Abstract
Sandhoff disease (SD) is a lysosomal disease caused by a mutation of the HEXB gene associated with excessive accumulation of GM2 ganglioside (GM2) in lysosomes and neurological manifestations. Production of autoantibodies against the accumulated gangliosides has been reported to be involved in the progressive pathogenesis of GM2 gangliosidosis, although the underlying mechanism has not been fully elucidated. The thymus is the key organ in the acquired immune system including the development of autoantibodies. We showed here that thymic involution and an increase in cell death in the organ occur in SD model mice at a late stage of the pathogenesis. Dramatic increases in the populations of Annexin-V+ cells and terminal deoxynucletidyl transferase dUTP nick end labeling (TUNEL) + cells were observed throughout the thymuses of 15-week old SD mice. Enhanced caspase-3/7 activation, but not that of caspase-1/4, -6 ,-8, or −9, was also demonstrated. Furthermore, the serum level of corticosterone, a potent inducer of apoptosis of thymocytes, was elevated during the same period of apoptosis. Our studies suggested that an increase in endocrine corticosterone may be one of the causes that accelerate the apoptosis of thymocytes leading to thymic involution in GM2 gangliosidosis, and thus can be used as a disease marker for evaluation of the thymic condition and disease progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sandhoff disease (SD, OMIM ID #268800) and Tay-Sachs disease (TSD, OMIM ID #272800) are lysosomal storage disorders classified as GM2 gangliosidoses that result from deficiencies of lysosomal β-hexosaminidase (Hex, EC 3.2.1.52) isozymes, associated with massive accumulation of GM2 ganglioside (GM2) in the central nervous system (CNS) (Gravel et al. 2001 and Sango et al. 1996). The major Hex isozymes are HexA (αβ heterodimer) and HexB (ββ homodimer) (Mahuran 1999), and only HexA can catalyze cleavage at the β-N-acetylgalactosamine residue of GM2 through co-operation with a substrate specific protein cofactor, GM2-activator protein (Wu et al. 1994 and Zarghooni et al. 2004).
Previously, Wu et al. reported that the activated macrophage/microglia population is responsible for the rapid neurodegenerative course in SD mice (Sango et al. 1996 and Wu and Proia 2004). Yamaguchi et al. showed that the production of autoantibodies against gangliosides should play an important role in the pathogenesis of GM2 gangliosidosis (Yamaguchi et al. 2004). Furthermore, bone marrow transplantation prolongs the life span and ameliorates neurological manifestations in SD mice, regardless of a slight increase in Hex activity, and slight reductions in the GM2 and GA2 levels (Norflus et al. 1998). These findings suggest abnormalities of the immune system in GM2 gangliosidosis.
The thymus is an important organ for the immune system for maturation of the T cell population. Physiological development of the thymus continues until puberty (around 4 to 6 weeks of age in mice). Alteration of the thymus has been observed in several LSD models such as the feline GM1 gangliosidosis model (Cox et al. 1998) and Krabbe disease model mice (twitcher mice) (Galbiati et al. 2007). Furthermore, Takahashi et al. reported atrophy of the thymus in an autopsied case (Takahashi et al. 1974). In SD mice, we found the thymic involution at a late stage of pathogenesis.
In this study, we show the elevation of apoptosis of thymocytes and the serum level of corticosterone at a late stage of pathogenesis in SD mice. Our results indicate that thymic involution is a novel pathogenic aspect and that an increase in endocrine corticosterone in serum may be used as a disease-specific marker for monitoring the thymic condition and progression of GM2 gangliosidosis.
Materials and methods
This study involving mice was performed according to recommendations of the Animal Care Committee of the University of Tokushima.
Mice
Sandhoff (Hexb−/−) mice (C57BL/6x129sv) were kindly provided by Dr R. L. Proia (NIH). Mice were bred by mating with wild-type C57BL/6 mice (SLC, Shimazu, Japan), and were housed under standard housing conditions and checked infectious/parasitic status at regular intervals according to the Animal Care Committee of the University of Tokushima (The sign of any disease status were not detected).
Annexin V/propidium iodide (PI) staining assay
Thymuses were immediately collected after PBS perfusion under anesthesia from wild-type (WT) and SD mice. The thymuses were cut into small pieces, which were gently passed through a nylon mesh (100 μm mesh). The dissociated cells were suspended in 1% NH4Cl/10 mM HEPES, pH 7.4, kept on ice for 5 min, and then centrifuged at 1,500 r.p.m. for 5 min, the supernatant being removed. The cells were resuspended in ice-cold RPMI 1640 containing antibiotics, 2 mM L-glutamine and 10% FCS, and then centrifuged at 1,500 r.p.m. for 5 min, the supernatant being removed. The cell pellet was stained with annexin V and propidium iodide (PI) according to the manufacturer’s protocol. A total of 100,000 events was analyzed by flow cytometry (FACS Epics Altra, Beckman Coulter, USA).
