Abstract
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by the presence of antiphospholipid antibodies, which may trigger vascular thrombosis with consecutive infarcts. However, cognitive dysfunctions representing one of the most commonest neuropsychiatric symptoms are frequently present despite the absence of any ischemic brain lesions. Data on the structural and functional basis of the neuropsychiatric symptoms are sparse. To examine the effect of APS on hippocampal neurogenesis and on white matter, we induced experimental APS (eAPS) in adult female Balb/C mice by immunization with β2-glycoprotein 1. To investigate cell proliferation in the dentate gyrus granular cell layer (DG GCL), eAPS and control mice (n = 5, each) were injected with 5-bromo-2′-deoxyuridine (BrdU) once a day for 10 subsequent days. Sixteen weeks after immunization, eAPS resulted in a significant reduction of BrdU-positive cells in the DG GCL compared to control animals. However, double staining with doublecortin and NeuN revealed a largely preserved neurogenesis. Ultrastructural analysis of corpus callosum (CC) axons in eAPS (n = 6) and control mice (n = 7) revealed no significant changes in CC axon diameter or g-ratio. In conclusion, decreased cellular proliferation in the hippocampus of eAPS mice indicates a limited regenerative potential and may represent one neuropathological substrate of cognitive changes in APS while evidence for alterations of white matter integrity is lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the antiphospholipid syndrome (APS), stroke is one of the most common causes of disability and death (Cervera et al. 2009, 2015). However, apart from thrombotic events in APS various non-stroke neuropsychiatric manifestations are consistently observed (Yelnik et al. 2016a, b). In this very heterogeneous group of syndromes cognitive dysfunctions are most frequently reported (Conti et al. 2012; Jacobson et al. 1999; Tektonidou et al. 2006; Yelnik et al. 2016a, b). Although certain aPL types and levels seem to be strongly associated with non-thrombotic neurological symptoms (Stojanovich et al. 2013; Jacobson et al. 1999), the underlying mechanisms remain to be clarified. While aPL are persistent markers in APS patients it is currently under discussion whether these autoantibodies may also be produced intrathecally suggesting a central nervous system-independent autoimmune process (Sokol et al. 2007).
When focusing on the structural and molecular basis of the cognitive alterations in APS, the experimental model of APS (eAPS) with β2-Glycoprotein 1 (β2-GPI) immunized mice represents a valuable tool (Shrot et al. 2002). After immunization, these mice show increased aPL titers and develop a hyperactive and cognitively impaired phenotype in the absence of any ischemic features in routine histology (Frauenknecht et al. 2015; Shrot et al. 2002). Nevertheless, additional investigations have revealed a regional breakdown of blood–brain barrier (BBB) integrity and IgG accumulation in neurons (Katzav et al. 2014) as well as alterations in 5-HT1A receptor binding densities, especially in the hippocampus (Frauenknecht et al. 2013). Furthermore, there is evidence of decreased synaptic spine density in hippocampal CA1 pyramidal neurons and a reduction of synaptopodin protein expression in the hippocampus (Frauenknecht et al. 2015).
Although inflammation and a distinct CNS phenotype are absent in eAPS at this stage of disease (Frauenknecht et al. 2013, 2014), dendritic atrophy and dendritic spine loss are features reminiscent of mouse models of systemic lupus erythematosus (SLE) (Sakic et al. 1998, 2000). Frequently, animal models of SLE display neurologic and behavioral manifestations, but the pathological changes observed in the brain are much more impressive than the changes seen in the eAPS model (Denenberg et al. 1992; Ma et al. 2006; Sakic et al. 1998, 2000). One might speculate that the changes seen in eAPS mice may reflect a form of subtle autoimmunity-induced neurodegenerative disease.
To further elucidate the structural and molecular changes underlying the cognitive decline in APS in the absence of stroke, we used the well-characterized mouse model of APS (eAPS) by immunization with β2-GPI and lack of ischemic damage (Shrot et al. 2002). In our present study, we first addressed the question of whether reduced dendritic complexity in the hippocampus may be associated with changes of the hippocampal volume. We further hypothesized that long exposure to aPL in eAPS may have an impact on the proliferation of cells as well as on neuroblast differentiation as it has been shown for other autoimmune disease models such as lupus (Kapadia et al. 2012; Stanojcic et al. 2009) and experimental autoimmune encephalomyelitis (EAE) (Huehnchen et al. 2011). For this purpose, we investigated the number of proliferating cells and generation of newborn neurons in the subgranular zone/granular cell layer of dentate gyrus of the hippocampus in eAPS and control mice. White matter abnormalities have been suggested to play a role in the pathophysiology of cognitive deficits (Filley 2010; Penke et al. 2012). We therefore expanded our analyses to include ultrastructural examination of callosal myelinated fibers in eAPS mice.
