Abstract
In the healthy human brain the hippocampus is known to work in concert with a variety of cortical brain regions. It has recently been linked to the default network of the brain, with the precuneus being its core hub. Here we studied the remote effects of damage to the hippocampus on functional connectivity patterns of the precuneus. From 14 epilepsy patients with selective, unilateral hippocampal sclerosis and 8 healthy control subjects, we acquired functional MRI data during performance of an object-location memory task. We assessed functional connectivity of a functionally defined region in the precuneus, which showed the typical properties of the default network: significant task-related deactivation, which was reduced in patients compared to control subjects. In control subjects, a largely symmetrical pattern of functional coherence to the precuneus emerged, including canonical default network areas such as ventral medial prefrontal cortex, inferior parietal cortex, and the hippocampi. Assessment of group differences within the default network areas revealed reduced connectivity to the precuneus in ipsilesional middle temporal gyrus and hippocampus in left hippocampal sclerosis patients compared to controls. Furthermore, left hippocampal sclerosis patients showed lower connectivity than right hippocampal sclerosis patients in left middle temporal gyrus, ventral medial prefrontal cortex, and left amygdala. We report remote effects of unilateral hippocampal damage on functional connectivity between distant brain regions associated with the default network of the human brain. These preliminary results underline the impact of circumscribed pathology on functionally connected brain regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The investigation of functional connectivity between brain regions with functional magnetic resonance imaging (fMRI) is a relatively new field within cognitive neuroscience researching the human brain in vivo. It allows researchers to identify networks of brain regions with similar blood oxygenation level-dependent (BOLD) signal fluctuations. This is taken as a surrogate marker of cooperation of brain regions and has been demonstrated to be detectable during different brain states, from instructed task performance [1] to resting states [2] and even during sleep [3]; for a review, see [4]. Functional coherence mapping approaches find an ideal target in the study of declarative memory, as this cognitive function requires the functional integrity of a widely distributed network working in concert [5].
In medial temporal lobe epilepsy (MTLE), declarative memory is typically the most profoundly affected cognitive function. The most common form of focal epilepsies is MTLE, and hippocampal sclerosis (HS) is its most frequent neuropathological substrate [6]. The underlying hippocampal pathology—though highly circumscribed—has an enormous impact on the patient’s daily life, which stems from seizures and declarative memory impairments. Present knowledge about human memory systems is built on seminal observations of memory deficits in a surgically treated MTLE patient [7], and material-specific memory deficits have been demonstrated in non-resected MTLE patients with hippocampal sclerosis [8, 9]. Investigations in this patient group might further elucidate memory network dysfunction at the brain systems level with the new technique of functional coherence mapping.
The impact of hippocampal sclerosis on remote brain structures has been shown previously, in terms of reduced gray matter concentration as assessed with MRI [10] and altered metabolism as measured with PET [11]. Using fMRI and functional connectivity analyses on regions of interest associated with verbal memory task processing, we were recently able to demonstrate that MTLE patients whose verbal memory performance declined after epilepsy surgery had a higher coupling of these brain regions prior to surgery, suggesting a greater functional integrity of the network compared to patients without postoperative memory impairment [12].
Often cognitive impairments in patients with HS are not restricted to declarative memory but include deficits in further cognitive domains [13]. This suggests an additional functional impairment of distant brain regions which do not show obvious structural alterations even with high-resolution MR imaging in these patients. Hippocampal damage as present in MTLE might thus be associated with changes in coherence patterns of areas with intense synaptic connections to the hippocampus.
Recent literature postulated that posterior midline cortex, comprising precuneus (PREC) and posterior cingulate cortex (PCC) constitutes the core hub within the default network of the human brain, with strong connections to ventral medial prefrontal cortex/anterior cingulate cortex (vmPFC/ACC), inferior lateral parietal cortex (IPC), and the hippocampi [14, 15]. We hypothesized that in MTLE with hippocampal sclerosis, damage to one node of the network might disturb connectivity between other nodes of this large-scale network. We thus aimed to test whether the precuneus would exhibit altered functional connectivity strengths to other parts of the network in MTLE patients.
