Abstract
Epilepsy may affect connectivity between the putamen and cortex even during the resting state. Putamen is part of the basal ganglia resting state network (BG-RSN) which is anti-correlated with the default mode network (DMN) in healthy subjects. Therefore, we aimed at studying the functional brain connectivity (FC) of the putamen with the cortical areas engaged in the DMN as well as with the primary somatomotor cortex which is a cortical region engaged in the BG-RSN. We compared the data obtained in patients with epilepsy with that in healthy controls (HC). Functional magnetic resonance imaging (fMRI) was performed in 10 HC and 24 patients with epilepsy: 14 patients with extratemporal epilepsy (PE) and 10 patients with temporal epilepsy (PT). Resting state fMRI data was obtained using the 1.5 T Siemens Symphony scanner. The Group ICA of fMRI Toolbox (GIFT) program was used for independent component analysis. The component representing the DMN was chosen according to a spatial correlation with a mask typical for DMN. The FC between the putamen and the primary somatomotor cortex was studied to assess the connectivity of the putamen within the BG-RSN. A second-level analysis was calculated to evaluate differences among the groups using SPM software. In patients with epilepsy as compared to HC, the magnitude of anti-correlation between the putamen and brain regions engaged in the DMN was significantly lower. In fact, the correlation changed the connectivity direction from negative in HC to positive in PE and PT. The disturbed FC of the BG in patients with epilepsy as compared with HC was further illustrated by a significant decrease in connectivity between the left/right putamen and the left/right somatomotor cortex, i.e. between regions that are engaged in the BG-RSN. The FC between the putamen and the cortex is disturbed in patients with epilepsy. This may reflect an altered function of the BG in epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in the role of the basal ganglia (BG) in epilepsy has increased recently in terms of their prospective use as deep brain stimulation targets for treating seizures. The BG exercise remote control on widespread cortical areas and may exercise control on cortical epileptic activities. According to several experimental and clinical studies (for review, see Rektor et al. 2012), including our direct ictal depth electrode recordings from the BG and putamen in particular (Rektor et al. 2002; Rektor et al. 2011), the putamen and other BG nuclei may be involved in the inhibition of epileptic seizures. In the present study, we hypothesize that the connectivity between the putamen and cortex is affected in epilepsy even during the resting state.
For this purpose we performed two functional connectivity (FC) studies using functional magnetic resonance imaging (fMRI). We studied the FC of the putamen at rest in patients with epilepsy as compared with healthy control subjects. We chose to study the connectivity between the putamen and the default mode network (DMN), and we also aimed at exploring the FC between the putamen and the primary somatomotor cortex. What was the reason for that? It has been shown that the putamen is one of the major nodes involved in the BG resting state network (BG-RSN) which is consistent with the cortico-subcortical motor control circuit of the basal ganglia and is robustly reproducible across subjects, scanning parameters and common behavioural conditions for resting state studies (Robinson et al., 2009). It has been clearly shown by the same group (Robinson et al., 2009) that the BG-RSN is anti-correlated with the DMN in healthy controls while the frequency spectrum of this component is most similar in frequency composition to the DMN. The DMN is indeed the major resting state brain network that has been largely studied recently, and which is known to be altered in different neurological and psychiatric diseases including the epilepsy (e.g. Fox and Raichle 2007; Raichle and Snyder 2007; Gotman 2008; Kobayashi et al. 2009; Salek-Haddadi et al. 2006, 2003; Wang et al. 2011; Zhang et al. 2010). We hypothesized that by studying and comparing the DMN connectivity in different groups of subjects we will be able to assess both the correlations between the core regions engaged in the DMN and the magnitude of anti-correlations between the DMN and the putamen.