In situ TUNEL assay
In situ TUNEL analysis was performed by a method based on labeling of the termini of fragmented DNA. Briefly, thymus sections were fixed in 4% paraformaldehyde (PFA) overnight, and then washed with PBS three times. Dead cells were detected with a DeadEndTM Colorimetric TUNEL System (Invitrogen, Madison, WI) according to the manufacturer’s instructions.
Caspase assay
Caspase activities were measured with a SensoLyter® AMC Caspase Substrate Sampler Kit Fluorometric (ANASPEC, San Jose, CA) and a modified protocol (Martin et al. 1995). Tissue extracts of thymuses were prepared by Dounce homogenization in lysis buffer and subsequently centrifuged at 13,200 g for 15 min at 4°C. The protein concentrations of the supernatants were adjusted to 1 mg/mL with lysis buffer. Fifty microlitters of a tissue extract was added to a black-walled 96-well plate and then caspase substrates were added, followed by incubation at RT for 1 h. The fluorescence (Ex/Em = 354 nm/442 nm) of each sample was measured with a Fluoroscan Ascent (Thermo, Kanagawa, Japan).
Western blotting to evaluate activated caspase-3
Tissue extracts of thymus were prepared by sonication in lysis buffer (1% TritonX-100/150 mM NaCl/20 mM Tris-HCl (pH 7.4)) containing protease inhibitors (1 μM pepstatin A, 20 μM leupeptin, 2 mM EDTA and 2 mM PMSF), and then centrifuged at 13,200 g for 15 min at 4°C. The resultant supernatant was designated as the tissue extract. For western blot detection of cleaved caspase-3, tissue extracts (15 μg protein) were subjected to 12.5 % SDS-PAGE. After protein transfer, the membranes were blocked with Blocking One (NACALAI TESQUE, Kyoto, Japan) in TBS (pH 7.4) for 3 hr at RT. One membrane was incubated with anti-cleaved caspase-3 monoclonal antibodies (R&D System, MN) in Can Get Signal solution 1 (1 : 2,000 dilution) overnight at 4°C. Another membrane was incubated with anti-β-actin monoclonal antibody (Sigma, St, Louis, MO) in Can Get Signal solution 1 (1 : 2,000 dilution) for 1 hr at RT. After washing with 0.1% Tween-20/TBS (pH 7.4), the membranes were incubated with HRP-linked anti-rabbit IgG antibodies or HRP-conjugated anti-mouse Ig’s antibodies in Can Get Signal solution 2 (1 : 2,000 dilutions) for 1 hr at RT. Specific proteins were visualized with Western LightningTM Plus-ECL (PerkinElmer, MA) using an LAS 4000 (FUJIFILM, Tokyo, Japan).
Corticosterone ELISA
Blood was quickly collected from the hearts of mice by means of a needle (27 G) during anesthetization with diethyl ether (at 9:00 a.m. to 11:00 a.m.). Serum was isolated by centrifugation and stored in aliquots at −80°C. The corticosterone (CC) concentrations were determined by corticosterone ELISA (IBL, Hamburg, Germany) according to the manufacturer’s instructions.
Statistical analysis was performed using ANOVA (Tukey’s post hoc test).
Results
Thymic involution
Thymic involution had been reported in several LSDs model mice, so we wondered whether or not thymic involution occurred in SD mice. First, we measured the thymus weights in WT- and SD mice. The sizes and weights of thymuses derived from the SD mice were smaller at 15-weeks-old, although there were no change in the 14-weeks-old SD mice (Fig. 1a and b).