Methods
Subjects
We included eight patients with left-sided HS (LHS), six patients with right-sided HS (RHS), and eight healthy control subjects (CON). Patients were recruited from the Freiburg University Epilepsy Center during presurgical evaluation. Written informed consent was obtained from each participant. The study was performed according to the Declaration of Helsinki of 1964 and approved by the Ethics Committee of the University of Freiburg.
Groups (LHS, RHS, CON) did not differ regarding age or handedness as assessed with the Edinburgh Handedness Questionnaire [16] (MANOVA, n.s.). Gender distribution was not different between groups (Chi-square test, n.s.). Patient groups did not differ concerning age at epilepsy onset nor duration of epilepsy (Mann–Whitney U test, n.s.; Table 1).
All patients had unilateral medial temporal lobe epilepsy and hippocampal sclerosis. The epileptogenic temporal lobe was identified by video-EEG-monitoring. Hippocampal sclerosis was diagnosed with MR imaging based on hippocampal atrophy plus increased signal on T2-weighted images [17]. HS was verified in all cases which were histopathologically inspected post surgery (seven LHS, six RHS patients). The degree of pathology was determined in six LHS and six RHS patients and was similar in the patient groups (Mann–Whitney U test, n.s.). In the LHS group one patient was classified as Wyler grade 1, one as grade 2–3, and four as grade 3. In the RHS group five patients were classified as Wyler grade 3, one as grade 3–4.
The anti-epileptic drugs (AEDs) used by these patients were lamotrigine (5/8 LHS; 5/6 RHS patients), levetiracetam (3/8 LHS; 2/6 RHS), oxcarbazepine (1/8 LHS; 2/6 RHS), pregabalin (1/8 LHS), valproate (1/8 LHS), and carbamazepine (3/8 LHS). Median drug load was 1.5 in both patient groups, ranging from one to three AEDs. Median seizure frequencies per month were five simple partial seizures (range 0–18), five complex partial seizures (range 1–18), and zero grand mal-seizures (range 0–4). Severity of epilepsy was similar in the two patient groups (Mann–Whitney U test, n.s.).
All patients were examined with a standardized neuropsychological test battery, parts of which were a verbal [18] and a visual memory test [19]. Verbal learning of 15 words over five trials was not statistically different between groups, visual learning of nine designs over five trials exhibited a tendency towards being worse in RHS patients (p = 0.08; Mann–Whitney U test).
MRI data acquisition
Functional images (Siemens Vision Magnetom (Siemens, Erlangen, Germany), 1.5T, TR/TE = 4000/64 ms, 104 brain volume GE-EPI scans per subject, 64 × 64 matrix, 4 × 4 mm in-plane resolution, 30 axial slices, 3 mm slice thickness plus 0.3 mm gap) were obtained during performance of a classical hippocampal memory task (Fig. 1, top). This spatial object-location memory task comprised alternating blocks of encoding, control condition, and recognition of object locations. The control condition was a tedious task which required the subjects to indicate by button press which of two visually presented objects was larger. This task was chosen because subjects find it extremely easy but still distracting from the memory blocks. The paradigm was created to elicit hippocampal activation in fMRI investigations of healthy subjects and epilepsy patients. It has been demonstrated to evoke asymmetrical hippocampal activation in MTLE patients depending on the side of pathology [20]. Additionally, structural MR data were acquired after functional runs (Siemens Vision 1.5T, TR/TE = 9.7/4 ms, 256 × 256 matrix, 1 × 1×1 mm3 resolution, 160–170 sagittal slices).
Top screenshots from the memory task and the control condition. Middle, activation (red) and deactivation patterns (blue) of the CON group (p < 0.001, uncorrected) overlaid on a canonical T2-weighted image. Bottom significantly reduced task-related deactivation in patients (LHS and RHS) compared to CON (p < 0.001, uncorrected; k = 5 contiguous voxels)
fMRI data analyses
fMRI data were analyzed using (1) a classical, model-driven approach which only served to functionally define a posterior midline region-of-interest (ROI) within the PREC area that would show the typical signal properties of the default network: task-related deactivation, reduced in patients compared to CON. This step was followed by (2) a functional connectivity analysis, aimed at identification of the entire default network, which was then submitted to further assessment regarding group differences. All MRI data processing was carried out using SPM5 (http://www.fil.ion.ucl.ac.uk) and MATLAB 7.0 (http://www.mathworks.com).
