Abstract
Experience with new-born screening (NBS) for disorders of fatty-acid oxidation (FAOD) is now becoming available from an increasing number of programs worldwide. The spectrum of FAOD differs widely between ethnic groups. Incidence calculations from reports from Australia, Germany, and the USA of a total of 5,256,999 newborns give a combined incidence of all FAOD of approximately 1:9,300. However, it appears to be much lower in Asians. Consequently, a significant prevalence and evidence for a clear benefit of NBS is proven for medium-chain acyl-CoA dehydrogenase deficiency (MCAD) only in countries with a high percentage of Caucasians, with very-long-chain acyl-CoA dehydrogenase deficiency (VLCAD) and long-chain 3-hydroxy acyl-CoA dehydrogenase deficiency (LCHAD) being additional candidates. The long-term benefit for many disorders has still to be evaluated and will require international collaboration, especially for the rarest disorders. Short-chain acyl-CoA dehydrogenase deficiency (SCAD) [as well as Systemic carnitine transporter deficiency (CTD) and dienoyl-CoA reductase deficiency (DE-RED)] are conditions of uncertain clinical significance, but most FAOD have a spectrum of clinical presentations (healthy–death). Confirmatory diagnostic procedures should be agreed upon to ensure international comparability of results and evidence-based modifications. The case of short-chain acyl-CoA dehydrogenase deficiency (SCAD) deficiency shows that even inclusion of conditions without a clearly known natural course may prove useful with respect to gain of knowledge and consecutive exclusion of a biochemical abnormality without clinical significance, although this line of argument implies the existence of structured follow-up programs and bears ethical controversies. As a final conclusion, the accumulated evidence suggests all FAOD should to be included into tandem mass spectrometry (MS/MS)-based NBS programs provided sufficient laboratory performance is guaranteed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Disorders of fatty-acid oxidation (FAOD) are a group of frequent inborn errors of metabolism with an estimated combined incidence of 1:9,000. In Caucasian populations, the frequency of medium-chain acyl-CoA dehydrogenase (MCAD) deficiency is about the same as that of phenylketonuria (PKU), the condition for which newborn screening (NBS) was implemented in the 1960s. Most FAOD are identified by rapid acylcarnitine profile analysis by flow injection electrospray ionization (ESI) tandem mass spectrometry (MS/MS). Furthermore, they are treatable by simple avoidance of fasting in most cases, whereas metabolic decompensations can be fatal in unsuspected patients. Given their frequency, due to the improved clinical outcome achieved by presymptomatic initiation of treatment and the ability to apply acylcarnitine analysis by MS/MS to high-throughput testing using dried blood spots (DBS), MCAD deficiency and other FAOD were included into many NBS programs since the mid-1990s (Zytkovic et al. 2001; Schulze et al. 2003). The current state of NBS for FAOD in various regions and countries was reviewed and discussed during one session of the symposium on Fatty Acid Oxidation–Clinical, Biochemical and Molecular Aspects held in Fulda, Germany, in November 2008. Particular focus was given to selection of conditions into NBS programs, the relevance of test performance metrics, epidemiological data, and follow-up practices of abnormal screening results. This short report summarizes the content of the presented lectures and the conclusions drawn in the subsequent roundtable discussion.
Selection criteria for inclusion of FAOD into NBS programs
To aid in the selection of diseases to be included into screening programs, screening principles were first developed by Wilson and Jungner on behalf of the World Health Organization in 1968 (Wilson et al. 1968). Although these principles were not specifically developed for NBS and had to be modified for this specific theme, they are still the most commonly cited selection criteria. In 2002, however, the American College of Medical Genetics (ACMG) was commissioned by the Maternal and Child Health Bureau of the Health Resources and Services Administration (HRSA) of the United States Department of Health and Human Services to review the scientific basis of NBS and develop recommendations as to which disorders should be included in NBS programs. The impetus for a comprehensive review of the status of NBS was the scattered implementation of MS/MS in US screening laboratories, which led to marked discrepancies in the number of screened conditions. Whereas several states provided NBS for only three diseases, those that implemented amino acid and acylcarnitine profiling by MS/MS were screening for more than 30 conditions. In 2006, and after gathering input from national and international stakeholders in NBS and experts of the conditions under review, the ACMG’s conclusions and recommendations were published (Watson et al. 2006). An initial scoring system grouped eight of 13 FAODs detectable by acylcarnitine analysis within those conditions that every baby should be screened for. Upon further review, only five FAOD were deemed appropriate for inclusion in what was termed primary or core conditions (Table 1). The reasons to move short-chain acyl-CoA dehydrogenase deficiency (SCAD) deficiency to the group of secondary targets was that significant controversy exists as to whether SCAD deficiency is merely a biochemical phenotype without clinical correlate. For glutaric acidemia type II and medium-/short-chain 3-OH-acyl-CoA dehydrogenase deficiency (M/SCHAD) deficiency, the natural history is not sufficiently understood.
