2015a; Klein et al. 2014; Nesvag et al. 2014; Palaniyappan and Liddle 2014) and at https://surfer.nmr.mgh.harvard.edu/fswiki/LGI. Briefly, an outer envelope that tightly wraps the pial cortical surface is created before local measurement of circular GI is computed for each vertex of the outer surface as the ratio of corresponding regions of interest (ROI) on the outer envelope and pial surface. Delineation of the ROI on both the outer surface (ROIO) and pial surface (ROIP) uses a matching algorithm based on geodesic constraints, so that the ROIP delineates the entire patch of the cortical surface within the circular perimeter of the ROIO. Finally, at the end of the computational process, individual LGI cortical maps quantify the amount of cortex buried within the sulcal folds in the surrounding circular region.
Statistical Analyses
Behavioral data were analyzed using the Statistical Package of the Social Sciences (SPSS version 22.0, SPSS Inc., Chicago, IL). Sociodemographic and clinical variables were described and compared between the three groups with unpaired t test or χ 2 test for categorical variables using conventional significance levels (p < 0.05). The between-group differences in cortical measures were explored with a ROI-based approach. For this purpose, cortical thickness, cortical area and LGI values of 68 structures (34 in the left and 34 in the right hemisphere) (“aparc.stats” files) based on Desikan–Killiany atlas (Desikan et al. 2006) were automatically extracted from FreeSurfer. Then, we used a statistical hierarchical approach that minimizes the problem of multiple comparisons (Fig. 1): First, normality distribution of NSS scores and cortical measures was evaluated with the Kolmogorov–Smirnov test. Second, we applied an omnibus test over the three study groups. Third, we tested bivariate differences between the study groups (SZ vs. AS, SZ vs. HC, AS vs. HC). In particular, differences in those NSS subscales and cortical measures that did not fit to a normal distribution according to Kolmogorov–Smirnov testing (p < 0.05) were analyzed using the nonparametric Mann–Whitney U test. NSS subscales and cortical measures that fitted to a normality distribution were analyzed using analyses of covariance (ANCOVA) including the potentially distorting factors age, gender, years of education and chlorpromazine equivalents. Third, p values of the identified NSS subscales and cortical measurements of significant cortical regions were Bonferroni corrected. Further, our behavioral and neuroimaging data have been analyzed twice, using correlational analysis (within-group) and multivariate logistic regression (between-group). First, the association between NSS scores and significant cortical regions was explored using partial correlations by treating age, gender, years of education and chlorpromazine equivalents as potential confounders. Second, univariate and multivariate logistic regression analyses were used to further characterize the significant association between NSS scores and cortical regions from the between-group analyses.
Results
Clinical Characteristics of Study Participants
Socio-demographic and clinical characteristics of the study sample are summarized in Table 1. The three groups of participants were matched according to age, gender, handedness and years of education. Subjects with AS and other study subjects were not matched for IQ, because in autism IQ is not a valid measure of such functioning owing to the atypical profile of subtest performance (Allen et al. 2004).
Group Difference in NSS Performance (Controlling for Age, Gender, Years of Education and Medication)
Schizophrenia vs. AS vs. Healthy Controls
Table 1 shows the prevalence of NSS across the three groups. First, ANCOVA of the three groups of participants revealed differences in NSS total score [F (6, 47) = 8.82; p < 0.001] and on the five NSS subscales MOCO [F (6, 47) = 7.63; p < 0.001], IF [F (6, 47) = 4.81; p = 0.001], COMT [F (6, 47) = 2.8; p = 0.022), RLSPO [F (6, 47) = 1.08; p = 0.38) and HS [F (6, 47) = 3.2; p = 0.011). The NSS total score and four NSS subscales (MOCO, IF, COMT and HS) reached statistical significance (p < 0.05). A multiple regression model was used to test whether NSS performance is predictive for diagnosis. The four subscale scores were included in the regression analysis. The NSS subscales significantly predicted diagnosis, F (4, 47) = 4.612, p < 0.003, R 2 = 0.3. However, only the HS subscale score added statistically significantly to this prediction, p < 0.05.
Schizophrenia vs. AS
In both study groups, NSS total scores and NSS scores on all five subscales were normally distributed according to Kolmogorov–Smirnov testing (p < 0.05). The ANCOVA showed that schizophrenia patients had significantly higher NSS scores on the subscale MOCO [F (5, 26) = 4.57; p = 0.004] when compared to individuals with AS. On the other hand, individuals with AS showed higher NSS total scores [F (5, 26) = 3.81; p = 0.01] and elevated NSS scores on the subscales IF [F (5, 26) = 4.93; p = 0.003], COMT [F (5, 26) = 1.4; p = 0.25], RLSPO [F (5, 26) = 0.36; p = 0.866] and HS [F (5, 26) = 0.6; p = 0.69]. Two NSS subscales (MOCO and IF) survived Bonferroni correction (p < 0.0041).
