Abstract
Electric source imaging (ESI) based on dense array electroencephalography (EEG) is a non-invasive technique for source localization of epileptic activity. However, the diagnostic accuracy of this tool is still debatable. In this study we aimed to investigate the source of epileptiform discharges in a group of epilepsy patients. For this, 20 patients (24.4 ± 8.0 years, 10 males) with drug-resistant focal epilepsy were included. All patients underwent 256-channel EEG recordings and were imaged on a 3T MRI scanner according to a predefined protocol. For spatio-temporal source reconstruction, LORETA (low resolution brain electromagnetic tomography) solution was applied to interictal averaged spikes. Electric sources of epileptiform discharges were detected in all 20 patients. In 18 (90%) patients source localization was concordant with patients’ seizure semiology. The most frequent source was identified in the temporal lobe. Dense array EEG is an accurate modality for localization of epileptogenic brain areas in the pre-surgical evaluation of drug-resistant epilepsy patients.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Epilepsy is defined as a disease of the brain [1] that affects about 1% of the population or approximately 78 millions people around the world. The epilepsy treatment with antiepileptic drugs has limited effectiveness and 25–30% of patients become drug-resistant [2]. The surgery is the superior option in the treatment of these patients and should be performed as earlier as possible. But in order to perform the surgery it is necessary to accomplish the pre-surgical evaluation of patients with drug-resistant epilepsy for the determination of the epileptogenic zone [3]. This assessment comprises mandatory and optional components. Electrical source imaging (ESI) of interictal epileptiform discharges is an emerging optional non-invasive tool for source reconstruction in drug-resistant epilepsy patients [4].
ESI based on dense array electroencephalography (EEG) is the simultaneous registration of the electric sources of epileptiform discharges with the seizure-generating brain structures as derived either from patient’s or template magnetic resonance imaging (MRI). But to render patients seizure freedom after the surgery, the source of patient’s epileptic activity should be precisely determined.
In our study we used low resolution brain electromagnetic tomography (LORETA) solution for the source localization of interictal spikes in patients with drug-resistant epilepsy.
2 Materials and Methods
2.1 Participants
We recruited 20 patients with focal epilepsy, who were consulted in the outpatient department of the National Center of Epileptology, Chisinau, Republic of Moldova. The diagnosis of epilepsy was based on clinical and EEG criteria according to International League against Epilepsy classification. All participants gave written informed consent prior to the study inclusion.
2.2 Dense Array EEG Acquisition
The interictal dense array EEG recordings was performed at rest and in an alert state for two hours in a dimly lit and quite room as described in [5]. The EEG electrodes were placed according to the international 10/5 system and included in a special net with 20–25 mm interelectrode distance (Hydrogel Geodesic Sensor Net 130, 256 electrodes, Electrical Geodesic, Inc., Eugene, OR) and in reference to anatomical landmarks. The sampling rate of recordings was set to 1000 Hz with low (0.3 Hz) and high (70 Hz) frequency filters. The recorded EEG data were stored using the Net Station 5 software package (Electrical Geodesic). The electrodes’ impedance was kept below 10 kΩ.
2.3 MRI Acquisition
All patients were imaged on a 3T MRI scanner (SIEMENS Skyra, Siemens Healthcare) with a 32-channel head coil according to a predetermined Epilepsy protocol used in [6, 7], which comprises 3D T1-weighted, T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences.
2.4 Source Reconstruction
The EEG recordings were visually checked and bad channels removed. Interictal spikes in artefact-free epochs (with a duration of ±500 ms) were manually selected. For each patient at least 20 spikes with similar morphology were marked at the point of maximum negativity. The source localization of interictal spikes was performed by using the LORETA solution [8, 9], which solves the inverse problem by assuming related orientations of neuronal sources and quantifies at each voxel of the grey matter the current density as the linear weighted sum of the scalp electrical potentials [10]. To solve the forward problem we used a template MRI for realistic head model construction (based on finite difference model, FDM). The pipeline of ESI is represented in Fig. 1.
