Abstract
Different neuroimaging techniques (fMRI, spectroscopy, PET) are being used to evaluate candidate drugs in pharmacological development. In patients with epilepsy fast propagation of the epileptiform activity between different brain areas occurs. Electric Source Imaging (ESI), in contrast to the aforementioned techniques, has a millisecond time resolution, allowing visualization of this fast propagation. The purpose of the current project was to use ESI to investigate whether introduction of an antiepileptic drug (levetiracetam, LEV) would change the propagation patterns of the interictal epileptiform activity. Thirty patients with epilepsy were subject to an EEG recording before (pre-LEV) and after (in-LEV) introduction of LEV. Interictal spikes with similar topographic distribution were averaged within each subject, and a distributed source model was used to localize the EEG sources of the epileptiform activity. The temporal development of the activity within 20 regions of interest (ROIs) was determined, and source propagation between different regions was compared between the pre-LEV and in-LEV recordings. Patients with epileptic seizures showed propagation in 22/24 identified spike types in the pre-LEV recordings. In the in-LEV recordings only 7/15 spike types showed propagation, and six of these seven propagating spikes were recorded in patients with poor effect of treatment. Also in patients without seizures LEV tended to suppress propagation. We conclude that the observed suppression of source propagation can be considered as an indicator of effective antiepileptic treatment. ESI might thus become a useful tool in the early clinical evaluation of new candidate drugs in pharmacological development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the evaluation of candidate drugs for neuropharmacological treatment, different neuroimaging techniques such as fMRI, NMR spectroscopy and PET have been used (Lee and Farde 2006; Borsook et al. 2006; Pellecchia et al. 2002; Wise and Tracey 2006). The key to success in drug development is to target the drugs to the main pathophysiological process (Spedding 2006). In patients with epilepsy, propagation of the epileptiform activity is likely to be an important factor for the development of clinical symptoms (Duncan et al. 2006; Elger and Schmidt 2008; Lehnertz et al. 2009). Fast spread of the epileptiform activity can occur, both within the temporal lobe and between distant areas (Alarcon et al. 1994, 1997; Emerson et al. 1995; Lantz et al. 2003b; Merlet et al. 1996; Sutherling and Barth 1989). The possibility to disentangle the temporal sequence of these complex activation patterns may be of considerable importance for understanding the epileptic process and for assessing the potential effectiveness of a certain candidate drug. Unfortunately, this important feature of the epileptic disorder is not easily visualized with hemodynamic methods, due to their inherently limited temporal resolution. On the other hand, several studies have demonstrated that it is indeed possible to visualize this propagation using modern quantitative EEG analysis techniques (Ebersole et al. 1995; Fuchs et al. 1999; Huppertz et al. 2001; Lantz et al. 2003b; Scherg et al. 1999; Michel et al. 1999).
Pharmaco-EEG has traditionally been one tool in studies of drug effects (Itil 1982; Saletu et al. 1987; Wauquier 2005). In recent years there has been a considerable methodological development concerning quantification and localization of brain electric activity (EEG) (for a review see, Michel et al. 2004b). Source reconstruction procedures, known as Electric Source Imaging (ESI), have found important applications both in research, trying to assess normal functions of the brain (sensory functions, cognition, for a review see (Michel et al. 2001)), and in patient oriented clinical research, especially epilepsy (Ebersole 1991; Fuchs et al. 1999; Lantz et al. 2003a, b, 1997, 1999; Merlet and Gotman 1999; Scherg et al. 1999; Sperli et al. 2006; Michel et al. 1999, 2004a). So far, however, few investigators have applied these new analysis methods in drug assessment studies.