AS vs. Healthy Controls (medication was not included as a covariable)
In both study groups, NSS total scores and NSS scores on the subscale MOCO and HS were not normally distributed according to Kolmogorov–Smirnov testing (p < 0.05). In both study groups, NSS scores on the subscale IF, COMT and RLSPO were normally distributed according to Kolmogorov–Smirnov testing (p > 0.05). Patients’ total NSS scores (p = 0.000024) and NSS scores on the subscale MOCO (p = 0.001) and HS (p = 0.002) were significantly increased when compared to controls (Mann–Whitney U non-parametric test). According to ANCOVA, patients’ NSS scores on the subscale IF [F (4, 27) = 4.29; p = 0.008], COMT [F (4, 27) = 2.98; p = 0.036] and RLSPO [F (4, 27) = 1.68; p = 0.18] were increased when compared to controls. NSS total, MOCO and HS scores survived Bonferroni correction (p < 0.0041).
Schizophrenia vs. Healthy Controls
In both study groups, NSS total scores and NSS scores on the subscale MOCO and HS were not normally distributed according to Kolmogorov–Smirnov testing (p < 0.05). In both study groups, NSS scores on the subscale IF, COMT and RLSPO were normally distributed according to Kolmogorov–Smirnov testing (p > 0.05). Patients’ total NSS scores (p = 0.002) and NSS scores on the subscale MOCO (p = 0.003) and HS (p = 0.01) were significantly increased when compared to controls (Mann–Whitney U non-parametric test). According to ANCOVA, patients’ NSS scores on the subscale IF [F (5, 26) = 1.94; p = 0.122], COMT [F (5, 26) = 2.21; p = 0.083] and RLSPO [F (5, 26) = 0.75; p = 0.59] were increased when compared to controls. NSS total and MOCO scores survived Bonferroni correction (p < 0.0041).
Group Differences in Cortical Measures (ROI-Based Analysis)
Schizophrenia vs. AS vs. Healthy Controls
First, ANCOVA of the three study groups revealed no differences in the cortical area. Second, ANCOVA of the three study groups revealed statistically significant differences in cortical thickness of the left caudal middle frontal gyrus [F (6, 47) = 3.186; p < 0.012], the left middle temporal gyrus [F (6, 47) = 2.730; p < 0.025], the left pars opercularis [F (6, 47) = 2.444; p < 0.041], the left precentral gyrus [F (6, 47) = 3.191; p < 0.012], the left superior frontal gyrus [F (6, 47) = 2.739; p < 0.025], the left supramarginal gyrus [F (6, 47) = 3.002; p < 0.016], the banks of the right superior temporal sulcus [F (6, 47) = 6.164; p < 0.001], the right lateral orbitofrontal gyrus [F (6, 47) = 2.694; p < 0.027], the right paracentral gyrus [F (6, 47) = 3.478; p < 0.007], and the right pars triangularis [F (6, 47) = 3.97; p < 0.017], respectively. Third, ANCOVA of the three groups revealed significant differences in LGI of the left caudal middle frontal gyrus [F (6, 47) = 3.245; p < 0.011], the left inferior parietal lobule [F (6, 47) = 2.441; p < 0.041], the left pericalcarine gyrus [F (6, 47) = 4.285; p < 0.002], the right inferior temporal gyrus [F (6, 47) = 2.691; p < 0.027], the right precentral gyrus [F (6, 47) = 2.328; p < 0.05], and the right temporal pole [F (6, 47) = 3.116; p < 0.013], respectively. There was no significant difference in the three cortical measurements in the other regions among the three groups (p < 0.05).
Schizophrenia vs. AS
In comparison to AS individuals, schizophrenia patients showed reduced cortical thickness in the left caudal middle frontal gyrus [F (5, 31) = 2.683; p < 0.044], the left middle temporal gyrus [F (5, 31) = 2.118; p < 0.095], the left pars opercularis [F (5, 31) = 2.105; p < 0.097], the left precentral gyrus [F (5, 31) = 5.218; p < 0.002], the left superior frontal gyrus [F (5, 31) = 1.47; p < 0.234], the left supramarginal gyrus [F (5, 31) = 1.156; p < 0.357], the banks of the right superior temporal sulcus [F (5, 31) = 3.077; p < 0.026], and the right pars triangularis [F (5, 31) = 2.274; p < 0.077], respectively. Schizophrenia patients showed greater cortical thickness in the right lateral orbitofrontal gyrus [F (5, 31) = 4.457; p < 0.005] and the right paracentral gyrus [F (5, 31) = 2.323; p < 0.072]. Only two regions (left precentral gyrus and right lateral orbitofrontal gyrus) survived Bonferroni correction (p < 0.0041). In comparison to schizophrenia patients, AS individuals showed reduced cortical gyrification in the left caudal middle frontal gyrus [F (5, 31) = 3.538; p < 0.014], the left inferior parietal lobule [F (5, 31) = 1.51; p < 0.221], the left pericalcarine gyrus [F (5, 31) = 4.231; p < 0.006], the right inferior temporal gyrus [F (5, 31) = 1.925; p < 0.124], and the right temporal pole [F (5, 31) = 0.232; p < 0.945], respectively. Patients with schizophrenia showed reduced LGI in the right precentral gyrus [F (5, 31) = 2.126; p < 0.094] when compared to individuals with AS. Only one region (left pericalcarine gyrus) survived Bonferroni correction (p < 0.0041).