Change in thymic mass. a Thymuses derived from WT- and SD mice at 15-weeks-old. b The thymus weights of thymuses from normal and SD mice at 14, 15 and 16 weeks of age. Each point is the result for a single animal, and between three and seventeen animals were used for each time point (**P < 0.01, Turkey’s post hoc test)
Analysis of apoptosis in the thymus
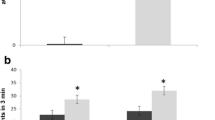
To examine the cell death features of the thymus, we performed flow cytometric analysis. Figure 2 presents representative flow cytometry data. The number of thymocytes derived from 15-week-old SD mice was decreased compared with that from WT mice. The morphological change of isolated thymocytes from 15-week-old SD mice exhibited typical apoptotic features in forward versus side light scatter plots (Fig. 2a). An increase in the binding of annexin V to the cell surface revealed on exposure of phosphatidylserine is an important marker of apoptotic cell death (Martin et al. 1995). We performed annexin V and PI staining of the cell surface of thymocytes to evaluate apoptosis and the DNA staining of dead cells. Figure 2a presents representative data on annexin V and PI staining. Thymocytes derived from 15-week-old SD mice exhibited high levels of early and late apoptotic stages, compared with ones from age-matched WT mice. Figure 2b summarizes quantitative data for annexin V and PI staining. The percentage of early apoptotic cells, annexinV+/PI-, was 15.1 ± 0.5 %, and that of late apoptotic or dead cells, annexinV+/PI+, was 17.0 ± 5.2 %, at 15 weeks of age. In contrast, such dramatic increases in apoptosis in the thymus could not be detected at 14-weeks-old.
Analysis of apoptosis in thymocytes. a Flow cytometric analysis of thymocytes isolated from WT- and SD mice at a late stage. The upper figure shows forward versus side light scatter plots, and the lower one shows Annexin-V versus PI for total cells. The results are representative WT- (n = 3) and SD mice (n = 3) samples. b Summary of the percentages of Annexin-V/PI-defined populations in all experiments. Error bars represent means ± SD (n = 3) (*P < 0.05, ***P < 0.001, Turkey’s post hoc test)
Next, to determine whether the apoptosis in thymus could be detected in situ or not, we performed in situ TUNEL analysis. We observed a physiological level of apoptosis in the cortex of the thymuses derived from WT mice. In contrast, the junction of the cortex and medulla could not be observed and abundant TUNEL+ apoptotic cells were detected throughout the thymuses derived from 15-week-old SD mice (Fig. 3).
Analysis of caspase activation
Next, we measured the activities of various caspases as executioners of apoptosis. Figure 4a shows that the caspase-3/7 activities in the thymuses from SD mice were higher than in those from WT mice, although there were no significant differences in the activities of other caspases. Consistent with the caspase activity assay results, the western blot experiment revealed that cleaved capase-3 was greatly increased in the thymus in 15-week-old SD mice but not in WT- or 14-week-old SD mice.
Caspase activition in thymuses from WT- and SD mice at 15-weeks-old. Thymus extracts were prepared from WT- and SD mice, and various caspase activities were measured. Error bars represent means ± SD (n = 4) (*P < 0.05, ***P < 0.001, Turkey’s post hoc test). Caspase activation was assayed by Western blot analysis with anti-cleaved caspase-3 and anti-β-actin antibodies
Determination of the corticosterone level in serum
We hypothesized that the apoptosis of thymocytes in SD was mice induced by an increased concentration of CC, a well known apoptotic inducer, and thus examined the CC level in serum. As shown in Fig. 5a, the level of CC in serum significantly increased in SD mice from 14 to 15 weeks, but not at an earlier age. In contrast, there was no change in the CC concentration in serum from the age-matched WT mice. Figure 5b indicates the relationship between the thymus weight and CC concentration in serum. The thymus weight was lower and the level of CC in serum was higher in 15- and 16-week-old SD mice compared with in age-matched WT mice. These data indicated the correlation between an increase in endocrine CC and elevation of apoptosis in the thymus at the late stage of the pathogenesis.
Variation of the corticosterone level in serum and the thymus weight. a Serum was collected from WT- and SD mice at each point and the corticosterone levels were determined by ELISA. Error bars represent means ± SD (at 11-weeks-old, n = 4–5; at 14-weeks-old, n = 5–7; at 15-weeks-old, n = 6–12, at 16-weeks-old, n = 3, respectively) (*P < 0.05, ***P < 0.001, Turkey’s post hoc test). b Summary of the thymus weight and the corticosterone level in serum at a late stage. SD mice at 13-weeks-old were starved for 72 h, serum was collected, and then the thymus weights and corticosterone levels measured
Discussion
In this study, we demonstrated that massive involution of the thymus in SD mice occurs in the disease at a late stage. The cell number in the thymus in SD mice rapidly decreased to about 3 % (2.5 ± 0.7 × 106 cells/organ) of that in WT mice at a late stage (data not shown). Our studies revealed that the massive cell death in the thymuses of SD mice is mediated by apoptotic events, including cell surface exposure of phosphatidiylserine, DNA degradation and caspase-3/7 activation. It has been reported that most thymocytes undergo apoptosis in the cortex (Surh and Sprent 1994). Histological analysis revealed that the structural boundary between the cortex and medulla could not be observed and apoptotic thymocytes were widely detected throughout the thymus at a late stage in SD mice. These results indicate that aberrant apoptotic events occur at the late stage of the pathogenesis in thymocytes from SD mice.