In case that the FC between the putamen and the core brain areas engaged in the DMN was altered in epilepsy as compared with healthy controls one may think of two possible explanations: 1 Changes in FC between the putamen and the DMN are caused purely by the DMN disturbance in epilepsy; 2 There is a more general impairment of the connectivity between the putamen and cortex in epilepsy. In order to shed additional light on this we also aimed at studying the FC between the putamen and the primary somatomotor cortex, i.e. between the subcortical region and the cortical area that are both engaged in the BG-RSN (Robinson et al. 2009).
Methods
Subjects
The study included 10 healthy control subjects (HC; 9 males, 1 female; mean age 25.1 ± 3.0 years) and 24 patients with epilepsy: 14 patients with extratemporal epilepsy (PE; 8 males, 6 females; mean age 29.9 ± 9.5 years), and 10 patients with temporal epilepsy (PT; 2 males, 8 females; mean age 40.6 ± 13.5 years). The patients with epilepsy were consecutive patients who underwent long-term diagnostic video-EEG monitoring at the Epilepsy Monitoring Unit in Brno. All underwent a standardized epileptologic assessment including ictal scalp or scalp-sphenoidal video/EEG monitoring, brain MRI, and neuropsychology. Further investigations were performed for some patients, e.g. SPECT, PET, advanced MRI methods, and invasive exploration. PE was heterogenic (frontal lobe epilepsy n = 8; parieto-occipital n = 4; idiopathic generalized epilepsy n = 1; progressive myoclonic epilepsy n = 1; cortical dysplasia n = 4; glioma n = 1, and non-lesional epilepsy n = 7 patients). PT was more homogenous: all of the patients had mesiotemporal epilepsy. In PT, 4 patients had hippocampal sclerosis, 1 had a temporobasal cystic tumour, and in 5 the epilepsy was non-lesional. Informed consent was obtained from each participant prior to the experiment. The study received the approval of the local ethics committee. A neuropsychological examination excluded severe cognitive disturbances and dementia in each patient. All the patients had normal motor performance, normal hearing, and normal or corrected-to-normal vision. All patients were on antiepileptic medication.
Study Design
Resting state fMRI data (lasted 15 min), acquired with no explicit task, were obtained from PE, PT, and HC. Subjects were instructed to keep their eyes closed and not to fall asleep during these resting state sessions. After functional measurement, structural images were obtained. All patients were asked for the presence of epileptic seizure during measurement; none reported any. During functional measurement, simultaneous EEG data were acquired for epileptic patients using the BrainAmp MR 32-channel EEG system. None of the patients fell asleep or had an epileptic seizure during the MRI scanning.
MR Imaging
Imaging was performed on a 1.5 T Siemens Magnetom Symphony scanner. We obtained 300 functional scans from each subject during one 15-min run. Functional images were acquired using a gradient echo echoplanar imaging (EPI) sequence with the following parameters: RT (scan repeat time) = 3,000 ms, TE = 40 ms, FOV = 220 mm, flip angle = 90°, matrix size 64 × 64, slice thickness = 3.5 mm, 32 transversal slices per scan. Following functional measurements, high-resolution anatomical T1-weighted images were acquired using a 3D sequence that served as a matrix for the functional imaging (160 sagittal slices, resolution 256 × 256 interpolated to 512 × 512, slice thickness = 1.17 mm, TR = 1,700 ms, TE = 3.96 ms, FOV = 246 mm, flip angle = 15°).
Data Processing: Independent Component Analysis (ICA)
Acquired data were pre-processed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/). Functional scans were realigned to the first scan of the series, spatially normalized into the standard anatomical space according to statistical parametric mapping as defined by the Montreal Neurological Institute (SPM-MNI), and spatially smoothed using a Gaussian filter with an FWHM of 8 mm. The voxel size generated from the above acquisition parameters was oversampled to 3 × 3 × 3 mm. Group ICA was used to separate the blood oxygen level-dependent (BOLD) signal into spatially independent components using the Group ICA of fMRI Toolbox (GIFT) program (http://icatb.sourceforge.net/) (Calhoun et al. 2001). ICA was performed separately for each group (HC, PT, and PE). Conjunction of masks from all participants was used for the separate ICAs in order to ensure that only common voxels would be analysed. Data was intensity normalized during GIFT pre-processing. Group ICA was performed with 2 reduction steps. The optimal number of components was chosen according to the minimum descriptive length (MDL) criteria (Li et al. 2007). The maximum MDL across subjects was used for the first reduction, and the mean MDL was used for the second one. Independent components were calculated using the Infomax algorithm. Back reconstruction from aggregate components was done using the GICA3 method. No scaling was applied to final independent components.