Materials and methods
Animal experiments and tissue processing for hippocampus volumetry and immunohistochemistry/immunofluorescence
Animal experiments have been approved Israeli Health Ministry (Ethical approval no. 775/12) and by the Chaim Sheba Medical Center Animal Welfare Committee. All experiments were in accordance with national and international guidelines and regulations. Female Balb/C mice (n = 10) were obtained from Harlan Laboratories Limited (Israel) and housed under standard conditions (23 °C ± 1 °C), 12 h light cycle (7 am ± 7 pm) with ad libitum access to food and water (Animal facility, Chaim Sheba Medical Center, Tel Hashomer).
For analysis of hippocampus volume, cell proliferation and neurogenesis in the DG GCL, female BALB/c mice at the age of 8 weeks were immunized once with 10 ug β2 GPI emulsified in complete Freund’s adjuvant (CFA) (n = 5) while controls were immunized with CFA alone (n = 5). For Measurement of aPL levels (β2-GPI-dependent aCL), 96-well plates were coated with cardiolipin (50 ug/ml in ethanol) and the ELISA technique was used as previously described (Bakimer et al. 1992).
Hyperactive behavior within a 3-min period was assessed 16 weeks after immunization by the staircase test as described (Pick et al. 1996; Simiand et al. 1984). After determination of aCL levels and hyperactive behavior in the staircase test, mice were injected with BrdU (100 mg/kg KG; Sigma-Aldrich Cat. B5002) once a day for ten subsequent days to assess cellular proliferation over a longer period. After the last BrdU injection, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg) followed by transcardial perfusion with PBS followed by 4% paraformaldehyde (PFA) in PBS. The brains were carefully removed, post-fixed in 4% PFA at 4 °C overnight and stored in 0.5% PFA. Brains were sliced into 40-µm coronal sections using a vibratome (VT1000S, Leica, Germany). Sections were collected in series of 6 throughout the entire rostral–caudal extent of the hippocampus. From each series the first section was Nissl-stained according to standard protocols and was then used for volumetry as described below. Section 2 was subjected to DCX-immunohistochemistry, section 3 to BrdU-immuno-fluorescence, section 4 to BrdU and DCX double staining and section 5 was used for BrdU and NeuN double staining.
For determination of hippocampus volume, Nissl-stained sections were scanned at a magnification of 1.6× with a Leica Microscope (Leica, Wetzlar, Germany) and digitized. Using the MCID image analysis system (Imaging Research Inc, St. Catharines, Ontario, Canada), hippocampal volumes were calculated by multiplying sectional areas by interval thickness and expressed as hippocampus volume in mm3 ± SEM.
For DCX immunohistochemistry (IHC) free-floating 40-µm-thick vibratome sections were incubated in 1 ml H2O2 (30%) in 100 ml methanol/TBS (1 + 1) to block endogenous peroxidases. Thereafter, the sections were washed for 10 min with TBS and twice for 10 min with TBS-T (0.2% Triton X-100) followed by a 30-min incubation step [5% normal goat serum (NGS)/TBS]. Free-floating sections were incubated with anti-DCX antibody [rabbit polyclonal anti-DCX antibody; ab18723; 1:2000 in 5% NGS/TBS; Abcam, UK] followed by 30 min incubation (RT) with biotinylated anti-rabbit antibody (1:50 5% NGS/TBS; Linares, Germany). After rinsing with TBS-T (4 × 10 min), immunoreactivity was visualized by the avidin–biotin–peroxidase complex method (Vectastain Kit Universal, Vector laboratories, UK) followed by 10 min washing steps with TBST and TBS. Subsequently, sections were developed with the DAB Labvision Kit (Thermoscientific, Germany) for 30 s at room temperature, rinsed with TBS, wet-mounted onto slides, dehydrated and cover-slipped using Cytoseal XYL (Thermoscientific, Germany). Negative controls were obtained by omitting the primary antibody.