Preprocessing
In order to correct for head movement during functional MR scanning, all images were realigned using six parameter rigid-body transformations. The resulting images were normalized to match a template brain (MNI152) in Talairach-space and resampled to 3 × 3×3 mm3 voxel size. Afterwards, images were spatially smoothed with a Gaussian kernel of 9 × 9×9 mm3 full width at half maximum.
Task-related activation/deactivation patterns
Single subject analyses
The preprocessed data were statistically analyzed on a voxel-by-voxel basis according to the general linear model approach for time series data [21]: a design matrix was created with one regressor for the memory task blocks (box-car function convolved with a canonical hemodynamic response function), the control condition being implicitly modeled. Before model estimation, data were band-pass filtered to remove high- and low-frequency noise. A statistical parametric map of brain activation was computed representing the contrast ‘memory task vs. control condition’. This was taken to the second level group analysis.
Group level analysis
A random-effects, voxel-wise ANOVA with the 3-levels factor Group was performed on task-related activation maps from LHS patients, CON, and RHS patients. Mean activation and deactivation patterns were obtained to visualize task-related brain activity. In order to identify a suitable ROI for subsequent connectivity analyses, differential activation between patients and CON was assessed. Brain activation was contrasted between CON and patients (LHS and RHS combined), thresholded at p < 0.001 (voxel-level) and cluster-size k = 5 contiguous voxels.
Functional connectivity analyses
Single subject level
In each subject, BOLD signal time series data were extracted from voxels inside the functionally defined PREC-ROI (see “Results” of task-related analysis). The extracted time course vector from the PREC-ROI was fed into a regression analysis, together with six realignment parameters as in the model-driven analysis. The PREC-ROI time series regressor as well as the data were band-pass filtered for artifact removal applying a third order Butterworth filter with a low-pass cut-off frequency of 0.08 Hz and a high-pass cut-off frequency of 0.0078 Hz. A statistical parametric map representing functional connectivity patterns of the PREC-ROI was obtained for the accomplishment of further group statistics. Previous studies have shown that because functional connectivity analysis is based on low-frequency fluctuations (<0.1 Hz), sparse temporal sampling in the range of seconds is sufficient [22].
Group level
For whole-brain data, an ANOVA with the 3-levels factor Group was performed on functional connectivity maps obtained from single subject analyses. The contrast revealing the pattern of functional connectivity to the PREC-ROI in CON was calculated, thresholded at p < 0.001 and k = 20 contiguous voxels. Regions of interest with 5 mm radius were built around peak voxels from clusters showing significant connectivity to the PREC in CON. For each ROI, we compared connectivity to the PREC between groups applying nonparametric, pairwise Mann–Whitney U tests rather than the standard parametric approach provided by the SPM software package. In each subject, voxel-wise functional connectivity values (effect size measures from single subject connectivity analyses) were averaged across voxels within the ROI. This average effect size per subject was then compared between groups in order to test for significant connectivity differences in each ROI. We chose this approach to adapt for the rather small sample sizes which led us to prefer non-parametric tests.
Results
Task-related activation/deactivation
Mean activation across groups was observed symmetrically in inferior, middle, and superior occipital cortex, right inferior temporal/fusiform gyrus, bilateral inferior (IFG) and middle frontal gyri (MFG), left hippocampus, and the cerebellar vermis (Fig. 1, middle, red).
Deactivation was observed in left and right lingual gyri, bilateral superior temporal cortex, spreading into amygdala and insula, ACC and middle cingulate cortex (MCC), bilateral inferior (ITG) and middle temporal gyri (MTG), right temporal pole and right pre- and postcentral gyri (Fig. 1, middle, blue).