Components related to the DMN (one each for HC, PE, and PT) were chosen from group components using the DMN mask included in GIFT program (Garrity 2007). The DMN component selected for HC was used in combination with the DMN mask to select proper components from PE and PT (using spatial multiple regression within GIFT). Subsequently, corresponding individual subject components from the ICA back-reconstruction process were selected to assess between-group effects in SPM5. The second-level statistics analysis was done using one-way ANOVA (full factorial design in SPM5 with 1 factor and 3 levels) for the 3 groups (HC, PE, and PT) with age and gender as covariates. The resulting SPM were thresholded at p < 0.05 using FWE correction for multiple comparisons and minimal cluster size was set up to 3 voxels.
Functional Connectivity (FC) Analysis Between Our Regions of Interest
FC calculated as correlation coefficients between selected nodes was studied to assess the connectivity of the putamen within the BG-RSN. Representative signals from left and right primary somatomotor cortex and left and right putamen were extracted. Voxels for the selected regions/nodes were selected using an anatomical mask as implemented in WFU_PickAtlas software (Maldjian et al. 2004, 2003). Brodmann area 4 (BA4) was used to define the primary somatomotor cortex. The putamen has a direct definition within the included AAL atlas. For each region, representative time-series within the region were calculated as an eigenvariate of the time-series from all voxels in this region. Correlation coefficients were calculated for each pair of representative signals. These coefficients were calculated for each subject, and then functional connectivity at the group level was studied using the Mann–Whitney U Test to assess if there were any significant differences between groups (HC vs. all patients with epilepsy, HC vs. PT, HC vs. PE). The false discovery rate (FDR) was set to p < 0.05 to correct for multiple comparisons.
Results
Demographic/Behavioural Results
The groups were not fully homogeneous, as the age difference was significant between HC and PT and between PE and PT (using ANOVA with p < 0.05 and t test with p < 0.05). There was a significantly different gender ratio between HC and PT (p = 0.0025, Mann–Whitney U test). We used age and gender as covariates for all of our second-level data analyses.
The DMN Connectivity Results: Differences Between Groups
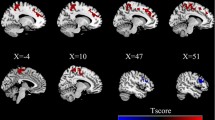
The DMN component for HC was significantly correlated with the DMN mask (r = 0.60). DMN components for PE and PT were significantly correlated both with the DMN mask (r = 0.56 and 0.58), and with the DMN component for HC (r = 0.82 and 0.87), respectively. Our results confirmed typical and robust DMN engagement in all 3 explored groups (see Fig. 1). The most involved regions included the posterior cingulate cortex and precuneus, medial frontal gyrus and anterior cingulate cortex, and superior and middle temporal gyri on both sides. The covariates age and gender were insignificant at all voxels (p < 0.05, FWE corrected).
The differences between HC, PE, and PT are summarized in Table 1. In PE/PT as compared with HC, the DMN component loadings were significantly decreased specifically in the cortical brain areas engaged in the DMN including the posterior core nodes of the DMN (i.e. in the posterior cingulate cortex and precuneus), the left superior frontal gyrus, left postcentral gyrus, and the right superior temporal gyrus, i.e. the connectivity within the DMN was decreased in patients with epilepsy as compared with HC. Conversely, in PE/PT as compared with HC, the DMN component loadings were significantly increased in the left lingual gyrus, left and right putamen, right insula/inferior frontal gyrus, and left inferior frontal gyrus, i.e. the connectivity between the DMN and specific brain areas outside of the DMN were increased in patients with epilepsy as compared with HC.