BRDU/DCX double-staining immunofluorescence was performed as follows: 40-µm-thick vibratome sections were washed for three times in TBS, incubated in 50% formamide/2x SSC for 1.5 h at 65 °C followed by two washing steps with 2xSSC. Afterwards, free-floating sections were incubated in 2 M HCl for 30 min at 37 °C and then neutralized with 0.1 M borate buffer pH 8.5. Sections were washed in TBS, blocked with 5% NGS in TBST and incubated overnight at 4 °C with the anti-BRDU antibody (Rat anti-BrdU antibody; clone BU1/75 (ICR1); 1:500 in 5% NGS/TBS-T; AbD Serotec USA). Thereafter, sections were washed thoroughly in TBS followed by incubation for 2 h at room temperature in Alexa Fluor 546 labeled anti-rat antibody (1:200 in 5% NGS/TBS). After that, sections were first washed in TBS and then three times in PBS, followed by blocking in 10% BSA in TBST and incubation overnight at 4 °C with the anti-DCX antibody (1:2000 in 5% NGS/TBS; Abcam, UK). Subsequently, sections were washed thoroughly in PBS-Tween (0.25% Tween 20) followed by incubation for 2 h at room temperature with an Alexa Fluor 488 anti-rabbit antibody (1:200 in 10% BSA/PBS). Thereafter, sections were washed in PBS-Tween then three times in PBS followed by incubation for 5 min at room temperature with DAPI (1:1000 in PBS). After washing in PBS, sections were wet-mounted onto slides and immediately cover-slipped using Pro Long Gold antifade. Negative controls were obtained by omitting the primary antibody.
BRDU/NeuN double-staining immunofluorescence was performed as in BrdU/DCX double staining. After that, sections were first washed in TBS and then three times in PBS followed by blocking in 10% BSA in PBS–0.2% Triton X-100 and incubation with the anti-NeuN antibody (1:500 in 10% BSA, 0,2% Triton X-100, PBS; clone A60 Millipore, Germany) for 2 h at room temperature. Afterwards sections were washed thoroughly in PBS-Tween (0.25% Tween 20) followed by incubation for 2 h at room temperature with an Alexa Fluor 488 anti-mouse antibody (1:400 in 10% BSA/PBS). After washing in PBS, sections were wet-mounted onto slides and immediately cover-slipped using Pro Long Gold antifade. Negative controls were obtained by omitting the primary antibody.
Cell counting
Counting of positively labeled cells was performed by two investigators (KF, PL) blinded to the group assignment of each animal using a Leica DM6000B microscope. Stereological quantification of immunoreactive cells was performed in every sixth section in each DG in the granule cell layer (GCL), including the subgranular zone. Seven sections per staining were used to determine the number of immunoreactive cells. DCX-, NeuN and BrdU-positive as well as double-positive cells were counted at 40× using a Leica microscope (Leica, Germany). Due to artificial damage of single DGs, the total number of positively labeled cells X per DG GCL per mouse was estimated by multiplying the counted cell number by a correction factor Z. Z was determined for each animal by dividing the number of 14 DG GCL (2 DG GCL per section multiplied by 7 sections = 14) by the available and undamaged number of DG GCL (X) per animal. Cell numbers were then multiplied by Z (Z = 14/X). The total number of positive cells per DG GCL animal could be determined by multiplying the corrected cell number by 6.
Tissue processing for light and electron microscopy
For light and electron microscopic assessment of callosal fibers, vibratome sections were obtained from 6 eAPS and 7 control mice brains from our previous study (Frauenknecht et al. 2015). Animal experiments from this previous study have been performed in the same manner as in the present study. One 40-µm-thick section per mouse at the level of the anterior commissure was fixed in 3% glutaraldehyde for 2 h at room temperature. Following several rinses in 0.2 M sodium cacodylate buffer (pH 7.3), tissue samples were post-fixed in 1% osmium tetroxide in cacodylate buffer for 2 h. The samples were then dehydrated through an ascending series of ethanol concentrations and embedded in resin with propylene oxide. Semi-thin (0.65 µm) sections for light microscopy and ultrathin (50 nm) sections for electron microscopy were cut in the sagittal plane on a Leica Ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany) starting at midline position (c.f. Fig. 3a for further details of the tissue processing). Semi-thin sections were stained with methylene blue and viewed with a Leica light microscope (DM2000, Leica Microsystems, Wetzlar, Germany). Ultrathin sections were stained with an alcoholic solution of 1% uranyl acetate and lead citrate in sodium hydroxide and examined with a Zeiss EM-910 transmission electron microscope (Carl Zeiss, Germany). Quantitative electron microscopy was performed on approximately 100–200 myelinated fibers/slice randomly photographed at a magnification of 10,000. Total fiber diameter (µm) and axon diameter (µm) were determined using MCID Analysis software. Subsequently, the g-ratio (axon diameter/outer diameter of the myelinated fiber) was calculated. Morphometric analysis of myelinated fibers was performed on five images. Further, the number of axons was counted and the axon density determined and expressed as mean number/µm2 ± SEM.