The purpose of the current study was to evaluate whether ESI could be used to extract features from the interictal epileptiform activity that could be used as markers for effective antiepileptic treatment. Given the known tendency of interictal spikes to propagate between different brain areas we considered this an appropriate feature to study. Propagation of epileptiform activity between distant brain areas is likely to be an important pathophysiological factor for the development of epileptic seizures (Bikson et al. 2003; Jefferys 2003; Pfurtscheller and Silva 1999; Suffczynski et al. 2001), and for this reason the faculty of an antiepileptic drug (AED) to suppress the tendency of a spike to propagate would be a strong indicator of efficacy. Consequently, if ESI could be shown to be capable of visualizing such a suppression of propagation, it would yield useful information about the change in brain connectivity due to the introduction of a specific AED. The hypothesis was that it should be possible (a) to visualize propagation of interictal epileptiform activity in a group of patients with no or inadequate antiepileptic medication, and (b) to document a change in these propagation patterns after introduction of an AED, in this case levetiracetam, that on clinical/electrophysiological grounds was considered to be efficient in a specific patient.
Patients and Methods
All patients (about 500) in our database with Focal Nocturnal Epileptiform Activity (FNEA) were reviewed. FNEA was defined as sleep enhanced focal epileptiform activity with a spike index above 30 (Larsson et al. 2009). From this group of patients, 30 fulfilling the following criteria were identified and included in the study: The patients should have been subject to two separate EEG recordings, one before (pre-LEV) and one after (in-LEV) introduction of levetiracetam (LEV), and they should be on LEV monotherapy during the in-LEV investigation. Of the 30 patients (21 males, 9 females, aged 4–14, mean 9 years) seventeen also had a history of focal epileptic seizures. Thirteen patients did not have seizures, but had ambulatory recording either due to unclear episodes or for quantification of FNEA. LEV was initiated due to the FNEA. The pre-LEV recording was performed between 0 and 8 (mean 0.7) months before introduction of LEV, and the in-LEV recording was performed between 2 and 31 (mean 6) months after introduction of the drug. At the time of the pre-LEV investigation, 19 of the patients were without antiepileptic drug medication, whereas 11 patients were treated with other AEDs (see Table 1). At the time of the in-LEV investigation none of the patient was treated with any other AED drug.
The EEG recordings were performed using one of three different recording systems: (1) An Embla®-system with 16 recording electrodes placed according to the 10–20 system except Fz,Cz and Pz. (2) A Track-it® system with 19 recording electrodes placed according to the 10–20 system. (3) A NicOne® system with 25 recording electrodes (“low rows” F9, T9, P9, F10, T10, P10 added). Before further processing, data from the different systems were standardized to the same 16 channel electrode configuration and downsampled to 100 Hz. The data was filtered off line with LFF 2 Hz, and HFF 30 Hz.
In each patient the two different EEG recordings were reviewed. Two hours of EEG, recorded just after the patient had fallen asleep, was scanned using the pattern recognition feature of the BESA® system in order to identify different interictal epileptiform potentials with different surface voltage distributions. With this algorithm, spikes are detected by template matching of the principal components derived from the current source density estimate of the patients’ EEG. Between one and three different surface distributions were identified in each investigation, and all the individual spikes or sharp waves of a certain distribution were averaged. These different surface distributions are in this manuscript referred to as spike types. The number of potentials averaged ranged between 85 and 9198 (mean 2824). Although not all spikes in the EEG were recognized by the analysis program, it was assured, through visual inspection of the traces, that no significant distributions had been overseen by the pattern recognition algorithm. In a few patients in whom the epileptiform activity was of too low amplitude to be reliably detected by the algorithm, the spike identification was performed manually.
A distributed source model (Radially Weighted Minimum Norm; Grave de Peralta Menendez and Gonzalez Andino 1998) was used to model the distribution of the electric activity within the brain during different phases of the spike or sharp wave. The solution space for the inverse solution, consisted of 3005 solution points, which were regularly distributed in the standard brain provided by the Montreal Neurological Institute (MNI). A spherical head model with anatomical constraints (SMAC; Spinelli et al. 2000) which transforms the MRI to the best fitting sphere, was used. The solution space was divided into 20 regions of interest (ROIs), and the average source activity of all solution points within a ROI was calculated for each time point during the course of a spike or a sharp wave. In this way the development of the activity within each ROI could be followed over time (Fig. 1). To avoid contamination from background noise only ROIs with a peak power of at least 25% of that of the region with the maximal power were considered to show a significant activation.