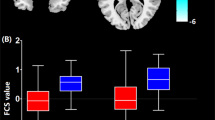
As outlined above, we were specifically interested in the connectivity of the DMN with the putamen. In HC, the putamen displayed significant negative values of connectivity within the DMN component (ICA component loadings), while in PE and PT these values were non-significant and slightly positive (see Fig. 2). The right and left putamen in PE and the right putamen in PT were significantly less anti-correlated with the DMN than in HC (see bar graphs in Fig. 2). Thus, the putamen is not significantly engaged within the DMN component in epileptic patients; moreover, its connectivity is changed from negative in HC to positive in PE and PT.
Differences between a HC and PE or b HC and PT within putamen. Statistical parametric maps of DMN components were thresholded at a level of significance p < 0.05, FWE corrected, with a minimal cluster size of 3 voxels. The bar graph on the right side shows subjects’ ICA loading for cluster maxima in the putamen (marked by a circle on the left side)
FC Between the Putamen and the Primary Somatomotor Area (BA4)
We tested if there were any significant differences between HC and patients (combined data from PE and PT) in the connectivity of the analysed regions. We found a significantly decreased connectivity between the left (right) putamen and both the left and right BA4 (p < 0.05 FDR) in the patients as compared with HC, see Fig. 3a.
Differences in connectivity between a HC and all patients (combined data from PE and PT), b HC and PE, c HC and PT. The solid lines depict connections with significant differences between groups (p < 0.05 FDR). The dotted lines depict connections that showed similar trends but did not reach statistical significance (p < 0.05 uncorrected). All correlations displayed decreased connectivity in patients with epilepsy as compared with HC
The same analysis was then done separately for comparing HC and PE, and HC and PT. The connectivity between the left (right) putamen and both the left and right BA4 were decreased in PE as compared with HC, but this difference did not reach statistical significance (Fig. 3b). The same was true for PT as compared with HC (Fig. 3c). We found significantly decreased connectivity between the two putamen in PT as compared with HC (p < 0.05 FDR), (Fig. 3c).
Discussion
It is well known that the epileptic process affects brain areas well beyond the epileptogenic cortex. The FC within large-scale physiological brain networks may be impaired in both localized and generalized epilepsies, and may be present either on the side of the epileptic zone or bilaterally (Bettus et al. 2010; Frings et al. 2009; Laufs et al. 2007; Morgan et al. 2010). It has been shown that the epileptic activity can lead to the inhibition of widespread physiological networks via subcortical structures (Blumenfeld 2003; Federico et al. 2005; Laufs et al. 2007).
Epilepsy has frequently been explored using spike-related EEG-fMRI measurements. Interictal discharges (IED) are consistently associated with the BOLD signal changes in the epileptic focus as well as in distant cortical and subcortical sites, including the BG (Federico et al. 2005; Archer et al. 2003; Hamandi et al. 2008). This study investigated the correlation of spontaneous activity within and between the two brain resting state networks in patients with epilepsy as compared with HC.
For studying the DMN, we used the ICA approach, which enables to explore the FC in the brain affected by epilepsy but does not rely on scalp-recorded IED. ICA has been largely used for studying the FC of coherent spontaneous neuronal activity including the DMN (Greicius et al. 2008; Luo et al. 2012; Wang et al. 2011; Zhang et al. 2010). In our study, in patients with epilepsy as compared with HC, the ICA analysis displayed significant decreases of the FC within regions typical for the DMN, particularly in the posterior cingulate and precuneus. This finding is in line with other studies (Frings et al. 2009; Zhang et al. 2010, 2009), and most likely reflects the intrinsic property of the DMN that is altered by epilepsy.