Workflow of the electric source imaging. Left column illustrates the EEG analysis pipeline: selection and averaging of spikes followed by generation of the electric maps (at the rising phase of the averaged spike). Right column illustrates the structural MRI analysis pipeline: segmentation of the brain and grey matter followed by generation of the head model with solution points within the grey matter
3 Results
3.1 Participants’ Characteristics
The mean age of patients was 24.4 ± 8.0 years and mean disease duration was 11.7 ± 7.4 years (Table 1). Seizure semiology varied across the patients, the most predominant type of focal seizures being focal seizures with impaired awareness, mainly due to temporal lobe lesions.
3.2 Neuroimaging Results
Patients presented different structural causes of their focal epilepsy syndromes. Eight (40%) patients displayed imaging signs of hippocampal sclerosis, two (10%) presented with grey matter heterotopia, four (20%) cases of regional gliosis, two (10%) cases of left postsurgical encephalomalacia, one (5%) case of left temporal infiltrative lesion, one (5%) case of right frontal porencephalic cyst and two (10%) cases of normal MRI scans.
3.3 Source Localization Results
Based on dense array EEG, the focal source of discharges was identified in all 20 patients. From ESI analysis we identified only one source of interictal discharges in 17 (85%) patients, two separate sources in two (10%) patients and three sources in one (5%) patient. In seven (35%) cases the source of epileptiform discharges was localized in Brodmann area (BA) 38, in five (25%) patients in BA, in two (10%) cases in BA 25, two (10%) cases in BA 37, one (5%) patient in BA 10, one (5%) in BA 42 and one (5%) in BA 28 (Table 2). Out of 20 patients, in 18 (90%) patients the source was concordant with the presumed epileptic discharges as detected on standard EEG and seizure semiology (Figs. 2 and 3) and in 2 (10%) patients was non-concordant.
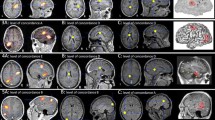
Example of correct dense array EEG source localization. a Segmented interictal spike, b topographic waveform plot of 256 EEG channels (common average reference), c the average of all selected spikes, d topographic map displaying the negative potential in blue color and positive potential in red color and e source imaging at the rising phase of the interictal spike with maximum of the estimated source in hippocampus
Example of correct dense array EEG source localization. a–d As in Fig. 2. e Source imaging at the rising phase of the interictal spike with maximum of the estimated source in temporoparietal junction
4 Discussion
For a successful epilepsy surgery, precise localization of electric sources of the brain’s epileptic activity is warranted. Dense array EEG electric source localization is a non-invasive imaging technique that reliably identifies the epileptogenic region. This modality in combination with inverse solution methods has already proved its clinical efficacy in detection of electric sources in patients with intractable epilepsy of temporal as well as extra-temporal origin [4, 10]. In this study we employed LORETA solution for the spatio-temporal source reconstruction of epileptiform activity based on the analysis of interictal spikes in a group of focal epilepsy patients. We were able to localize the sources in all 20 patients but with comparable accuracy, since in two cases we obtained erroneous results, which apparently were non-concordant with patients’ clinical profile.
From a methodological point of view electric source imaging consists of a series of steps, whose proper consideration is key for precise localization. The methods aimed for source estimation rely on a theoretical model of propagation of epileptic activity from the brain compartment through skull tissues towards the scalp, which have different conductivity properties [10]. Overall, these methods offer a relatively high diagnostic accuracy. According to a recent systematic review, the accuracy of interictal dense array EEG detection of electric sources for epilepsy surgery work-up, reaches a sensitivity of 87% (95% CI: 77–93%) and a specificity of 61% (95% CI: 45–74%) [11]. However, in particular cases the existence of constraints inherent to this methodology hinders the use of dense array EEG solely and requires multimodal imaging, frequently invasive ones.