Contrasting groups, patients revealed significantly less deactivation in bilateral superior temporal gyri (STG), right amygdala, and bilateral PREC (peak voxel coordinates of the two PREC clusters: [+3 −60 +30], [−6 −63 +30]; Fig. 1, bottom).
Functional connectivity analyses
Functional connectivity analyses were performed in two stages: (1) The ‘regular’ PREC connectivity pattern was identified in CON subjects in order to find brain areas functionally connected to the PREC in the healthy brain. We assumed that this pattern would reveal the entire default network in CON. (2) ROI were derived from this pattern’s peak regions (see “Methods”). Connectivity values (in terms of beta weights from the ANOVA described in “Group level”) were compared between groups applying nonparametric tests using the MATLAB Statistics Toolbox.
Whole-brain PREC connectivity
The PREC connectivity pattern in CON subjects that emerged from the group level analysis consisted of 14 clusters significantly connected to the PREC-ROI (thresholded at p < 0.001, k = 20 contiguous voxels; Table 2; Fig. 2). Besides a larger cluster centered around the PREC-ROI and spreading into PCC, MCC, and parts of the visual cortex, significant functional connectivity to the PREC was observed in the following regions, which include the canonical nodes of the default network: bilateral vmPFC and ACC, bilateral IPC, left and right hippocampus, bilateral middle (MTG) and STG, left amygdala, left inferior frontal gyrus, left Insula, and bilateral MCC (cf. Fig. 2). All peak voxels in this analysis survived a threshold of p = 0.05 (FDR corrected).
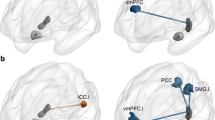
Functional connectivity pattern of the PREC in CON subjects. Red dots indicate respective peak voxel within a cluster, which were used as centers for subsequent ROI analyses. Red and green circles mark ROI which exhibited group differences regarding connectivity to the PREC-ROI. Note the resemblance to the canonical default network as described in the literature
ROI analyses of group differences
For each of the spherical ROI, derived from the preceding whole-brain analysis, pairwise nonparametric Mann–Whitney U tests were calculated in order to detect group differences regarding connectivity to the PREC-ROI. These revealed significantly reduced connectivity in the left middle temporal gyrus (MTG) in LHS patients compared to CON as well as RHS patients (Fig. 3, top right). Connectivity in the left amygdala was lower in LHS than RHS patients (Fig. 3, bottom left). Furthermore, tendencies towards significantly reduced connectivity values in the left hippocampus (LHS < CON; Fig. 3, bottom right) and in the left vmPFC/ACC (LHS < RHS; Fig. 3, top left) were observed.
Contrast estimates (mean ± SD) from the group level functional connectivity analysis for each of the four regions of interest with group differences regarding functional connectivity to the precuneus. Star indicates significant group difference (p < 0.05, two-tailed Mann–Whitney U test), triangle indicates tendency towards significance (p < 0.075)
Discussion
In the current study we used fMRI data acquired during a visuospatial memory task to identify the network of brain regions anchored to the PREC in healthy subjects. The pattern that emerged was largely congruent with previous descriptions of the default network based on PET [23] and resting state fMRI data [14]. We were able to detect reduced functional coherence within this network in LHS patients compared to healthy controls in the left lateral temporal cortex and in the pathologically affected left hippocampus. Furthermore, LHS patients showed lower PREC connectivity compared to RHS patients in left MTG, vmPFC/ACC, and left amygdala.
Our analysis of functional connectivity to the PREC-seed was based on prior identification of a posterior midline cortical area that showed the typical properties of default network areas: deactivation during a highly attention-demanding task compared to a tedious control condition, and reduced deactivation of this area in patients.
Default network and cognition
We refer to the network we identified in healthy subjects by functional connectivity analysis with the PREC as a seed region (cf. Fig. 2) as the default network. It needs, however, to be stated that typical studies investigating the default network rely on data acquired during rest rather than task performance. Nevertheless, the following points led us to interpret this network as the default network: (1) Regions identified by our analysis are functionally connected to the PREC, which is the central node of the default network, and showed the characteristic signal properties of the default network. (2) The network we identified comprises the canonical parts of the default network (PREC/PCC, vmPFC/ACC, IPC, MTL/hippocampi). (3) It has previously been demonstrated that functional coherence within the default network persists in both rest and task performance blocks [1].