AS vs. Healthy Controls
In comparison to healthy controls, AS individuals showed increased cortical thickness in the left caudal middle frontal gyrus [F (5, 31) = 3.052; p < 0.034], the left middle temporal gyrus [F (5, 31) = 0.631; p < 0.644], the left pars opercularis [F (5, 31) = 1.766; p < 0.165], the left precentral gyrus [F (5, 31) = 0.862; p < 0.492], the left superiorfrontal gyrus [F (5, 31) = 3.283; p < 0.026], the left supramarginal gyrus [F (5, 31) = 0.969; p < 0.44], the banks of the right superior temporal sulcus [F (5, 31) = 1.757; p < 0.167], the right lateral orbitofrontal gyrus [F (5, 31) = 0.637; p < 0.641], the right paracentral gyrus [F (5, 31) = 1.512; p < 0.227], and the right pars triangularis [F (5, 31) = 1.602; p < 0.202], respectively. No region survived Bonferroni correction (p < 0.0041). In comparison to healthy controls, AS individuals showed reduced cortical gyrification in the left caudal middle frontal gyrus [F (4, 31) = 1.445; p < 0.247], the left inferior parietal lobule [F (4, 31) = 1.704; p < 0.178], the left pericalcarine gyrus [F (4, 31) = 3.1; p < 0.032], the right inferior temporal gyrus [F (6, 47) = 4.083; p < 0.010], the right precentral gyrus [F (6, 47) = 1.531; p < 0.221], and the temporal pole [F (6, 47) = 3.837; p < 0.014]. No region survived Bonferroni correction (p < 0.0041).
Schizophrenia vs. Healthy Controls
In comparison to healthy controls, schizophrenia patients showed reduced cortical thickness in the left caudal middle frontal gyrus [F (5, 32) = 0.746; p < 0.597], the left middle temporal gyrus [F (5, 32) = 3.417; p < 0.017], the left pars opercularis [F (5, 32) = 2.663; p < 0.045], the left precentral gyrus [F (5, 32) = 3.028; p < 0.028], the left superior frontal gyrus [F (5, 32) = 2.182; p < 0.087], the left supramarginal gyrus [F (5, 32) = 5.690; p < 0.001], the banks of the right superior temporal sulcus [F (5, 31) = 6.215; p < 0.001], the right lateral orbitofrontal gyrus [F (5, 31) = 3.511; p < 0.015], the right paracentral gyrus [F (5, 31) = 3.230; p < 0.021], and the right pars triangularis [F (5, 31) = 2.831; p < 0.036], respectively. Only one region (left supramarginal gyrus) survived Bonferroni correction (p < 0.0041). In comparison to healthy controls, schizophrenia patients showed reduced cortical gyrification in the left caudal middle frontal gyrus [F (5, 31) = 4.982; p < 0.001], the left inferior parietal lobule [F (5, 31) = 4.829; p < 0.002], the left pericalcarine gyrus [F (5, 31) = 3.1; p < 0.081], the right inferior temporal gyrus [F (5, 31) = 1.666; p < 0.178], the right precentral gyrus [F (5, 31) = 2.853; p < 0.035], and the temporal pole [F (5, 31) = 2.251; p < 0.079]. Two regions (left caudal middle frontal gyrus and left inferior parietal lobule) survived Bonferroni correction (p < 0.0041).
Correlational Analysis (Schizophrenia and AS)
In patients with schizophrenia, there was a significant correlation between higher MOCO scores and reduced cortical thickness in the left precentral gyrus (r: −0.620; p = 0.032). IF subscale scores were associated with reduced cortical thickness in the left precentral gyrus (r: −0.637; p = 0.026). Significant correlations between higher COMT subscale scores and reduced cortical thickness in the left precentral gyrus (r: −0.664; p = 0.018) and reduced LGI values in the right temporal pole (r: −0.610; p = 0.035) were found. Higher RLSPO scores were associated with reduced cortical thickness in the left precentral gyrus (r: −0.820; p = 0.001) and reduced LGI values in the left caudal middle frontal gyrus (r: −0.659; p = 0.02), the left inferior parietal gyrus (r: −0.812; p = 0.001), the right precentral gyrus (r: −0.749; p = 0.005) and the temporal pole (r: −0.613; p = 0.034). No other associations between NSS levels and cortical regions reached statistical significance at the chosen threshold (p < 0.05) (Fig. 2).
Cortical regions based on the Desikan–Killiany Atlas (Desikan et al. 2006) showing significant associations between NSS scores on the subscale RLSPO and MOCO and reduced cortical thickness and LGI in recent-onset schizophrenia
In individuals with AS, higher NSS total scores were associated with increased cortical thickness in the left caudal middle frontal gyrus (r: 0.654; p = 0.015). Higher MOCO scores were associated with increased cortical thickness in the left caudal middle frontal gyrus (r: 0.817; p = 0.001) (Fig. 3). COMT scores were associated with increased cortical thickness in the left caudal middle frontal gyrus (r: 0.589; p = 0.034) and precentral gyrus (r: 0.588; p = 0.035) (Fig. 3). No other associations between NSS levels and cortical regions reached statistical significance at the chosen threshold (p < 0.05).