Statistical analysis
Statistical analysis was performed using the general statistics module of Analyse-it™ for Microsoft Excel (Analyse-it Software, Ltd., Leeds, UK). Normality was checked using the Shapiro–Wilk test. Significant effects were confirmed by the Student’s t test (normal distribution) or Mann–Whitney test (not normally distributed) with a significance level at p < 0.05. Results are presented as mean ± SEM. The investigators carrying out the analyses were blinded to group identity. Additionally, effect sizes were calculated according to Cohen (1988) as small (d = 0.1), medium (d = 0.3) or large (d = 0.5).
Results
aPL, staircase test
Analysis of β2-GPI-dependent aCL antibodies revealed significantly higher ODs as measures of antibody binding in the ELISA assay in eAPS mice compared to controls (Control vs. eAPS: OD 0.0258 ± 0.12951 vs. 1.1162 ± 0.12951; p = 0.0003, t test; d = 3.129) (Fig. 1a). In behavioral measures of a 3-min staircase test, eAPS mice showed a significantly higher number of stairs climbed as well as of rearing movements compared to control animals (Control vs. eAPS; stairs: 24.2 ± 1.62 vs. 32.0 ± 1.62; p = 0.0093; d = 1.034; rearing movements: 22.8 ± 1.75 vs. 28.6 ± 1.75; p = 0.0468; t test; d = 0.814) (Fig. 1b).
Evidence for successful induction of eAPS in mice. a eAPS mice developed significantly higher levels of β2-GPI-dependent anti-cardiolipin antibodies (aCL) compared to controls immunized with adjuvant alone. b Mice immunized with β2-GPI exhibited the typical behavioral alterations in this ePAS model, namely a significantly higher number of stairs climbed as well as of rearing movements compared to control animals (*p < 0.05). OD optical density
Hippocampus volumetry
Volume analysis of the hippocampus of eAPS and control mice revealed no significant differences between the experimental groups (Control vs. eAPS: 2.7 mm3 ± 0.08 vs. 2.6 mm3 ± 0.1; p = 0.6142; t test; d = 1.104) (not shown).
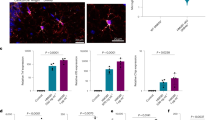
Proliferation (BrdU), post-mitotic neuroblasts and immature neurons (DCX), and mature neurons (NeuN)
Overall, the number of BrdU-positive proliferating cells was significantly lower in the DG of eAPS mice (1183 ± 245) compared to control mice (1994 ± 245) (p = 0.0473; t test; d = 1.38) (Fig. 2). Interestingly, the number of neuroblasts/immature DCX + cells was not significantly reduced in eAPS mice DGs compared to control mice (Control vs. eAPS 3791 ± 277 vs. 3677 ± 328; p = 0.8200; t test; d = 0.162) (Fig. 2). The number of DCX and BrdU double-positive cells was not significantly different for eAPS mice (492 ± 120) vs. control mice (803 ± 120; p = 0.1046; t test; d = 1.046) (Fig. 2). The same was seen for NeuN and BrdU double-positive cells (eAPS mice (1022 ± 332) vs. control mice (749 ± 248); p = 0.1528; t test; d = 0.53) (Fig. 2).