The secondary targets include conditions that did not meet all selection criteria. Most of them are nevertheless identified because they are included in the differential diagnosis of screening results observed in core conditions (Table 1). After all, screening tests do not primarily determine disease status but measure analytes that, in most cases, are not specific for a particular condition. For example, when screening by acylcarnitine analysis for MCAD deficiency, which achieved the highest score among all conditions that were considered in the ACMG report, it is expected to also uncover cases of M/SCHAD deficiency, medium-chain ketothiolase (MCKAT) deficiency and glutaric acidemia type 2. The estimated incidence of conditions was also considered by the ACMG, and higher scores were given to the more common conditions, whereas overall, this criterion was weighted less than some others, such as simplicity and benefit of early treatment on outcome and test characteristics (Table 2) (Watson et al. 2006).
Similar to the situation in the USA, the implementation of MS/MS into NBS programs has prompted several other countries to standardize their NBS menu, whereas others remain skeptical of the benefit of NBS for FAOD, except MCAD deficiency. For example, in Germany, a commission was established by the Ministry for Health and Social Security to determine the conditions for which every newborn should be screened. Different from the ACMG recommendations, six FAODs were included, and the fact that screening for these conditions could uncover cases of three additional FAODs was ignored (Table 1).
Implications of test performance on NBS
The performance of NBS programs with respect to false positive/negative rates (FPR, FNR), positive/negative predictive values (PPV, NPV), and detection rates has not received significant attention until multianalyte testing in the form of acylcarnitine and amino acid profiling by MS/MS was applied to NBS. Until then, the primary goal was 100% sensitivity with sufficient specificity, and parameters such as FPR and PPV were only considered for those conditions that were particularly affected by a large number of FPR, such as screening for congenital adrenal hyperplasia (van der Kamp and Wit 2004). NBS by MS/MS generating profiles of metabolites that by themselves are often not specific disease markers is not easily amenable to simple application of age-appropriate cutoff values but often also requires result interpretation. Accordingly, expansion of NBS panels increases the responsibility of laboratories to provide testing with highest sensitivity and specificity to allow identification of affected patients while minimizing the FPR (Rinaldo et al. 2006). With respect to FAOD, this requires laboratories to become familiar with the relevant metabolites and metabolite ratios expected to be abnormal in a particular condition and to set cutoffs at appropriate levels. This is now achievable by learning from the experience of programs that have been using MS/MS for several years and by participating in projects such as the Region 4 Collaborative Project, which aims at improving the analytical quality of NBS by MS/MS and is accessible to any interested screening laboratory (http://www.region4genetics.org/msms_data_project). Exemplary performance metrics for FAOD of three programs with multiyear experience are shown in Table 3.
It is extremely difficult to asses FNRs, as screening laboratories usually will not be informed about an FAOD diagnosis in an older, symptomatic child. False negative cases have been reported for MCAD (Maier et al. 2009) and are imminent in all FAODs, as acylcarnitine profiles may normalize in older infants (Boneh et al. 2006).