We also detected functionally significant connectivity to the PREC in CON in bilateral MTG. Although the lateral temporal cortex is not a canonical part of the classical default network, it has recently been linked to it [14, 15].
Cortical midline areas, being part of the default network, serve the recollection of the past [24] as well as planning/simulating the future of oneself [14, 25]. That makes the MTL with its main structure, the hippocampus, inevitably a crucial component of the default network. Consequently, it has recently been stated that patients with hippocampal amnesia have difficulties imagining new experiences [26]. Extensive overlap between the declarative memory network and the default network has been demonstrated earlier [27].
Furthermore, both the medial PFC and the posterior parietal cortex have been associated with self-referential mental activity [28, 29]. It seems highly plausible that this self-referential thinking is (1) linked to the declarative memory system (and hence the hippocampus), and (2) suspended or attenuated when it comes to goal-directed, externally stimulated behavior.
Affection of the default network does not seem to be specific to one type of perturbation of brain physiology. Instead, it has been reported in dementia [30, 31], pain [32] schizophrenia [33], and other neuropsychiatric disorders [34]. Therefore, it might signal the extent of brain damage and concomitant cognitive impairment.
Asymmetry
From our data it appeared that LHS patients were more affected regarding functional coherence of the PREC than RHS patients, who did not show reduced connectivity to the PREC at any site—neither compared to CON nor to LHS. This might reflect the lack of statistical power as the sample size was smaller in RHS than LHS patients. However, exploration of connectivity strengths of each group at regions with reduced connectivity in LHS patients showed rather an opposite effect: in regions where LHS patients exhibited reduced coherence to the PREC, RHS patients showed increased connectivity. Results might indicate, as well, that the connections of the hippocampi to the default network are not equally strong. Fransson and Marrelec [15] recently demonstrated that while the left and right hippocampi are tightly interconnected, they are connected to the rest of the default network only via the left hippocampus, the right hippocampus being only indirectly functionally connected. Furthermore, the left hippocampus has been ascribed a special role in terms of being the hub of the autobiographical memory network [35].
Additionally, clinical data support the unique role of the left (language-dominant) hippocampus: Gleissner and colleagues [36] reported that after resection of the left hippocampus, patients suffered from more noticeable and persistent memory disturbances than patients who underwent right-sided resections.
In the same vein, previous structural MRI studies have described an asymmetry in the hippocampally based memory network as well: assessing the effect of unilateral hippocampal atrophy, left hippocampal damage produced a more widespread extra-hippocampal gray matter reduction than right hippocampal damage—among the remote areas affected were medial frontal and parietal areas [37]. Carrete and colleagues [38] investigated temporal pole abnormalities in terms of altered gray-white matter demarcation in hippocampal sclerosis patients and reported significantly more frequent occurrence in left-sided than right-sided hippocampal sclerosis. Our data thus confirm the special role of the left hippocampus regarding its integration into large-scale networks.
Compensatory increase of connectivity
Addis and colleagues [35] recently applied analyses of effective connectivity on functional MRI data acquired from left-sided MTLE patients during an autobiographical memory task. The intriguing finding they reported was an increased connectivity between distant brain regions (posterior and anterior midline cortex), which the authors interpreted as a compensatory mechanism for structural deficits in the MTL. This was not reproduced by our study, which might be due to the different analysis technique (effective connectivity analysis in the article by Addis). It might also be due to the different tasks during which activity was recorded: Addis et al. [35] used an autobiographical memory task, which relies mainly on the left hippocampus. The spatial memory task from our study is relatively more demanding for the right hippocampus and typically elicits rather bilateral activation [20]. Thus, left hippocampal damage might provoke more compensatory brain activity during ‘left hippocampal tasks’. On the other hand, RHS patients in our study exhibited tighter coupling to the precuneus than LHS patients in left MTG, left amygdala, and anterior midline cortex. Connectivity measures at these sites were higher in RHS than in CON (though not to a statistically significant degree), which might indicate a compensatory up-regulation of connectivity in a rather ‘right hippocampal task’. However, this needs to be elucidated in further studies with larger patient groups.