The production of autoantibodies to gangliosides accelerated disease progression (Yamaguchi et al. 2004). Hexb −/−/Fcrγ −/− mice exhibit a partial recovery of the thymic mass and attenuation of disease progression, including the production of autoantibodies. In the case of SD mice, Kanzaki et al. reported that thymic alteration in SD mice is partially dependent of the FcRγ chain, including severe decreases in CD4+/CD8+ T cells and chemotaxis of B1 cells toward CXCL13 in the thymus (Kanzaki et al 2010). These findings suggested that the production of autoantibodies can be attributed to thymic alterations. Previously, Cox et al. reported thymic involution and alteration of the growth hormone/insulin-like growth factor I pathway in feline GM1 gangliosidosis (Cox et al. 1998 and 1999), and Galbiati et al. indicated that autonomic denervation of lymphoid organs parallels progressive and severe lymphopenia in Krabbe disease model mice (Galbiati et al. 2007). These findings suggested that the nervous system exerts a significant influence on immune organs through the action of hormones and through direct innervation. Moreover, apoptosis could be induced by GM2, GM3 and GM1 in thymocytes (Zhou et al. 1998). At this time, we cannot exclude that autonomic innervation, alteration of neuroendocrine/immune axis and direct effects of gangliosides possibility contribute to the process of thymic involution in SD mice. In case of the SD mice, however, since GM2 accumulation was observed in the thymus at an early stage and the thymic involution was the result of a significant increase in thymic cell death with apoptosis between 14- and 15-weeks-old. Other factors may contribute to induce apoptosis at the late stage of thymic involution.
We found that the CC level in serum of SD mice was significantly higher than that in WT mice at a late stage of the pathogenesis. CC is a potent inducer of apoptosis of thymocytes (Thompson 1999), CD4+/CD8+ T cells most being significantly affected by CC in the T cell subpopulation (Weigers et al. 2001).
Some reports have indicated that excessive accumulation of substrates such as GM2 and GA2 in neuronal cells is in the case of GM2 gangliosidosis (Jean-Pyo et al. 2007 and Kawashima et al. 2009), and apoptotic cells have been observed in the central nervous system at a late stage of the pathogenesis (Jing-Qi et al. 1997 and Jeyakumar et al. 2003). It is postulated that substrate accumulation in the brain leads to neural cell death and/or neurodegeneration, and thus could disturb the feed back system for CC mediated by the hypothalamus-pituitary-adrenal axis. The course of the increase in endocrine CC in SD mice is not clear, because SD mice at 14 to 15 weeks of age do not exhibit ingestive failure and their body weights change little. Here we hypothesize that elevation of the concentration of CC in serum of SD mice induces a drastic increase in apoptosis of thymocytes, leading to thymic involution.
Previously, Gadola et al. reported the impaired selection of invariant natural killer T (iNKT) cells in thymuses from SD mice (Gadola et al. 2006). NKT knock out mice can not defensively reject microorganisms (Kinjo et al. 2005), infectants/parasites (Nieuwenhuis et al. 2009), or fungus (Matsuda et al. 2008). From the aspect of therapy, dysfunctions, including of NKT cells, are an important issue not only in GM2 gangliosidosis patients but also in other lysosomal disease patients.
So far, a biomarker in serum for monitoring the efficiency of treatment for lysosomal diseases has not been developed, although several experimental and clinical trials have been performed (Shapiro et al. 2009 and Jean-Pyo et al. 2007). In contrast, chitoriosidase (Hollak et al. 2001) and the chemokine CCL18 (Boot et al. 2004) in plasma are measured to evaluate the effectiveness of enzyme replacement therapy for Gaucher disease.
We propose that the thymic involution and the increase in the CC concentration in serum of SD mice are new aspects of the pathology, although further research is needed, including reversal of involution of the thymus by means of therapeutic approaches such as enzyme replacement therapy and cell therapy. The CC level in serum should be useable as a disease-specific marker for monitoring the thymic condition and progression of GM2 gangliosidosis.