Newborn screening experiences and incidences of FAOD
Experience with NBS for FAOD for about a decade is now available from an increasing number of programs worldwide. Wilcken and colleagues reported a comprehensive outcome study revealing a reduction by 74% of severe metabolic decompensations and/or death in patients with MCAD deficiency who were identified by NBS (Wilcken et al. 2009). Reduced morbidity and mortality at an incremental cost below the range for accepted health care interventions has been shown for MCAD in The Netherlands and the UK (Pandor et al. 2004). Van der Hilst and colleagues calculated an incremental cost-effectiveness ratio of € 1,653 for every life year gained, which compares favorably to other preventive health measures (van der Hilst et al. 2007).
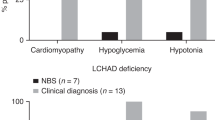
SCAD deficiency provides a different example following years of NBS. Whereas this condition was regarded a potentially life-threatening disorder prior to expanded NBS, patients identified by NBS seem to remain asymptomatic despite confirmation of severe enzyme deficiency. Accordingly, inclusion of this condition in NBS programs has been questioned and was removed from the screening panel in a few programs (van Maldegem et al. 2006; Waisbren et al. 2008; Wilcken et al. 2009). On the other hand, without inclusion of this disorder into NBS programs worldwide, this information may have eluded discovery to the detriment of patients who have been labelled with a diagnosis of SCAD deficiency when, indeed, an alternative or additional etiology of their clinical presentation should have been pursued. It may be argued then, that all FAOD should be included in NBS programs because evidence-based screening is only achievable by screening for these conditions and long-term follow-up of identified patients.
Incidence calculations from reports from Australia, Germany, and the USA of a total of 5,256,999 newborns give a combined incidence of all FAODs of approximately 1:9,300. Data from recent screening efforts in countries with different ethnic backgrounds reveal significantly different incidences (Table 2). This raises the question of whether an FAOD that is rarely identified in a particular population should be excluded from the screening panel, e.g., in Asians. Obviously, carefully evaluated pilot projects must be performed and evaluated in non-Caucasian populations before embarking on population screening. If screening for each FAOD would require significant effort, a separate DBS punch analysis, or even dedicated equipment, exclusion of such rare conditions seems reasonable. The situation is different for FAOD, however, because if any FAOD is deemed significant enough to be included into an NBS panel, there is no real additional effort, time, or cost accrued by screening for all detectable FAODs by acylcarnitine analysis. Screening for all conditions detectable by combined acylcarnitine and amino acid analysis by MS/MS can be expected to be more cost effective than screening for a single condition (Pandor et al. 2004). Of course, this is only true as long as the screening test performance is acceptable, which—as indicated above—is readily achievable given the growing number of NBS laboratories experienced in the use of MS/MS.
Recommendations for confirmatory diagnostic workup
Recommendations for the diagnostic workup of an abnormal NBS result have been developed, but—given the rarity of some of the conditions—are based mostly on expert opinion or consensus and lack unequivocal evidence. In the past, such procedural guidelines had been created by metabolic specialists to be used in their respective centers but for the most part were not made available to outsiders. Therefore, the ACMG established a work group of experts to develop guidelines for the follow-up of each primary and secondary condition it had recommended for inclusion into NBS programs. Following peer and ACMG board review, this work group’s suggested ACTion (ACT) sheets and diagnostic algorithms were published online (www.acmg.net) to be available for the primary care provider who may be faced with the task of follow-up of a presumptively positive screening result but does not have immediate access to a metabolic center. These guidelines are updated on a regular—yet not yearly—basis, and programs are encouraged to adopt them as they see fit and provide feedback for improvements.