Variations and alterations in functional connectivity
Functional connectivity as measured with fMRI has been demonstrated to indicate the functional integrity of brain networks involved in specific cognitive functions [12, 39]. Ranganath et al. [40] provided evidence for the importance of hippocampal-neocortical coupling, showing that BOLD signal connectivity between the hippocampi and neocortical regions (e.g., orbitofrontal, and medial parietal) is enhanced during successful as compared with unsuccessful memory formation in healthy subjects.
Previous imaging studies in epilepsy patients provided evidence for altered activity in brain regions remote from the epileptic focus—brain regions which are strikingly congruent to the regions we detected in our analyses. Van Paesschen and colleagues used single photon emission computed tomography (SPECT) in TLE patients with HS and demonstrated diminished cerebral perfusion during complex partial seizures predominantly in the frontal lobes and the precuneus [41]. Combining EEG and fMRI in examining patients with idiopathic generalized epilepsies, Gotman et al. [42] were able to show BOLD signal decreases during epileptic discharges in medial frontal, PREC/PCC, and lateral parietal areas. Laufs et al. [43] applied EEG–fMRI as well to demonstrate BOLD signal decreases associated with epileptic discharges in the classical default network anterior and posterior midline areas in a patient with absence seizures, and in a group of TLE patients, in contrast to non-TLE patients [44]. Beyond fMRI signal alterations related to epileptic discharges, our data point towards a durable negative effect of epileptic seizures originating in the (left) temporal lobe on distant brain areas, which is in line with previously reported alterations of these areas, in terms of atrophy [10] or asymmetrical metabolism [11].
At least three mechanisms in our view are worth considering as underlying remote area effects: (1) Wallerian degeneration due to reduced input from the pathological hippocampus might lead to structural alteration of connected areas. (2) Spread of ictal and interictal activity could cause dysfunctions of synaptically connected regions. (3) Reduced involvement of synaptically connected areas may induce a lower level of activity of these brain areas (similar to the concept of diaschisis), which may result in reduced involvement in other network functions as well.
Limitations of the study
Although we report strong effects that reached statistical significance, it should be noted that the size of the sample of data sets in which analyses were performed was too small (14 patients, eight healthy controls) to draw definitive conclusions pertaining to the population of patients with temporal lobe epilepsy and unilateral hippocampal sclerosis. Whereas observations like altered fMRI activation in the posterior midline cortex of patients are strongly corroborated by previous similar reports in the literature, altered connectivity in HS patients is a novel finding that needs to be examined in larger patient groups, and should thus be considered a preliminary result.
As all patients were treated with AEDs, effects of medication on fMRI activation differences compared to healthy controls cannot be excluded. Concerning carbamazepine, an effect on fMRI activation has been reported by Jokeit and colleagues [45]. Effects of antiepileptic medication on functional connectivity assessed with fMRI have not been demonstrated yet, but remain a possible confounding factor. The precise contribution of severity of epilepsy in terms of seizure types and frequencies cannot be determined by our study. However, the two patient groups were largely similar regarding drug load and seizure frequency. Thus, differences between patient groups are not likely due to these factors. Further studies with larger sample sizes might disentangle the effects of drug load or severity of epilepsy on altered connectivity measured with fMRI.
Conclusion
The hippocampus is part of the default network of the human brain with the precuneus being its core hub. In MTLE patients with left hippocampal sclerosis, functional connectivity between precuneus and ipsilesional lateral temporal cortex and hippocampus was reduced compared to healthy subjects.
We thereby demonstrate that damage to one node of this large-scale network affects the interplay between other nodes of the network, which can be distant from the site of structural pathology. FMRI here revealed extensive functional alterations which may have behavioral relevance: conceptually, consequences may range from specific cognitive deficits to the development of diffuse impairments as in so-called epileptic encephalopathies.