As for the BG-RSN, we replicated the results of Robinson et al. (2009) in showing that in HC the putamen is functionally anti-correlated with typical DMN regions. We demonstrated for the first time that in epilepsy patients the putamen was not significantly anti-correlated with the DMN regions. Moreover, the FC changed from negative (observed in HC) to positive (seen in PE and PT). In addition to this result, we also showed for the first time that the connectivity between the putamen and BA 4 (i.e. the subcortical and cortical areas that are engaged in the BG-RSN) is decreased in epilepsy patients as compared to HC. Of note, we found similar disturbances in the PE and PT groups as compared to HC.
In epilepsy, the bulk of the reported data in the literature concern the striatum (for review Rektor et al. 2012). The BG form a very complex system of nuclei and pathways. The BG may act as an integrated system due to the unidirectional character of the major connections and the information being transformed and transmitted from the cortex via the BG back into the cortex. Increased FC between the nuclei composing the BG was observed in idiopathic generalized epilepsies (Luo et al. 2012), indicating that also in epilepsy the BG act as an integrated system, probably within the well-known BG-thalamocortical circuitries.
We conclude that the two independent analyses performed in the same cohorts show that the FC between the putamen and cortical areas involved in the DMN and the BG-RSN are altered in epilepsy. The disturbed connectivity between the putamen and cortex at rest may reflect an altered inhibitory function of the BG in patients with epilepsy but this study does not provide specific evidence for this assumption.
There were several limitations to this study. It is unlikely that the heterogeneity of the studied groups in terms of their age and gender influenced the results since these regressors had no significant impact on fMRI results. Nevertheless, the possible effects of age and gender cannot be fully excluded. More questionable is the fact that our patients were treated with antiepileptic drugs. The functional connectivity within the DMN was significantly decreased during conscious sedation with midazolam in healthy subjects (Greicius et al. 2008). Further studies are needed to effectively determine the possible impact of antiepileptic drugs on the FC within different resting state networks. Another possible confounder is the impact of epilepsy on the cognitive status of patients. Although our patients did not suffer from serious cognitive impairment, epilepsy may lead to subtle cognitive deficits. Diseases with cognitive impairment (and neurodegenerative brain diseases such as Alzheimer’s disease in particular) may have a negative impact on the DMN connectivity (e.g. Broyd et al. 2009; Greicius et al. 2004; Rektorova et al. 2012) but this may rather be caused by the pathological protein aggregation within the core DMN regions (Buckner et al. 2005).
Furthermore, due to the limited sample size, we were not able to perform an analysis of the possible impact of seizure-related factors such as type, frequency, duration, and underlying pathology.
Conclusion
We used fMRI in order to study the FC between the putamen and the cortex in patients with extra-temporal and temporal lobe epilepsies. We found that in epileptic patients as compared with HC, both the physiological anti-correlation between the putamen and the core regions of the DMN and the physiological positive correlation between the putamen and the cortical region of the BG-RSN were decreased. This observation may be interpreted as a sign of an altered or modified function of the BG in epilepsy (for review, see Rektor et al. 2012). Restoring the functional integrity of the BG circuitries as a treatment of epilepsy, e.g. by deep brain stimulation (Saillet et al. 2009), deserves further research.