Cortical regions based on the Desikan–Killiany Atlas (Desikan et al. 2006) showing significant associations between NSS scores on the subscale COMT and MOCO and increased cortical thickness in individuals with Asperger-Syndrome
Multiple Regression Analysis
According to linear regression analysis, reduced cortical thickness in the left precentral gyrus and reduced LGI in the temporal pole had a significant effect on NSS levels on the subscale MOCO [F (2, 15) = 4.249, p < 0.038, R 2 = 0.629] in schizophrenia (Fig. 2). Furthermore, reduced cortical thickness in the left precentral gyrus and reduced LGI values in the left caudal middle frontal gyrus, the left inferior parietal lobule, the right precentral gyrus and the temporal pole had a significant effect on NSS levels on the RLSPO subscale [F (2, 15) = 4.249, p < 0.0001, R 2 = 0.936] in schizophrenia (Fig. 2). In individuals with AS, increased cortical thickness in the left caudal middle frontal gyrus had no statistically significant effect on NSS total scores [F (1, 15) = 0.198, p < 0.663, R 2 = 0.118] and NSS levels on the subscale MOCO [F (1, 15) = 0.218, p < 0.647, R 2 = 0.124]. In individuals with AS, increased cortical thickness in the left precentral gyrus had no significant effect on NSS levels on the subscale COMT [F (1, 15) = 3.456, p < 0.084, R 2 = 0.445]. In addition, multiple regression analysis was used to investigate the effects of NSS performance (MOCO and RLSPO) and cortical regions (reduced cortical thickness in the left precentral gyrus and reduced LGI in the left caudal middle frontal gyrus, the left inferior parietal lobule, the right precentral gyrus and the temporal pole) predicting the patient group (SZ and AS). All variables significantly predicted the patient group, F (7, 31) = 4.336, p < 0.003, R 2 = 0.748. However, only cortical thickness in the left caudal middle frontal gyrus added statistically significantly to the prediction (p < 0.05).
Furthermore, the effects of diagnosis and structural morphology in cortical regions on predicting the NSS performance were assessed using multiple regression analysis in all subjects. A multiple regression analysis was run to predict NSS total score and NSS scores on all subscales from diagnosis and cortical morphology (thickness and LGI) in 16 significant cortical regions. However, we only found one statistically significant relationship: Cortical thickness in the left supramarginal gyrus and the diagnosis predicted the NSS total score F (17, 47) = 2.218, p < 0.027, R 2 = 0.746. Both variables significantly added to the prediction (nominal p < 0.05). In addition, multiple regression analysis was used to investigate the effects of NSS performance (MOCO and IF) and cortical regions (16 areas) predicting the diagnosis. All variables significantly predicted the diagnosis, F (11, 47) = 3.307, p < 0.003, R 2 = 0.709. However, only cortical thickness in the left caudal middle frontal gyrus added statistically significantly to the prediction (p < 0.05).
Discussion
This study aimed at investigating the neuroanatomical correlates of NSS in recent-onset schizophrenia and AS using high-resolution MRI at 3 T. Three main findings emerged: Schizophrenia patients showed reduced cortical thickness and LGI in fronto-temporo-parietal regions. These atrophic patterns contributed significantly to higher NSS scores in schizophrenia. Although AS individuals showed reduced LGI in fronto-temporal areas, these atrophic patterns were not associated with NSS performance. Higher NSS in AS individuals were associated with increased cortical thickness in fronto-temporal regions.