The analysis procedure. a From the raw EEG spikes are identified. b Several spikes with a similar distribution are averaged. c A source reconstruction is performed for several time points throughout the spike-wave complex. d The solution space of the inverse solution is divided into 20 Regions of Interest (ROIs). e The average estimated current density of all solution points within a ROI is calculated and the time course of the average current density within each ROI is displayed
For each patient the number of spikes, and the maximum source amplitude of the averaged spike, was compared between the pre-LEV and in-LEV investigations. Reduction in number of spikes and reduction of source amplitude between the two investigations, together with the clinical judgment of whether the patient was optimally treated on LEV monotherapy, was then used as indicators of treatment efficacy (Table 1).
Subsequently, the time course of the reconstructed cortical activation within the different ROIs were inspected, and the delay between the activity peak in the ROI of primary activation and that of the other ROIs was measured, and interpreted in terms of propagation between the different brain regions. Then, for each patient, any change in spike propagation from the pre-LEV to the in-LEV study (Table 2) was assessed and related to the three other indicators of treatment efficacy (spike number, source amplitude, and clinical yield, Table 1).
Results
Patients with a History of Epileptic Seizures
Seventeen of the 30 patients had a history of epileptic seizures. In these 17 patients, a total of 24 different spike types were obtained in the pre-LEV investigations (Table 2). In 22 of these 24 spike types source localization revealed a time lag of 10–50 ms between the peak activity of the primary source and that of one or several other regions (Fig. 2).
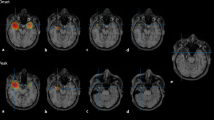
Results in Patient 6 who is considered optimally treated with LEV. Upper part: Raw EEG against a common reference. Middle part: Time course of the reconstructed cortical activation in the 20 ROIs. Lower part: Source reconstruction of active regions at different single time points. Colors show source strength within the different ROIs at the given time point (red strongest activation, green weakest activation). Red circle: primary activation area, blue circle: propagation area. Left side = left hemisphere. a Results for one spike average in the pre-LEV condition. The spike shows propagation from the right occipital to mainly the left occipito-temporal area. b Results for one spike average in the in-LEV condition. There is an important reduction in spike number (not shown) and spike/source waveform amplitudes compared to the pre-LEV investigation, and a suppression of the propagation to the left occipito-temporal area
In five of the 17 patients (pats 1–5) the epileptiform activity completely disappeared after introduction of LEV (Table 1). In one patient (Pat 11) epileptiform activity remained in a first but disappeared in a subsequent recording without changing the dose of the drug. In another five patients (pats 6–10) introduction of the drug lead to marked reduction in spike index (on the average—73%) and/or source amplitude (on the average—64%). These eleven patients were all considered to be optimally treated with LEV monotherapy.
In the five patients with remaining spikes but with a marked reduction in spike index/amplitude (pats 6–10), seven different spike types were encountered in the in-LEV recordings but for only one of these spikes was propagation between different areas seen (Pat 9, Table 2). Consequently, in cases where introduction of the drug did not suppress the epileptiform activity completely, the remaining epileptiform activity was not only less frequent and lower in amplitude compared to the pre-LEV study, but was also almost completely void of the tendency to propagate between different regions that had been observed in the pre-LEV investigations.
In six of the 17 patients with a seizure history (pats 12–17) the results were less clear. In these patients either the spike frequency, source amplitude or both remained unchanged or even increased after introduction of LEV treatment. Similar to the other patients with a history of epileptic seizures, these six patients all showed propagation between different brain areas in the pre-LEV investigation. The in-LEV investigations in these patients on the other hand revealed a mixture of source distributions with and without propagation (Table 2). As opposed to the previous group, these six patients were not clinically considered to be optimally treated. In one of these patients the LEV dose has been increased, but the effect of this is not yet known. In the remaining five patients the LEV medication was considered to be insufficient, and was either supplemented with nitrazepam (3 cases) or terminated (2 cases).