References
Archer JS, Abbott DF, Waites AB, Jackson GD (2003) FMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage 20:1915–1922
Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M (2010) Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 81:1147–1154
Blumenfeld H (2003) From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy. Epilepsia 44(Suppl 2):7–15
Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS (2009) Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 33(3):279–296
Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R et al (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717
Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14(3):140–151
Federico P, Archer JS, Abbott DF, Jackson GD (2005) Cortical/subcortical BOLD changes associated with epileptic discharges—an EEG-fMRI study at 3 T. Neurology 64:1125–1130
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosc 8:700–711
Frings L, Schulze-Bonhage A, Spreer J, Wagner K (2009) Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol 256:2021–2029
Garrity (2007) Aberrant ‘default mode’ functional connectivity in schizophrenia. Am J Psychiatry 164:1123
Gotman J (2008) Epileptic networks studied with EEG-fMRI. Epilepsia 49:42–51
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642
Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V (2008) Persistent default-mode network connectivity during light sedation. Hum Brain Mapp 29:839–847
Hamandi K, Laufs H, Noth U, Carmichael DW, Duncan JS, Lemieuxa L (2008) BOLD and perfusion changes during epileptic generalised spike wave activity. Neuroimage 39:608–618
Kobayashi E, Grova C, Tyvaert L, Dubeau F, Gotman J (2009) Structures involved at the time of temporal lobe spikes revealed by interindividual group analysis of EEG/fMRI data. Epilepsia 50:2549–2556
Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L (2007) Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp 28:1023–1032
Li YO, Adali T, Calhoun VD (2007) Estimating the number of independent components for functional magnetic resonance Imaging data. Hum Brain Mapp 28:1251–1266
Luo C, Li Q, Xia Y, Lei X, Xue K, Yao Z, Lai Y, Marti Nez-Montes E, Liao W, Zhou D et al (2012) Resting state basal ganglia network in idiopathic generalized epilepsy. Hum Brain Mapp 33:1279–1294
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239
Maldjian JA, Laurienti PJ, Burdette JH (2004) Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 80(21):450–455
Morgan VL, Gore JC, Abou-Khalil B (2010) Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res 88(2–3):168–178
Raichle ME, Snyder AZ (2007) A default mode of brain function: a brief history of an evolving idea. Neuroimage 37:1083–1090
Rektor I, Kuba R, Brazdil M (2002) Interictal and ictal EEG activity in the basal ganglia: an SEEG study in patients with temporal lobe epilepsy. Epilepsia 43:253–262
Rektor I, Kuba R, Brázdil M, Halámek J, Jurák P (2011) Ictal and peri-ictal oscillations in the human basal ganglia in temporal lobe epilepsy. Epilepsy Behav 20:512–517
Rektor I, Kuba R, Brázdil M, Chrastina J (2012) Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav 25:56–59
Rektorova I, Krajcovicova L, Marecek R, Mikl M (2012) Default mode network and extrastriate visual resting state network in patients with Parkinson’s disease dementia. Neurodegener Dis 10(1–4):232–237
Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin-Exner I, Bauer H, Moser E (2009) A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci 10:137
Saillet S, Langlois M, Feddersen B, Minotti L, Vercueil L, Chabardes S, David O, Depaulis A, Deransart C, Kahane P (2009) Manipulating the epileptic brain using stimulation: a review of experimental and clinical studies. Epileptic Disord 11:100–112
Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR (2003) Functional magnetic resonance imaging of human absence seizures. Ann Neurol 53:663–667
Salek-Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, Duncan J, Fish D, Lemieux L (2006) Hemodynarnic correlates of epileptiform discharges: an EEG-fMRI study of 63 patients with focal epilepsy. Brain Res 1088:148–166
Wang ZG, Lu GM, Zhang ZQ, Zhong YA, Jiao Q, Zhang ZJ, Tan QF, Tian L, Chen GH, Liao W et al (2011) Altered resting state networks in epileptic patients with generalized tonic-clonic seizures. Brain Res 1374:134–141
Zhang ZQ, Lu GM, Zhong Y, Tan QF, Yang ZG, Liao W, Chen ZL, Shi JX, Liu YJ (2009) Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett 458:97–101
Zhang ZQ, Lu GM, Zhong Y, Tan QF, Liao W, Wang ZG, Wang ZQ, Li K, Chen HF, Liu YJ (2010) Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res 1323:152–160
Acknowledgments
The study was supported by the project “CEITEC—Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from European Regional Development Fund and by the grant GACR P304/11/1318.
Conflicts of interest
None of the authors has any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rektor, I., Tomčík, J., Mikl, M. et al. Association Between the Basal Ganglia and Large-Scale Brain Networks in Epilepsy. Brain Topogr 26, 355–362 (2013). https://doi.org/10.1007/s10548-012-0272-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-012-0272-8