Cortical Thickness and Area
As predicted, we found reduced cortical thickness in fronto-temporo-parietal regions (left caudal middle frontal gyrus, left supramarginal gyrus and left inferior parietal lobule) in schizophrenia patients. These findings provide further evidence for widespread cortical thinning in the early stages of schizophrenia (Rimol et al. 2010; Schultz et al. 2010a, b). Further, they corroborate previous sMRI studies, which suggested that changes in cortical morphology may represent a characteristic brain phenotype in schizophrenia (Rimol et al. 2010; Schultz et al. 2010a, b). Another important finding of the current study is that AS subjects showed increased cortical thickness in fronto-temporal regions when compared to healthy controls. These findings are in line with previous structural studies on autism that reported age-related grey matter volume increase in frontal lobe (DeRamus and Kana 2015). This finding is remarkable, because individuals with autism exhibit morphological changes of frontal regions responsible for visuo-spatial working memory and sustained attention, cognitive functions that are involved in motor sequencing and imitation (Teixeira et al. 2014). Furthermore, temporal regions are commonly regarded as core regions for the “mirror neuron system” (Gallese et al. 2004). Correspondingly, previous sMRI studies in autism showed structural alterations in language-related areas located mainly in the temporal lobe (DeRamus and Kana 2015; Goch et al. 2014). Correspondingly, our results are in line with previous sMRI studies that showed an increased cortical thickness in individuals with autism (Hardan et al. 2006; Raznahan et al. 2010; Wallace et al. 2013). However, caution is needed when comparing previous investigations with the present study. While the majority of previous sMRI studies on schizophrenia and autism found statistically significant differences in cortical area between both disorders and healthy controls (Rimol et al. 2012), we did not find any differences in cortical area among the three study groups. Since cortical area might counteract the cortical thinning in neurodevelopmental disorders (Rimol et al. 2012), morphological differences between the three study groups might be exclusively driven by changes of cortical thickness rather than cortical area (Rimol et al. 2012). Nevertheless, other variables may also account for the divergence between our results and findings from other studies: First, previous sMRI studies investigated larger number of subjects. This may increase power yet at the same time it may also increase the likelihood of trivial effects (Friston 2012). Second, in contrast and unlike in our study, previous sMRI studies included subsets of schizophrenia patients with different duration of illness and autistic individuals with different years of age and education (Zielinski et al. 2014). In the present study, all subjects were diagnosed with AS and had normal intelligence. Therefore, our autistic sample represents a clinically homogenous group with a high level of functionality requiring only basic support from others (Freitag 2014). Third, the comparison of our results with previous data is limited given that no other sMRI study simultaneously investigated cortical area in schizophrenia patients and AS individuals. From a methodological point of view, the majority of previous studies used voxel-based morphometry (VBM) to study differences in brain structure between schizophrenia and autism (Cheung et al. 2010). However, VBM alone may not capture the full spectrum of subtle morphological changes of the grey matter volume as defined by cortical thickness and area in both disorders (Palaniyappan and Liddle 2012b). Also, there is a little evidence that grey matter volume differences are more likely driven by cortical thickness changes rather than cortical area dysmaturation (Raznahan et al. 2010). In our study, individuals with AS exhibited brain regions with increased cortical thickness when compared to healthy individuals. Although these results did not survived Bonferroni correction for multiple comparisons, they are in line with previous sMRI studies that showed increased cortical thickness in individuals with autism (Hardan et al. 2006; Raznahan et al. 2010; Wallace et al. 2013). However, other sMRI studies showed decreased cortical thickness in autism when compared to healthy individuals (Chung et al. 2005; Hadjikhani et al. 2006). Since the interindividual differences in cortical thickness might also be related to age, intelligence (Shaw et al. 2006) and genetic factors (Shaw et al. 2007), MRI studies on individuals with schizophrenia and autism might be confounded by various disorder-specific factors which we are not yet aware of.
The main issue of this study was the comparison of schizophrenia patients and AS individuals with regard to neuroanatomical correlates of NSS. In the present study, NSS levels in schizophrenia were associated with reduced cortical thickness mainly in the precentral gyrus. Therefore, the present data provide further evidence for cortical patterns underlying NSS in schizophrenia and are in line with the theory of motor-dysfunction within the schizophrenia-spectrum (Hirjak et al. 2014c; Wheeler and Voineskos 2014; Zhao et al. 2014). On the other hand, AS individuals with higher NSS levels showed statistically significant associations between NSS levels and increased cortical thickness. This finding suggests that changes of cortical thickness might play an important role in the development of NSS in AS individuals. In particular, it is possible that this finding reflects compensatory mechanism to maintain proper sensorimotor and language functions, perhaps due to reduced grey matter volume in fronto-temporal regions as suggested by previous sMRI studies (DeRamus and Kana 2015). At present, however, the neural substrates underlying NSS in individuals with AS are still poorly defined.
Local Gyrification Index
The findings of reduced LGI in the left caudal middle frontal gyrus, the left inferior parietal lobule, the left pericalcarine gyrus, the right precentral gyrus and the right temporal lobe are not surprising given the wealth of neuroimaging studies on reduced gyrification in schizophrenia (Nanda et al. 2014; Nesvag et al. 2014). Although our findings are in good accordance with previous sMRI studies (Nanda et al. 2014; Nesvag et al. 2014) that used the same analysis method, only two regions reached statistical significance. Therefore it is noteworthy that distinct variables such as sample size, demographics and psychometric heterogeneity may account for this divergence. Furthermore, we confirm that individuals with AS have a decreased LGI in the left caudal middle frontal gyrus and the right inferior temporal gyrus when compared to healthy individuals, in line with the theory of brain gyrification decrease during typical development and recent sMRI study using LGI measure in autism (Schaer et al. 2013). Furthermore, a review of existing evidence indicates that significant decrease of gyrification is detectable with increasing age (Raznahan et al. 2010). Here, LGI decrease in AS did not survive Bonferroni correction for multiple comparisons. To a certain extent, these findings are in line with previous sMRI studies, which did not report significant LGI differences in autism compared to healthy individuals (Casanova et al. 2009; Kates et al. 2009). Nevertheless, comparisons with previous studies should be treated with caution considering different LGI calculation methods and population differences (children or adolescents vs. adults).