Analysis of hippocampal cell proliferation. a BrdU-positive proliferative cell number was significantly lower in the DG of eAPS mice compared to control mice (*p < 0.05), whereas the number of neuroblasts/immature DCX + neurons as well as of DCX and BrdU double-positive and of NeuN and BrdU double-positive cells was largely preserved. Representative photomicrographs of BrdU-stained sections of Control (b) and eAPS (c) mice (scale bar 50 µm) as well as of DCX-stained section (d) (scale bar 200 µm; inlet 50 µm) and BrdU and DCX double-positive stained section (e) from one control animal (scale bar 50 µm; inlet 25 µm). Photomicrographs were adjusted to brightness
Electron microscopy
Experimental APS and control mice from our previous study (Frauenknecht et al. 2015) showed largely the same myelinated callosal fiber density/µm2 (Control vs. eAPS: 1.97 ± 0.13 vs. 1.95 ± 0.14; p = 0.9101, t test). The size–frequency distributions for axon and fiber area showed a shift towards larger axon diameter in eAPS mice compared to control mice (Fig. 3). However, overall axon and fiber size as well as g-ratio did not significantly differ (Control vs. eAPS: axon size 0.58 ± 0.019 vs. 0.60 ± 0.016 p = 0.545, t test; d = 0.349; fiber size 0.80 ± 0.028 vs. 0.81 ± 0.020, p = 0.254, t test; d = 0.101; p = 0.179, t test; d = 0.803; 0.71 ± 0.008 vs. 0.73 ± 0.002; p = 0.179, t test; d = 0.421) (Fig. 3). Myelin abnormalities including demyelination, excessive myelin figures or collapsed myelin sheaths were not detectable.
Ultrastructural analysis of myelinated axons in the corpus callosum. a Gives an impression of tissue processing for ultrastructural analysis. Representative images of callosal fibers from one control (top) and one eAPS mouse (bottom) are shown on the right side (bar corresponds to 1 µm). b The size–frequency distributions for axon and fiber area show a shift to larger axon diameters in eAPS mice. c However, g-ratio, axon diameter, fiber diameter and myelin thickness (not shown) did not significantly differ between eAPS and control mice
Discussion
One unresolved question in both human patients and animal models of the antiphospholipid syndrome is the cause of the behavioral abnormalities and cognitive alterations in the absence of an obvious morphological substrate. We could previously show that a reduction of the dendritic complexity of hippocampal CA1 neurons may contribute to cognitive deficits (Frauenknecht et al. 2015). A similar pattern of neuronal atrophy and reduced spine density has been reported in the murine lupus model (Sakic et al. 2000). However, in contrast to the lupus model (Sakic et al. 2000; Sled et al. 2009), long exposure to aPL was not accompanied by any changes in hippocampal volume. This may also explain why associations between aPL levels and hippocampal volume among APS patients with neurological manifestations have not been described.
The major finding of the present study was that cellular proliferation in the hippocampal dentate gyrus of eAPS mice was reduced while neuroblast differentiation remained largely preserved. Continuous adult neurogenesis in the DG is a complex process including multiple steps of cell maturation (Kempermann et al. 2015; Mandyam et al. 2007). Many studies have shown that different pathologic and physiologic stimuli including seizures, stress, learning, and exercise modulate the rate of neurogenesis (Eisch et al. 2008; Gould et al. 1999; Kronenberg et al. 2003; Nakagawa et al. 2000; Parent et al. 2006; van Praag et al. 1999). Reduced numbers of BrdU-positive proliferating cells in the hippocampus have been shown in rodents after acute and chronic LPS treatment (Bastos et al. 2008; Fujioka and Akema 2010). In autoimmune diseases such as SLE, disturbed cellular proliferation in the DG has been shown in MRL/lpr mice. These mice display decreased BrdU-positive cells in the taste buds indicating impaired cellular renewal (Kim et al. 2012). Using the same animal model, Stanojicic et al. (2009) found impaired proliferative capacity in the brain of this lupus-like disease.
To our knowledge, no data exists concerning the effect of APS/aPL on hippocampal cell proliferation and neurogenesis. The significant reduction of hippocampal cell proliferation in our present study, reflected by a 59% decrease of BrdU-labeled cells in eAPS mice is in accordance with the above-mentioned alterations in the lupus mouse model. As we assessed the numbers of BrdU-labeled cells after 10 days of BrdU treatment, we assume that not only proliferation but also survival of newborn cells is impaired by eAPS. Interestingly, the number of neuroblasts/nascent neurons as well as mature neurons was largely preserved in eAPS mice. This indicates that the differentiation process itself is not affected by aPL exposure. This finding also suggests a compensatory shift towards neuronal maturation despite the decreased regenerative potential. In future studies it would be interesting to find out whether higher levels of aPL or longer exposure to aPL may exceed this capacity, resulting in a decrease of DG neuroblasts and neurons.