The delimitation of the epileptogenic zone is more accurate when patient’s individual MRI but not a brain template (e.g. Montreal Neurological Institute (MNI)) is integrated into the source localization algorithm. This point becomes a substantial issue especially in patients, which have skull or brain defects disturbing tissue conductibility and providing wrong solutions [4]. Here we used the MNI template to construct realistic head models (based on FDM) that is one of our study’s limitations.
5 Conclusions
Our findings show that electric source imaging based on dense array scalp EEG is an accurate tool to localize the epileptogenic focus. This technique holds the promise to reduce the need for invasive EEG but we should be confident that at this time point independent diagnostic support from other source localization modalities might be required to proceed to resective surgery in patients with drug-resistant epilepsy.
References
Fisher, R., Acevedo, C., Arzimanoglou, A., et al.: ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55(4), 475–482 (2014). https://doi.org/10.1111/epi.12550
Brodie, M., Kwan, P.: Staged approach to epilepsy management. Neurology 58(8 suppl 5), S2–S8 (2002). https://doi.org/10.1212/WNL.58.8_suppl_5.S2
Rosenow, F., Lüders, H.: Presurgical evaluation of epilepsy. Brain 124(9), 1683–1700 (2001). https://doi.org/10.1093/brain/124.9.1683
Brodbeck, V., Spinelli, L., Lascano, A., et al.: Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain 134(10), 2887–2897 (2011). https://doi.org/10.1093/brain/awr243
Chiosa, V., Groppa, S., Ciolac, D., et al.: Breakdown of thalamo-cortical connectivity precedes spike generation in focal epilepsies. Brain Connect. 7(5), 309–320 (2017). https://doi.org/10.1089/brain.2017.0487
Chiosa, V., Ciolac, D., Groppa, S., et al.: Large-scale network architecture and associated structural cortico-subcortical abnormalities in patients with sleep/awake-related seizures. Sleep 42(4) (2019). https://doi.org/10.1093/sleep/zsz006
Michel, C., Murray, M., Lantz, G., et al.: EEG source imaging. Clin. Neurophysiol. 115(10), 2195–2222 (2004). https://doi.org/10.1016/j.clinph.2004.06.001
Birot, G., Spinelli, L., Vulliémoz, S., et al.: Head model and electrical source imaging: a study of 38 epileptic patients. NeuroImage: Clin. 5, 77–83 (2014). https://doi.org/10.1016/j.nicl.2014.06.005
Holmes, M.: Dense array EEG: methodology and new hypothesis on epilepsy syndromes. Epilepsia 49, 3–14 (2008). https://doi.org/10.1111/j.1528-1167.2008.01505.x
Lascano, A., Vulliemoz, S., Lantz, G., et al.: A review on non-invasive localisation of focal epileptic activity using EEG source imaging. Epileptologie 29, 80–89 (2012)
Mouthaan, B., Rados, M., Boon, P., et al.: Diagnostic accuracy of interictal source imaging in presurgical epilepsy evaluation: a systematic review from the E-PILEPSY consortium. Clin. Neurophysiol. 130(5), 845–855 (2019). https://doi.org/10.1016/j.clinph.2018.12.016
Acknowledgements
We would like to thank all our colleagues who helped us in acquiring and analyzing the data and all our patients who agreed to participate.
Conflict of Interest The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Groppa, S.A., Ciolac, D., Vataman, A., Chiosa, V. (2020). Dense Array Electroencephalography-Based Electric Source Imaging of Interictal Epileptiform Discharges. In: Tiginyanu, I., Sontea, V., Railean, S. (eds) 4th International Conference on Nanotechnologies and Biomedical Engineering. ICNBME 2019. IFMBE Proceedings, vol 77. Springer, Cham. https://doi.org/10.1007/978-3-030-31866-6_83
Download citation
DOI: https://doi.org/10.1007/978-3-030-31866-6_83
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31865-9
Online ISBN: 978-3-030-31866-6
eBook Packages: EngineeringEngineering (R0)