In summary, 22/24 spikes (92%) in the pre-LEV investigation of patients with a history of epileptic seizures showed propagation. In contrast, only 7/15 (47%) of the spikes in the in-LEV investigation showed propagation, and six of these seven spikes were recorded in patients with poor or insufficient effect of the treatment (Table 2).
The results in two of the patients with a history of seizures are shown in Figs. 2 and 3. In Patient 6 (Fig. 2) the pre-LEV study shows clear propagation, from the right occipital to mainly the left occipito-temporal area. Introduction of LEV gives a marked reduction in spike number and spike amplitude, and the propagation is suppressed. This patient is considered optimally treated with LEV. In Patient 12 (Fig. 3), with a left temporo-parietal focus, the spike number and spike amplitudes are not reduced after introduction of the drug, but there is a clear suppression of propagation to bifrontal areas. Based on persisting behaviour abnormalities and due to the unchanged spike number/amplitude, the patient was not considered optimally treated, and nitrazepam was added.
Results in Patient 12. Upper part: Time course of the reconstructed cortical activation in the 20 ROIs. Lower part: Source reconstruction of active regions at different single time points. Colors show source strength within the different ROIs at the given time point (red strongest activation, green weakest activation). Red circle: primary activation area, blue circle: propagation area. a Results for one spike average in the pre-LEV condition. The primary activation is in the left temporo-parietal area, but with important propagation to mainly left, but also right frontal regions. b Results for one spike average in the in-LEV condition. Compared to the pre-LEV condition there is no reduction in spike number (not shown) or spike amplitude, but there is a clear suppression of the bifrontal propagation. Due to the unchanged spike number/amplitude the patient was not considered optimally treated, and nitracepam was added
Patients Without a History of Epileptic Seizures
In the remaining 13 patients (pats 18–30) there was no history of seizures, and treatment with AEDs was initiated because the interictal epileptiform activity during sleep was considered to interfere significantly with the patient’s cognitive or psychosocial functions. In ten of these patients the treatment could be considered successful in the sense that the epileptiform activity completely disappeared after start of LEV-treatment (pats 18–25), or decreased considerably in number and amplitude (pats 26 and 27). As opposed to the patients with a seizure history, only four of the ten patients in this group showed propagation between different ROIs in the pre-LEV investigation (Table 2), and the patients showing propagation tended to be those with the most active epileptic focus (Table 1). In the last three patients (pats 28–30) the LEV treatment did not manage to suppress the epileptiform activity, neither in terms of number of spikes or spike amplitude, and the treatment was terminated. Similarly to the non successful cases in the group with seizures, there was for these patients a mixture of source distributions with and without time lag both in the pre-LEV and in-LEV investigations (Table 2).
In the non seizure group only 10/23 spikes (43%) in the pre-LEV recording, and 2/9 spikes (22%) in the in-LEV recording showed propagation (Table 2). Consequently there was a tendency for LEV to suppress propagation also in these patients, although this tendency was obscured by the fact that fewer of the spikes showed propagation in the first place.
Discussion
The aim of the present study was to assess the changes in propagation patterns of the interictal epileptiform activity when introducing LEV to patients. This was done by means of Electric Source Imaging. Apart from more intuitively comprehensible markers of drug efficacy, such as decrease in number of spikes and decrease in amplitude of the epileptic source, we noted a clear decrease in propagation of interictal activity between different brain areas as an effect of drug treatment.
In patients with FNEA the goal of antiepileptic treatment is not only freedom from seizures but also suppression of the interictal epileptiform activity which is considered to hamper the patients cognitive functions and hence, their daily life. It is often observed that successful treatment with AEDs initially leads to a decrease in number and amplitude of the interictal spikes, and that subsequent increase of the dose of the drug may lead to complete suppression of the epileptiform activity. There may also be several different epileptic foci that are simultaneously active, and the most active focus may vary from one investigation to the next, irrespective of whether any change in medication has been made. This means that in this specific patient group, it is difficult to determine exactly what effect a pharmacological substance has on one particular focus area. In some of the patients in this study the focus area was virtually unchanged from pre-LEV to the in-LEV investigation, whereas in some of the patients the pre- and post treatment focus locations were completely different. However, irrespective of whether the focus was the same before and during treatment or not, the tendency of this focus to propagate was suppressed by the treatment.