Next, we examined association between NSS performance and LGI in both diagnostic groups. The analysis of cortical folding might provide complementary information regarding the exact nature of potential cortical changes in subjects with higher NSS levels, which have been suggested by previous NSS studies (Hirjak et al. 2014b, c). However, there is a paucity of evidence regarding the exact pattern and degree of cortical folding underlying NSS in schizophrenia and AS (Hirjak et al. 2014a). In the present study, higher NSS scores on the subscales COMT and RLSPO were significantly associated with reduced LGI in the left caudal middle frontal gyrus, left the inferior parietal lobule, the right precentral gyrus and the temporal pole in schizophrenia patients. These findings corroborate previous sMRI studies that supported the hypothesis that morphological changes in fronto-temporo-parietal regions might account for the development of sensorimotor NSS (Heuser et al. 2011; Hirjak et al. 2015a; Thomann et al. 2009). Our data are in line with functions of frontal brain areas that are involved not only in motor functions and sensory information processing but also in executive functioning and attention. Furthermore, these associations fit with previous postulations that reduced LGI in schizophrenia reflects the early-disrupted cortical growth and might represent a useful cytoarchitectural measure to assess microstructural patterns underlying early sensory-motor abnormalities and vulnerability to schizophrenia (Haukvik et al. 2012; Nesvag et al. 2014; Rimol et al. 2010). Also, our findings of significant associations between reduced LGI and NSS performance are partly consistent with previous studies which investigated cortical gyrification in psychotic disorders such as schizophrenia (Hirjak et al. 2015a; Nanda et al. 2014; Nesvag et al. 2014; Palaniyappan and Liddle 2012a). Taken together, the requirements of the COMT and RLSPO subscales correspond with reduced LGI of fronto-temporo-parietal cerebral sites found to be associated with them.
In AS individuals, we found no statistically significant relationship between higher NSS scores and reduced LGI. These findings are somehow surprising. Although there was a widespread reduction of LGI in AS individuals, these atrophic patterns account not for higher NSS scores. This suggests that reduced LGI is a result of early neurodevelopmental process associated with the diagnosis of autism rather than a specific pathomechanism of NSS in AS.
In summary, it appears plausible that not just one but a number of cortical parameters can enhance our understanding of the neurobiological underpinnings of NSS in neurodevelopmental disorders such as schizophrenia and AS. While higher NSS levels in schizophrenia were associated with reduced cortical thickness and LGI, AS individuals with higher NSS showed cortical thickening in fronto-temporal areas. With regard to the relationship between NSS performance and cortical thickness changes, the association directionality substantially differed between schizophrenia and AS. Reasons for opposed associations might point towards disorder-specific neural mechanisms underlying NSS. In conjunction, the present results indicate that NSS in patients with schizophrenia and AS individuals could be related to different brain phenotypes reflecting different neurodevelopmental pathways. This dimensional approach might provide substantial information on a “motor phenotype” within schizophrenia and AS in terms of behaviour and brain structure (Hirjak et al. 2015b). Last but not least, the results of the present study provide preliminary evidence that the combination of behavioral data and cortical measurements might be useful to predict the diagnosis of schizophrenia and AS. Since motor symptoms and signs are present in a number of neuropsychiatric disorders, we strongly advocate larger multimodal neuroimaging studies across psychiatric diagnoses combining dimensions of behavior and neurobiology in order to identify novel and biologically reliable phenotypes in psychiatry (Hirjak et al. 2015b).
Strengths and Limitations
This study has both strengths and limitations. A major strength involves the perfect match for age, gender, handedness and years of education. However, it was not possible to match the three study groups for IQ. For these reasons, we can reasonably conclude that possible differences in IQ might have affected our results. Another strength of this study is the use of comprehensive set of cortical surface parameters in schizophrenia patients and AS individuals exhibiting NSS. Finally, our findings are bolstered by the rigorous correction for multiple comparisons. Despite the study’s strengths certain limitations may constrain the generalizability of our results: First, the cross-sectional design may be seen as a limitation of our study. Second, the size of our three study groups may be seen as a further limitation and implies that the findings cannot be generalized to all subjects suffering from neurodevelopmental disorders. Third, it is difficult to compare our findings with those presented by recent neuroimaging studies on NSS because of the use of different data analysis techniques (voxel-based morphometry vs. surface-based methods) and missing autistic cohort. In this respect, we strongly advocate studies, both in healthy individuals and populations with high-functioning autism.
Conclusion
Structural MRI acquisition and data analysis methods with fine-grained clinical examination can result in a comprehensive characterization of structural brain changes that are presumed to underlie NSS in schizophrenia and AS. Our results support the hypothesis that schizophrenia patients and individuals with AS share disorder-specific structural substrates underlying NSS. The combination of behavioral data with markers of cortical integrity might be useful to predict the diagnosis of schizophrenia and AS. With respect to NSS, this approach could be also helpful in delineating distinct “motor endophenotypes”, thus increasing precision and reliability in diagnosing neurodevelopmental disorders.