Abbreviations
- NBS:
-
Newborn screening
- FAOD:
-
Fatty-acid oxidation disorders
- MCAD:
-
Medium-chain acyl-CoA dehydrogenase deficiency
- VLCAD:
-
Very-long-chain acyl-CoA dehydrogenase deficiency
- LCHAD/mTFP:
-
Long-chain 3-hydroxy acyl-CoA dehydrogenase deficiency/mitochondrial trifunctional protein deficiency
- CTD:
-
Systemic carnitine transporter deficiency
- SCAD:
-
Short-chain acyl-CoA dehydrogenase deficiency
- CPT I:
-
Carnitine palmitoyltransferase 1 deficiency
- CPT II:
-
Carnitine palmitoyltransferase-2 deficiency
- CACT:
-
Carnitine acylcarnitine translocase deficiency
- MAD/GA II:
-
Multiple acyl-CoA dehydrogenase deficiency/glutaric aciduria type II (synonym)
- MCKAT:
-
Medium-chain ketothiolase
- M/SCHAD:
-
Medium-/short-chain 3-OH-acyl-CoA dehydrogenase deficiency
- DE-RED:
-
Dienoyl-CoA reductase deficiency
- MS/MS:
-
Tandem mass spectrometry
References
Boneh A, Andresen BS, Gregersen N et al (2006) VLCAD deficiency: pitfalls in newborn screening and confirmation of diagnosis by mutation analysis. Mol Genet Metab 88:166–170
Huang HP, Chu KL, Chien YH, Wie ML, Wu ST, Wang SF, Hwu WL (2006) Tandem mass neonatal screening in Taiwan—report from one center. Formos Med Assoc 105:882–886
Maier EM, Pongratz J, Muntau AC et al (2009) Validation of MCADD newborn screening. Clin Genet 76:179–187
Nichols MJ, Saavedra-Matiz CA, Pass KA, Caggana M (2008) Novel mutations causing medium chain acyl-CoA dehydrogenase deficiency: under-representation of the common c.985 A > G mutation in the New York state population. Am J Med Genet A 146A:610–619
Pandor A, Eastham J, Berverley C et al (2004) Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review. Health Technol Assess 8:1–152
Rinaldo P, Zafari S, Tortorelli S, Matern D (2006) Making the case for objective performance metrics in newborn screening by tandem mass spectrometry. Ment Retard Dev Disabil Res Rev 12:255–261
Schulze A, Lindner M, Kohlmüller D et al (2003) Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics 111:1399–1406
van der Hilst CS, Derks TGJ, Reijngoud DJ et al (2007) Cost-effectiveness of neonatal screening for medium chain acyl-CoA dehydrogenase deficiency: the homogenous population of the Netherlands. The J Pediatr 151:115–120
van der Kamp HJ, Wit JM (2004) Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol 151(Suppl 3):U71–U75
van Maldegem BT, Duran M, Wanders RJA (2006) Clinical, biochemical, and genetic heterogeneity in short-chain acyl-coenzyme a dehydrogenase deficiency. JAMA 296:943–952
Waisbren SE, Levy HL, Noble M, Matern D, Gregersen N, Pasley K, Marsden D (2008) Short-chain acyl-CoA dehydrogenase (SCAD) deficiency: an examination of the medical and neurodevelopmental characteristics of 14 cases identified through newborn screening or clinical symptoms. Mol Genet Metab 95:39–45
Watson MS, Mann MY, Lloyd-Puryear MA et al (2006) Newborn screening: towards a uniform screening panel. Genet Med 8:s1–s11
Wilcken B, Haas M, Joy P, Wiley V, Bowling F, Carpenter K, Christodoulou J, Cowley D, Ellaway C, Fletcher J, Kirk EP, Lewis B, McGill J, Peters H, Pitt J, Ranieri E, Yaplito-Lee J, Boneh A (2009) Expanded newborn screening: outcome in screened and unscreened patients at age 6 years. Pediatr 124:e241–e248
Wilson JM, Jungner G (eds) (1968) Principles and practice of screening for disease. World Health Organization. Available at http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. Accessed 21 Mar 2010
Zytkovic T, Fitzgerald EF, Marsden D et al (2001) Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England newborn screening program. Clin Chem 47:1945–1955
Acknowledgements
We thank all colleagues who participated and contributed to the discussion, especially Dr. Olaf Bodamer, Austria, Dr. Andreas Schulze, Canada, Dr. Ute Spiekerkötter, Germany, and Dr. Bridget Wilcken, Australia. The work of Dr. Lindner and Dr. Hoffmann is generously sponsored by the Dietmar-Hopp-Stiftung, Walldorf GmbH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Verena Peters
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Lindner, M., Hoffmann, G.F. & Matern, D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis 33, 521–526 (2010). https://doi.org/10.1007/s10545-010-9076-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9076-8