An interesting observation is that propagation between different brain areas in the pre-LEV EEG, was much more frequently seen in patients with a history of epileptic seizures. A relation between relative epileptogenicity and the complexity of focal spikes has also been reported by other authors (Rodin et al. 1995). We hypothesize that propagation is a feature of a more aggressive and complex disease. An epileptic seizure is a clinical manifestation of the electric activity in the brain. The pathological electromagnetic activity must comprise enough brain substrate to give visible symptoms. Hence, propagation of the activity is a prerequisite in most seizures. The fact that the rather few patients in the non seizure group that exhibited propagation in the pre-LEV study tended to be those with the most active interictal epileptic focus, also indicates a relation between tendency for propagation and severity of the disease. However, since MRI investigations were not available in all patients this could not be checked in this study.
It has been suggested that, in many circumstances, interictal epileptiform discharges can be the result of inhibitory processes that suppress the interictal-ictal transition, and that the strong after inhibition produced by interictal spikes actually protects against the occurence of ictal activity (De Curtis and Avanzini 2001). If this were the case, suppression of spike propagation might actually lower the ictal threshold rather than increasing it, and an efficient antiepileptic drug should rather be one that increases the spread of interictal discharges. The rather clear relation between suppression of spike propagation and clinical improvement in this study rather talk against such a protective role of interictal spiking.
In this study we interpret the time difference between activation of different regions in terms of source propagation. This interpretation can be debated. A complex activation of several independent regions with a certain time asynchrony would also explain our results. In Pat 10 there is in fact little or no time asynchrony between the different active regions in the pre-LEV investigation, but still an important suppression of the activity in several areas after drug introduction. On the other hand, the areas that are suppressed by the drug tend to be not the primarily activated ones, but rather those with a latency with respect to the primarily activate area. This fact would indicate that the mechanism is indeed suppression of propagation. In any case, the results indicate that introduction of LEV limits the extension of the areas that are activated by the epileptic process, whether the extended activation is a result of propagation or not.
An important question is whether the change in propagation is a specific feature of LEV and similar substances, or whether similar effects might be observed with other neurotropic drugs with different mechanisms of action. Some of the patients in this study were indeed treated with other AEDs (sodium valproate, topiramate and others, Table 1) at the time of the pre-LEV investigation, but it was not until levetiracetam treatment was introduced that the propagation disappeared and improvement in other EEG and clinical parameters occurred. Even so, although our study only demonstrates this effect for LEV treatment, it can not be excluded that what we are observing is the result of an efficient antiepileptic treatment irrespective of the type of drug as LEV was introduced due to lack of effect of prior AED medication.
Another important issue is whether the suppression of propagation occurs more promptly after administration of the AED than decrease in spike frequency and amplitude and, in that case, whether propagation changes could be used as a marker of acute drug effect, for instance after administration of a test dose. Another question is to what extent the pharmacological effect on source propagation is specific to patients with good results of treatment. In this study the number of patients with poor effect of LEV treatment was rather small, and the drug effect on propagation and other parameters in non responders has to be more thoroughly investigated.
In the present study it was necessary to use data that had been recorded in a clinical routine setting which leads to some methodological drawbacks. The limited number of recording electrodes (16 channels) limits the spatial resolution making source separation on a sub lobar level difficult (Lantz et al. 2003a). The relatively low sampling rate (100 Hz) also limits the possibility to visualize fast propagation. In some cases we have noted a propagation latency of only 10 ms between active areas (Table 2), which is on the limit of what our methodology allows us to detect. These factors lead to a rather limited accuracy, both spatially and temporally, of the results. The purpose of this study, however, was rather to visualize temporal separation of the activity in different major brain areas, a task which is less hampered by a limited number of recording electrodes. In addition, although quite short propagation latencies were encountered in a few patients, the average latency in the pre LEV recordings of patient with a seizure history was above 20 ms (Table 2), which is well within the limits of what can be detected with a 100 Hz sampling rate. Another potentially confounding factor is the fact that some of the patients were treated with other AEDs although, as mentioned above, this does not seem to have any major effect on the parameters we are measuring.