References
Allen G, Muller RA, Courchesne E (2004) Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol Psychiatry 56:269–278
Andreasen NC (1983) The Scale for the Assessment of Negative Symptoms (SANS). University of Iowa, Iowa City, Iowa
Andreasen NC (1984) The Scale for the Assessment of Positive Symptoms (SAPS). University of Iowa, Iowa City, Iowa
Andreasen NC, Paradiso S, O DS (1998) “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 24:203–218
Bachmann S, Bottmer C, Schroder J (2005) Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry 162:2337–2343
Baron-Cohen S, Hoekstra RA, Knickmeyer R, Wheelwright S (2006) The Autism-Spectrum Quotient (AQ)—adolescent version. J Autism Dev Disord 36:343–350
Bernard JA, Mittal VA (2015) Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 45(13):2685–2689. doi:10.1017/S0033291715000872
Bhojraj TS, Sweeney JA, Prasad KM, Eack S, Rajarethinam R, Francis AN et al (2011) Progressive alterations of the auditory association areas in young non-psychotic offspring of schizophrenia patients. J Psychiatr Res 45:205–212
Bombin I, Arango C, Buchanan RW (2005) Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull 31:962–977
Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R et al (2009) Reduced gyral window and corpus callosum size in autism: possible macroscopic correlates of a minicolumnopathy. J Autism Dev Disord 39:751–764
Chan RC, Gottesman II (2008) Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci Biobehav Rev 32:957–971
Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q et al (2010) Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS One 5:e12233
Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC (2005) Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage 25:1256–1265
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194
DeRamus TP, Kana RK (2015) Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. NeuroImage Clin 7:525–536
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Esposito G, Pasca SP (2013) Motor abnormalities as a putative endophenotype for Autism Spectrum Disorders. Front Integr Neurosci 7:43
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9:195–207
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH et al (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22
Freitag CM (2014) Autism Spectrum Disorder in DSM-5—concept, validity, and reliability, impact on clinical care and future research. Z Kinder Jugendpsychiatr Psychother 42:185–192
Friston K (2012) Ten ironic rules for non-statistical reviewers. Neuroimage. 61:1300–1310
Gallese V, Keysers C, Rizzolatti G (2004) A unifying view of the basis of social cognition. Trends Cogn Sci 8:396–403
Goch CJ, Stieltjes B, Henze R, Hering J, Poustka L, Meinzer HP et al (2014) Quantification of changes in language-related brain areas in autism spectrum disorders using large-scale network analysis. Int J Comput Assist Radiol Surg 9(3):357–365
Guy E (1976) Abnormal involuntary movement scale. Rockwille, MD
Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H (2006) Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 16:1276–1282
Hall RC (1995) Global assessment of functioning. A modified scale. Psychosomatics. 36:267–275
Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ (2006) An MRI study of increased cortical thickness in autism. Am J Psychiatry 163:1290–1292
Haukvik UK, Schaer M, Nesvag R, McNeil T, Hartberg CB, Jonsson EG et al (2012) Cortical folding in Broca’s area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol Med 42:1329–1337
Heuser M, Thomann PA, Essig M, Bachmann S, Schroder J (2011) Neurological signs and morphological cerebral changes in schizophrenia: an analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res 192:69–76
Hirjak D, Wolf RC, Koch SC, Mehl L, Kelbel JK, Kubera KM et al (2014a) Neurological abnormalities in recent-onset schizophrenia and asperger-syndrome. Front Psychiatry 5:91
Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Thomann PA (2014b) Multiparametric mapping of neurological soft signs in healthy adults. Brain Struct Funct. doi:10.1007/s00429-014-0964-9
Hirjak D, Wolf RC, Stieltjes B, Hauser T, Seidl U, Schroder J et al (2014c) Cortical signature of neurological soft signs in recent onset schizophrenia. Brain Topogr 27:296–306
Hirjak D, Kubera KM, Wolf RC, Thomann AK, Hell SK, Seidl U et al (2015a) Local brain gyrification as a marker of neurological soft signs in schizophrenia. Behav Brain Res 292:19–25
Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC (2015b) Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. doi:10.1016/j.schres.2015.10.022
Kates WR, Ikuta I, Burnette CP (2009) Gyrification patterns in monozygotic twin pairs varying in discordance for autism. Autism Res 2:267–278
Khan AR, Wang L, Beg MF (2008) FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using Large Deformation Diffeomorphic Metric Mapping. Neuroimage 41:735–746
Klein D, Rotarska-Jagiela A, Genc E, Sritharan S, Mohr H, Roux F et al (2014) Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One 9:e84914
Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F et al (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60:878–888
Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L et al (1989) Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 19:185–212
Mayoral M, Merchan-Naranjo J, Rapado M, Leiva M, Moreno C, Giraldez M et al (2010) Neurological soft signs in juvenile patients with Asperger syndrome, early-onset psychosis, and healthy controls. Early Interv Psychiatry. 4:283–290
Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J et al (2003) Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29:703–715
Nanda P, Tandon N, Mathew IT, Giakoumatos CI, Abhishekh HA, Clementz BA et al (2014) Local gyrification index in probands with psychotic disorders and their first-degree relatives. Biol Psychiatry 76:447–455
Nesvag R, Schaer M, Haukvik UK, Westlye LT, Rimol LM, Lange EH et al (2014) Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr Res 152:333–338