References
Greicius MD, Menon V (2004) Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16:1484–1492
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541
Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ et al (2008) Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG–fMRI study. Hum Brain Mapp 29:671–682
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711
Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50
Babb TL, Brown WJ (1987) Pathological findings in epilepsy. In: Engel J (ed) Surgical treatment of the epilepsies. Raven Press, New York, pp 511–540
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Saling MM, Berkovic SF, O’Shea MF, Kalnins RM, Darby DG, Bladin PF (1993) Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. J Clin Exp Neuropsychol 60:8–618
Focke NK, Thompson PJ, Duncan JS (2008) Correlation of cognitive functions with voxel-based morphometry in patients with hippocampal sclerosis. Epilepsy Behav 12:472–476
Bonilha L, Alessio A, Rorden C, Baylis G, Damasceno BP, Min LL et al (2007) Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp 28:1376–1390
Jokeit H, Seitz RJ, Markowitsch HJ, Neumann N, Witte OW, Ebner A (1997) Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain 120:2283–2294
Wagner K, Frings L, Halsband U, Everts R, Buller A, Spreer J et al (2007) Hippocampal functional connectivity reflects verbal episodic memory network integrity. Neuroreport 18:1719–1723
Marques CM, Caboclo LO, da Silva TI, Noffs MH, Carrete HJ, Lin K et al (2007) Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy Behav 10:477–485
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Fransson P, Marrelec G (2008) The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 42:1178–1184
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Van Paesschen W (2004) Qualitative and quantitative imaging of the hippocampus in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimaging Clin N Am 14:373–400 vii
Helmstaedter C, Durwen HF (1990) The Verbal Learning and Retention Test. A useful and differentiated tool in evaluating verbal memory performance. Schweiz Arch Neurol Psychiatr 141:21–30
Lamberti G, Weidlich S (1999) DCS—a visual learning and memory test for neuropsychological assessment. Hogrefe and Huber, Seattle
Frings L, Wagner K, Halsband U, Schwarzwald R, Zentner J, Schulze-Bonhage A (2008) Lateralization of hippocampal activation differs between left and right temporal lobe epilepsy patients and correlates with postsurgical verbal learning decrement. Epilepsy Res 78:161–170
Friston KJ, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R (1995) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL (2008) Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100:129–139
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682
Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005) Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453
Schacter DL, Addis DR, Buckner RL (2008) Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci 1124:39–60
Hassabis D, Kumaran D, Vann SD, Maguire EA (2007) Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA 104:1726–1731
Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL et al (1995) Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152:1576–1585
Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001) Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583
Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P (2005) Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp 26:231–239
Dickerson BC, Sperling RA (2008) Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia 46:1624–1635
Baliki MN, Geha PY, Apkarian AV, Chialvo DR (2008) Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 28:1398–1403
Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ et al (2003) Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361:281–288
Greicius M (2008) Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 24:424–430
Addis DR, Moscovitch M, McAndrews MP (2007) Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain 130:2327–2342
Gleissner U, Helmstaedter C, Schramm J, Elger CE (2004) Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia 45:960–962
Bonilha L, Rorden C, Halford JJ, Eckert M, Appenzeller S, Cendes F et al (2007) Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 78:286–294
Carrete HJ, Abdala N, Lin K, Caboclo LO, Centeno RS, Sakamoto AC et al (2007) Temporal pole signal abnormality on MR imaging in temporal lobe epilepsy with hippocampal sclerosis: a fluid-attenuated inversion-recovery study. Arq Neuropsiquiatr 65:553–560
He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M (2007) Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53:905–918
Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J (2005) Functional connectivity with the hippocampus during successful memory formation. Hippocampus 15:997–1005
Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A (2003) SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain 126:1103–1111
Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F (2005) Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102:15236–15240
Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K (2006) Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia 47:444–448
Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L (2007) Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp 28:1023–1032
Jokeit H, Okujava M, Woermann FG (2001) Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol 1:6
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frings, L., Schulze-Bonhage, A., Spreer, J. et al. Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol 256, 2021–2029 (2009). https://doi.org/10.1007/s00415-009-5233-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5233-0