We conclude that it is indeed possible to use ESI to visualize effects of antiepileptic medication on the interictal epileptiform activity, and the findings seem to be rather stable also in individual patients. We suggest that changes in the tendency of an epileptic focus to propagate between different brain regions could be used as an additional parameter when judging the efficacy of AED treatment, and this technique might thus become a useful tool in the evaluation of new candidate drugs in pharmacological development.
References
Alarcon G, Guy CN et al (1994) Intracerebral propagation of interictal activity in partial epilepsy: implications for source localisation. J Neurol Neurosurg Psychiatry 57:435–449
Alarcon G, Seoane JJG et al (1997) Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain 120:259–282
Bikson M, Fox JE et al (2003) Neuronal aggregate formation underlies spatiotemporal dynamics of nonsynaptic seizure initiation. J Neurophysiol 4(89):2330–2333
Borsook D, Becerra L et al (2006) A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov 5(5):411–424
De Curtis M, Avanzini G (2001) Interictal spikes in focal epileptogenesis. Prog Neurobiol 63:541–567
Duncan JS, Sander JW et al (2006) Adult epilepsy. Lancet 367:1087–1100
Ebersole JS (1991) EEG dipole modelling in complex partial epilepsy. Brain Topogr 4:113–123
Ebersole JS, Hawes S et al (1995) Intracranial EEG validation of spike propagation predicted by dipole models. Electroencephalogr Clin Neurophysiol 95:18
Elger CE, Schmidt D (2008) Modern management of epilepsy: a practical approach. Epilepsy Behav 12:501–539
Emerson RG, Turner CA et al (1995) Propagation patterns of temporal spikes. Electroencephalogr Clin Neurophysiol 94(5):338–348
Fuchs M, Wagner M et al (1999) Linear and nonlinear current density reconstructions. J Clin Neurophysiol 16:267–295
Grave de Peralta Menendez R, Gonzalez Andino SL (1998) A critical analysis of linear inverse solutions. IEEE Trans Biomed Eng 45:440–448
Huppertz HJ, Hoegg S et al (2001) Cortical current density reconstruction of interictal epileptiform activity in temporal lobe epilepsy. Clin Neurophysiol 112(9):1761–1772
Itil TM (1982) The significance of quantitative pharmaco-EEG in the discovery and classification of psychotropic drugs. In: Herrmann WM (ed) Electroencephalography in drug research. Fischer, Stuttgart , pp 131–158
Jefferys JGR (2003) Models and mechanisms of experimental epilepsies. Epilepsia 44:44–50
Lantz G, Michel CM et al (1997) Extracranial localisation of intracranial interictal epileptiform activity using LORETA (Low Resolution Electromagnetic Tomography). Electroencephalogr Clin Neurophysiol 102:414–422
Lantz G, Michel CM et al (1999) Frequency domain EEG source localization of ictal epileptiform activity in patients with partial complex epilepsy of temporal lobe origin. Electroencephalogr Clin Neurophysiol 110:176–184
Lantz G, Grave de Peralta R et al (2003a) Epileptic source localization with high density EEG: how many electrodes are needed? Clin Neurophysiol 114(1):63–69
Lantz G, Spinelli L et al (2003b) Propagation of interictal epileptiform activity can lead to erroneous source localizations: a 128 channel EEG mapping study. J Clin Neurophysiol 20(5):311–319
Larsson PG, Wilson J et al (2009) A new method for quantification and assessment of epileptiform activity in EEG with special reference to focal nocturnal epileptiform activity. Brain Topogr 22:52–59
Lee CM, Farde L (2006) Using positron emission tomography to facilitate CNS drug development. Trends Pharmacol Sci 27:310–316