The exact mechanism whereby aPL influences cell proliferation is not yet clear. Apart from a direct interaction of aPL with hippocampal progenitor cells leading to a decrease in proliferating cells, there are also hints for indirect mechanisms of action. Altered gene expression and dysregulated Sonic hedgehog, β-catenin and Notch signaling pathways have been shown to modulate hippocampal neurogenesis in experimental autoimmune encephalomyelitis (Huehnchen et al. 2011). Alternatively, reduced hippocampal cell proliferation in eAPS mice may also be caused by alterations in binding densities of serotonergic 5-HT1A receptors and GABAA receptors in the hippocampus (Frauenknecht et al. 2013). Both receptors contribute to neurogenesis in the SGZ (Alenina and Klempin 2015; Banasr et al. 2004; Tozuka et al. 2005; Wang et al. 2005). Blockade of 5-HT1A receptors by specific inhibitors or depletion of serotonin has been shown to reduce the number of BrdU + cells in the SGZ (Klempin et al. 2010; Radley and Jacobs 2002; Ueda et al. 2005).
Apart from the hippocampus, the white matter plays a central role in cognition and transfer of information between different brain regions (Filley 2010; Penke et al. 2012; Ulrich-Lai and Herman 2009). Studies evaluating the effect of aPL/APS on white matter in humans with SLE describe the association of neurological alterations with WM changes that mainly include WM hyperintensities related to ischemic events. (Kaichi et al. 2014; Valdés-Ferrer et al. 2008). However, there is also evidence that WM may be susceptible to elevated aPL titers in the absence of ischemic features. In a study with neurologically asymptomatic female APS patients with obstetric complications without signs of ischemic events, elevated titers for anti-ß2GPI and/or Lupus anticoagulant were associated with subtle white matter changes (Pereira et al. 2016). Using diffusion tensor imaging the authors provided evidence for impairment of myelin and/or axonal structures (Pereira et al. 2016).
In the present eAPS model, neither direct axonal damage nor myelin degeneration was detectable. Although we found ultrastructurally a shift towards larger axon diameter in eAPS, axon diameter and g-ratio did not significantly differ between eAPS and control mice. Thus, there is no evidence that white matter changes may be responsible for the cognitive alterations in the eAPS model used.
Conclusion
The present study contributes new information toward understanding the biological basis of the behavioral and cognitive anomalies occurring in the absence of ischemic alterations in both APS and the murine disease model.
Abbreviations
- aCL:
-
Anti-cardiolipin antibodies
- aPL:
-
Antiphospholipid antibodies
- APS:
-
Antiphospholipid syndrome
- BBB:
-
Blood–brain barrier
- BrdU:
-
5-Bromo-2′-deoxyuridine
- BSA:
-
Bovine serum albumin
- CA:
-
Cornu ammonis
- CNS:
-
Central nervous system
- CC:
-
Corpus callosum
- CFA:
-
Complete Freund’s adjuvant
- DCX:
-
Doublecortin
- DG GCL:
-
Dentate gyrus granular cell layer
- EAE:
-
Experimental autoimmune encephalomyelitis
- eAPS:
-
Experimental antiphospholipid syndrome
- LPS:
-
Lipopolysaccharide
- NGS:
-
Normal goat serum
- OD:
-
Optical density
- PBS:
-
Phosphate buffered saline
- PFA:
-
Paraformaldehyde
- RT:
-
Room temperature
- SEM:
-
Standard error of mean
- SLE:
-
Systemic lupus erythematosus
- SSC:
-
Saline-sodium citrate
- TBS:
-
Tris-buffered saline
- TBS-T:
-
TBS with Triton X-100
- WM:
-
White matter
References
Alenina N, Klempin F (2015) The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res 277:49–57
Bakimer R, Fishman P, Blank M, Sredni B, Djaldetti M, Shoenfeld Y (1992) Induction of primary antiphospholipid syndrome in mice by immunization with a human monoclonal anticardiolipin antibody (H-3). J Clin Invest 89:1558–1563
Banasr M, Hery M, Printemps R, Daszuta A (2004) Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the DG and the subventricular zone. Neuropsychopharmacology 29:450–460
Bastos GN, Moriya T, Inui F, Katura T, Nakahata N (2008) Involvement of cyclooxygenase-2 in lipopolysaccharide-induced impairment of the newborn cell survival in the adult mouse dentate gyrus. Neuroscience 155:454–462
Cervera R, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Kiss E et al (2009) Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 68:1428–1432
Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E et al (2015) Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 74:1011–1018
Cohen J (1988) Statistical power analysis for the behavioral sciences 2. Lawrence Erlbaum Associates, Hillsdale
Conti F, Alessandri C, Perricone C, Scrivo R, Rezai S, Ceccarelli F et al (2012) Neurocognitive dysfunction in systemic lupus erythematosus: association with antiphospholipid antibodies, disease activity and chronic damage. PLoS One 7:e33824
Denenberg VH, Sherman GF, Rosen GD, Morrison L, Behan PO, Galaburda AM (1992) A behavior profile of the MRL/Mp lpr/lpr mouse and its association with hydrocephalus. Brain Behav Immun 6:40–49
Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS (2008) Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci 28:11785–11791
Filley CM (2010) White matter: organization and functional relevance. Neuropsychol Rev 20:158–173
Frauenknecht K, Katzav A, Grimm C, Chapman J, Sommer CJ (2013) Neurological impairment in experimental antiphospholipid syndrome is associated with increased ligand binding to hippocampal and cortical serotonergic 5-HT1A receptors. Immunobiology 218:517–526
Frauenknecht K, Katzav A, Grimm C, Chapman J, Sommer CJ (2014) Altered receptor binding densities in experimental antiphospholipid syndrome despite only moderately enhanced autoantibody levels and absence of behavioral features. Immunobiology 219:341–349
Frauenknecht K, Katzav A, Weiss Lavi R, Sabag A, Otten S, Chapman J et al (2015) Mice with experimental antiphospholipid syndrome display hippocampal dysfunction and a reduction of dendritic complexity in hippocampal CA1 neurones. Neuropathol Appl Neurobiol 41:657–671
Fujioka H, Akema T (2010) Lipopolysaccharide acutely inhibits proliferation of neural precursor cells in the dentate gyrus in adult rats. Brain Res 1352:35–42
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265
Huehnchen P, Prozorovski T, Klaissle P, Lesemann A, Ingwersen J, Wolf SA et al (2011) Modulation of adult hippocampal neurogenesis during myelin-directed autoimmune neuroinflammation. Glia 59:132–142
Jacobson MW, Rapport LJ, Keenan PA, Coleman RD, Tietjen GE (1999) Neuropsychological deficits associated with antiphospholipid antibodies. J Clin Exp Neuropsychol 21:251–264
Kaichi Y, Kakeda S, Moriya J, Ohnari N, Saito K, Tanaka Y et al (2014) Brain MR findings in patients with systemic lupus erythematosus with and without antiphospholipid antibody syndrome. AJNR 35:100–105
Kapadia M, Stanojcic M, Earls AM, Pulapaka S, Lee J, Sakic B (2012) Altered olfactory function in the MRL model of CNS lupus. Behav Brain Res 234:303–311
Katzav A, Menachem A, Maggio N, Pollak L, Pick CG, Chapman J (2014) IgG accumulates in inhibitory hippocampal neurons of experimental antiphospholipid syndrome. J Autoimmun 55:86–93
Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult Hippocampus. Cold Spring Harb Perspect Biol 7:a018812
Kim A, Feng P, Ohkuri T, Sauers D, Cohn ZJ, Chai J et al (2012) Defects in the peripheral taste structure and function in the MRL/lpr mouse model of autoimmune disease. PLoS One 7:e35588
Klempin F, Babu H, de Pietri Tonelli D, Alarcon E, Fabel K, Kempermann G (2010) Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2010.00014
Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M et al (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463
Ma X, Foster J, Sakic B (2006) Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J Neuroimmunol 179:26–36
Mandyam CD, Harburg GC, Eisch AJ (2007) Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 146:108–122
Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer PL et al (2000) Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia 41:10–18
Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH (2006) Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol 59:81–91
Penke L, Maniega SM, Bastin ME, Valdés Hernández MC, Murray C, Royle NA et al (2012) Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry 17:1026–1030
Pereira FR, Macri F, Jackowski MP, Kostis WJ, Gris JC, Beregi JP et al (2016) Diffusion tensor imaging in patients with obstetric antiphospholipid syndrome without neuropsychiatric symptoms. Eur Radiol 4:959–968
Pick CG, Peter Y, Terkel J, Gavish M, Weizman R (1996) Effect of the neuroactive steroid alpha-THDOC on staircase test behavior in mice. Psychopharmacology 128:61–66