References
Boot RG, Verhoek M, Fost ND et al. (2004) Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate maker for assessing therapeutic intervention. Blood 103:33–39
Cox NR, Ewald SJ, Morrison NE et al. (1998) Thymic alteration in feline GM1 gangliosidosis. Vet Immunol Immunopathol 63:335–353
Cox NR, Morrison NE, Sartin JL et al. (1999) Alterations in the growth hormone/insuline-like growth factor I pathway in feline GM1 gangliosidosis. Endocrinology 140:5698–5704
Gadola SD, Silk JD, Jeans A et al. (2006) Impaired selection of invariant natural killer cells in diverse mouse models of glycosphingolipid lysosomal storage disease. J Exp Med 203:2293–2303
Galbiati F, Basso V, Ludovico C et al. (2007) Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J Neurosci 27:13730–13738
Gravel A, Kaback M, Proia RL et al. (2001) The GM2 gangliosidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited diseases, 8th edn. McGraw-Hill, New York, pp 3827–3877
Hollak CE, Maas M, Aerts JM (2001) Clinically relevant therapeutic endpoints in type I Gaucher disease. J Inherit Metab Dis 24(Supplement 2):97–105
Jean-Pyo L, Jeyakumar M, Gonzalez R et al. (2007) Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med 13:439–447
Jeyakumar M, Thomas R, Elliot-Smith E et al. (2003) Central nervous system inflammation is a hallmark of pathogenesis in mouse of GM1 and GM2 gangliosidosis. Brain 126:974–987
Jing-Qi H, Trasler JM, Igdoura S et al. (1997) Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases. Hum Mol Genet 6:1879–1885
Kanzaki S, Yamaguchi A, Yamaguchi K et al. (2010) Thymic alterations in GM2 gangliosidoses model mice. PLoS ONE 8:e12105
Kawashima N, Tsuji D, Okuda T et al. (2009) Mechanism of abnormal growth in astrocytes derived from a mouse model of GM2 gangliosidosis. J Neurochem 111:1031–1041
Kinjo Y, Wu D, Kim G et al. (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 24:520–525
Mahuran DJ (1999) Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochem Biophys Acta 1455:105–138
Martin SJ, Reutelingsperger CP, McGahon AJ et al. (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–1556
Matsuda JL, Mallevaey T, Scott-Browne J et al. (2008) CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol 20:358–368
Nieuwenhuis EE, Matsumoto T, Lindenbergh D et al. (2009) CD1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest 119:1241–1250
Norflus F, Tifft CJ, McDonald MP et al. (1998) Bone marrow transplantation prolongs life span and ameliorates neurogenic manifestations in Sandhoff disease mice. J Clin Invest 101:1881–1888
Sango K, McDonald MP, Crawley JN et al. (1996) Mice lacking both subunits of lysosomal β-hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nat Genet 14:348–352, s
Shapiro BE, Pastores GM, Gianutsos J et al. (2009) Miglustat in late-onset Tay-Sachs disease: a 12-month, randomized, controlled clinical study with 24 months of extended treatment. Genet Med 11:425–433
Surh CD, Sprent J (1994) T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 372:100–103
Takahashi A, Saito K, Kuizumu Y (1974) An autopsy case of Sandhoff disease. Beitr Pathol 152:418–428
Thompson EB (1999) Mechanism of T-cell apoptosis induced by glucocorticoids. Trends Endocrinol Metab 10:353–358
Weigers GJ, Knoflach M, Bock G et al. (2001) CD4CD8TCRlow thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis. Eur J Immunol 31:2293–2301
Wu YP, Proia RL (2004) Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc Natl Acad Sci USA 101:8425–8430
Wu YY, Locker JM, Sugiyama E et al. (1994) Expression and specificity of human GM2 activator protein. J Biol Chem 269:6276–6283
Yamaguchi A, Katsuyama K, Nagahama K et al. (2004) Possible role of autoantibodies in the pathophysiology of GM2 gangliosidoses. J Clin Invest 113:200–208
Zarghooni M, Bukovac S, Tropak M et al. (2004) An alpha-subunit loop structure is required for GM2 activator protein binding by beta-hexosaminidase A. Biochem Biophys Res Commun 324:1048–1052
Zhou J, Shao H, Cox NR et al. (1998) Gangliosides enhance apoptosis of thymocytes. Cell Immunol 183:90–98
Funding
This work was supported by the Program the Promotion of Fundamental Studied Health Sciences of the National Institute of Biomedical Innovation (NIBIO) (Osaka, Japan), and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Ed Wraith
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Matsuoka, K., Tsuji, D., Taki, T. et al. Thymic involution and corticosterone level in Sandhoff disease model mice: new aspects the pathogenesis of GM2 gangliosidosis. J Inherit Metab Dis 34, 1061–1068 (2011). https://doi.org/10.1007/s10545-011-9316-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-011-9316-6