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Palaniyappan L, Liddle PF (2012a) Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci: JPN. 37:399–406
Palaniyappan L, Liddle PF (2012b) Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 60:693–699
Palaniyappan L, Liddle PF (2014) Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull 40:675–684
Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH (2010) Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 49:539–551, 51 e1–51 e4
Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q et al (2010) Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex 20:1332–1340
Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ Jr, Pung CJ et al (2010) Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 68:41–50
Rimol LM, Nesvag R, Hagler DJ Jr, Bergmann O, Fennema-Notestine C, Hartberg CB et al (2012) Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry 71:552–560
Rinehart NJ, Tonge BJ, Bradshaw JL, Iansek R, Enticott PG, McGinley J (2006) Gait function in high-functioning autism and Asperger’s disorder: evidence for basal-ganglia and cerebellar involvement? Eur Child Adolesc Psychiatry 15:256–264
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH et al (2002) Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 58:695–701
Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran JP, Eliez S (2012) How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J Vis Exp: JoVE. doi:10.3791/3417
Schaer M, Ottet MC, Scariati E, Dukes D, Franchini M, Eliez S et al (2013) Decreased frontal gyrification correlates with altered connectivity in children with autism. Front Hum Neurosci 7:750
Schroder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M et al (1991) Neurological soft signs in schizophrenia. Schizophr Res 6:25–30
Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, Schachtzabel C et al (2010a) Complex pattern of cortical thinning in schizophrenia: results from an automated surface based analysis of cortical thickness. Psychiatry Res 182:134–140
Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C et al (2010b) Reduced cortical thickness in first episode schizophrenia. Schizophr Res 116:204–209
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK et al (2004) A hybrid approach to the skull stripping problem in MRI. NeuroImage. 22:1060–1075
Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. (2006) Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63(5):540–549.
Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. (2007) Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol 6(6):494–500
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Strauss JS, Carpenter WT Jr (1974) The prediction of outcome in schizophrenia. II. Relationships between predictor and outcome variables: a report from the WHO international pilot study of schizophrenia. Arch Gen Psychiatry 31:37–42
Tani P, Lindberg N, Appelberg B, Nieminen-von Wendt T, von Wendt L, Porkka-Heiskanen T (2006) Clinical neurological abnormalities in young adults with Asperger syndrome. Psychiatry Clin Neurosci 60:253–255
Teixeira S, Machado S, Velasques B, Sanfim A, Minc D, Peressutti C et al (2014) Integrative parietal cortex processes: neurological and psychiatric aspects. J Neurol Sci 338:12–22
Thomann PA, Wustenberg T, Santos VD, Bachmann S, Essig M, Schroder J (2009) Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med 39:371–379
Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A (2013) Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 136:1956–1967
Weiß RH (2006) CFT 20-R Grundintelligenztest Skala 2—Revision. Hogrefe, Göttingen
Wheeler AL, Voineskos AN (2014) A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci 8:653
Whyatt C, Craig C (2013) Sensory-motor problems in Autism. Front Integr Neurosci 7:51
Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M (1997a) SKID-I: Strukturiertes klinisches Interview für DSM-IV. Hogrefe, Göttingen
Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M (1997b) SKID-I: Strukturiertes klinisches Interview für DSM-IV. Hogrefe, Göttingen
Woodbury-Smith MR, Robinson J, Wheelwright S, Baron-Cohen S (2005) Screening adults for Asperger Syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. J Autism Dev Disord 35:331–335
Zhao Q, Li Z, Huang J, Yan C, Dazzan P, Pantelis C et al (2014) Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ALE meta-analysis. Schizophr Bull. 40(3):626–641
Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS et al (2014) Longitudinal changes in cortical thickness in autism and typical development. Brain. 137:1799–1812
Acknowledgments
The authors cordially thank all participants for participating and their interest in this study. We also thank Maik Sieber (SALO GmbH) for his help with the recruitment of AS individuals. Further, we thank the two anonymous reviewers for their valuable comments. This work was supported by the Marie-Curie Initial Training Network TESIS: “Toward an Embodied Science of InterSubjectivity” (FP7-PEOPLE-2010-ITN, 264828).
Author contributions
DH designed the study, performed image processing and statistical analyses, undertook neurological, psychopathological and psychometric assessments, and wrote the manuscript. RCW supervised image processing and statistical analyses and contributed to the interpretation of the results. BS and KHM implemented the image acquisition sequence and supervised MRI procedures. IP, KMK and AKT were involved in designing the study and in the interpretation of the results. PAT designed the study, supervised image processing and statistical analyses, wrote the manuscript, and supervised diagnostic procedures. All authors contributed to and have approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Rights and permissions
About this article
Cite this article
Hirjak, D., Wolf, R.C., Paternoga, I. et al. Neuroanatomical Markers of Neurological Soft Signs in Recent-Onset Schizophrenia and Asperger-Syndrome. Brain Topogr 29, 382–394 (2016). https://doi.org/10.1007/s10548-015-0468-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-015-0468-9