Radley JJ, Jacobs BL (2002) 5-HT1A receptor antagonist administration decreases cell proliferation in the DG. Brain Res 955:264–267
Sakic B, Szechtman H, Denburg JA, Gorny G, Kolb B, Whishaw IQ (1998) Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J Neuroimmunol 87:162–170
Sakic B, Kolb B, Whishaw IQ, Gorny G, Szechtman H, Denburg JA (2000) Immunosuppression prevents neuronal atrophy in lupus-prone mice: evidence for brain damage induced by autoimmune disease? J Neuroimmunol 111:93–101
Shrot S, Katzav A, Korczyn AD, Litvinju Y, Hershenson R, Pick CG et al (2002) Behavioral and cognitive deficits occur only after prolonged exposure of mice to antiphospholipid antibodies. Lupus 11:736–743
Simiand J, Keane PE, Morre M (1984) The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology 84:48–53
Sled JG, Spring S, van Eede M, Lerch JP, Ullal S, Sakic B (2009) Time course and nature of brain atrophy in the MRL mouse model of central nervous system lupus. Arthritis Rheum 60:1764–1774
Sokol DK, O’Brien RS, Wagenknecht DR, Rao T, McIntyre JA (2007) Antiphospholipid antibodies in blood and cerebrospinal fluids of patients with psychosis. J Neuroimmunol 190:151–156
Stanojcic M, Burstyn-Cohen T, Nashi N, Lemke G, Sakic B (2009) Disturbed distribution of proliferative brain cells during lupus-like disease. Brain Behav Immun 23:1003–1013
Stojanovich L, Kontic M, Smiljanic D, Djokovic A, Stamenkovic B, Marisavljevic D (2013) Association between non-thrombotic neurological and cardiac manifestations in patients with antiphospholipid syndrome. Clin Exp Rheumatol 31:756–760
Tektonidou MG, Varsou N, Kotoulas G, Antoniou A, Moutsopoulos HM (2006) Cognitive deficits in patients with antiphospholipid syndrome: association with clinical, laboratory, and brain magnetic resonance imaging finding. Arch Int Med 166:2278–2284
Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47:803–815
Ueda S, Sakakibara S, Yoshimoto K (2005) Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning. Neuroscience 135:395–402
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409
Valdés-Ferrer SI, Vega F, Cantú-Brito C, Ceballos-Ceballos J, Estañol B, García-Ramos G et al (2008) Cerebral changes in SLE with or without antiphospholipid syndrome: a case–control MRI study. J Neuroimaging 18:62–65
van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270
Wang LP, Kempermann G, Kettenmann H (2005) A subpopulation of precursor cells in the mouse DG receives synaptic GABAergic input. Mol Cell Neurosci 29:181–189
Yelnik CM, Kozora E, Appenzeller S (2016a) Cognitive disorders and antiphospholipid antibodies. Autoimmun Rev 15:1193–1198
Yelnik CM, Kozora E, Appenzeller S (2016b) Non-stroke central neurological manifestations in antiphospholipid syndrome. Curr Rheumatol Rep (2016) 18:11
Acknowledgements
The technical expertise of Magdeleine Herkt and Nicole Roder is kindly acknowledged. The authors are grateful to Professor Elisabeth Jane Rushing (University Hospital Zurich, Switzerland) for critically reading the manuscript.
Funding
The present work was supported by grants from the MAIFOR program (intramural funding, Medical Center of the Johannes Gutenberg University Mainz, Germany) from the Chief Scientist in the Israel Ministry of Health and from Israel Science Foundation. Katrin Frauenknecht is the recipient of a Career Development Award by the Stavros Niarchos Foundation (https://www.snf.org/22474).
Author information
Authors and Affiliations
Contributions
Experimental design: KF, AK, CJS; animal experiments: AK, RW; histology/volumetry/morphometric studies: KF, PL, HDP; figures: KF, PL, HDP; manuscript KF, PL, JC, CJS. All authors read and approved the final manuscript. The authors declare no competing interests.
Corresponding author
Ethics declarations
Ethical approval
Animal experiments have been approved Israeli Health Ministry (Ethical approval no. 775/12) and by the Chaim Sheba Medical Center Animal Welfare Committee. All experiments were in accordance with national and international guidelines and regulations. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Frauenknecht, K., Leukel, P., Weiss, R. et al. Decreased hippocampal cell proliferation in mice with experimental antiphospholipid syndrome. Brain Struct Funct 223, 3463–3471 (2018). https://doi.org/10.1007/s00429-018-1699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1699-9