Lehnertz K, Bialonski S et al (2009) Synchronization phenomena in human epileptic brain networks J Neurosci Methods 183:42–48
Merlet I, Gotman J (1999) Reliability of dipole models of epileptic spikes. Clin Neurophysiol 110:1013–1028
Merlet I, Garcia-Larrea L et al (1996) Source propagation of interictal spikes in temporal lobe epilepsy. Correlations between spike dipole modelling and [18F]fluorodeoxyglucose PET data. Brain 119(Apr):377–392
Michel CM, Grave de Peralta R, Lantz G, Gonzalez Andino S, Spinelli L, Blanke O, Landis T, Seeck M (1999) Spatio-temporal EEG analysis and distributed source estimation in presurgical epilepsy evaluation. J Clin Neurophysiol 16:225–238
Michel CM, Thut G, Morand S, Khateb A, Pegna AJ, Grave de Peralta R, Gonzalez S, Seeck M, Landis T (2001) Electric source imaging of human brain functions. Brain Res Rev 36:108–118
Michel CM, Lantz G, Spinelli L, Grave de Peralta Menendez R, Landis T, Seeck M (2004a) 128-channel EEG source imaging in epilepsy: clinical yield and localization precision. J Clin Neurophysiol 21:71–83
Michel CM, Murray M, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R (2004b) EEG source imaging. Clin Neurophysiol 115:2195–2222
Pellecchia M, Sem DS et al (2002) NMR in drug discovery. Nat Rev Drug Discov 1(3):211–219
Pfurtscheller G, Silva FHL (1999) Functional meaning of event-related desynchronization (ERD) and synchronization (ERS). In: Pfurtscheller G, Silva FHL (eds) Event-related desynchronization, vol 6. Elsevier, Amsterdam, pp 51–65
Rodin E, Litzinger M et al (1995) Complexity of focal spikes suggests relative epileptogenicity. Epilepsia 36:1078–1083
Saletu B, Anderer P et al (1987) Topographic brain mapping of EEG in neuropsychopharmacology. Part II. Clinical applications (pharmaco EEG imaging). Methods Find Exp Clin Pharmacol 9(6):385–408
Scherg M, Bast T et al (1999) Multiple source analysis of interictal spikes: goals, requirements, and clinical value. J Clin Neurophysiol 16(3):214–224
Spedding M (2006) New directions for drug discovery. Dialogues Clin Neurosci 8(3):295–301
Sperli F, Spinelli L et al (2006) EEG source imaging in pediatric epilepsy surgery: a new perspective in presurgical workup. Epilepsia 47(6):981–990
Spinelli L, Gonzalez Andino S et al (2000) Electromagnetic inverse solutions in anatomically constrained spherical head models. Brain Topogr 13:115–125
Suffczynski P, Kalitzin S et al (2001) Computational model of thalamo-cortical networks: dynamical control of alpha rhythms in relation to focal attention. Int J Psychophysiol 43:25–40
Sutherling WW, Barth DS (1989) Neocortical propagation in temporal lobe spike foci on magnetoencephalography and electroencephalography. Ann Neurol 25:373–381
Wauquier A (2005) EEG and neuropharmacology. In: Niedermeyer E, Lopes da Silva F (eds) Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins, Philadelphia, pp 660–670
Wise RG, Tracey I (2006) The role of fMRI in drug discovery. J Magn Reson Imaging 23(6):862–876
Acknowledgements
We thank Denis Brunet for the development of the analysis software CarTool, and Svein Johannessen for valuable discussions. GL is a part time employee of Electrical Geodesic Inc. MS has received consulting fees from Pfizer and Janssen-Cilag. None of the other authors has any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larsson, P.G., Eeg-Olofsson, O., Michel, C.M. et al. Decrease in Propagation of Interictal Epileptiform Activity After Introduction of Levetiracetam Visualized with Electric Source Imaging. Brain Topogr 23, 269–278 (2010). https://doi.org/10.1007/s10548-010-0150-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-010-0150-1