Abstract
It is the purpose of this article to briefly review the initial development and subsequent evolution of newborn screening programs to detect infants with congenital hypothyroidism (CH) and then to provide an update of the advantages and disadvantages of the main test strategies. Pilot programs began screening newborn populations in North America in the mid-1970s using either primary thyroxine (T4)-follow-up thyroid stimulating hormone (TSH) or primary TSH testing. Many programs in the United States and around the world continue to prefer a primary T4-follow-up TSH test strategy. This approach has the advantage of detecting infants with primary CH, as well as cases of hypopituitary hypothyroidism, by follow-up of infants with a T4 below an absolute cutoff or with a persistently low T4 level, necessitating a higher recall rate. With increasing assay sensitivity and specificity, several programs in the United States and worldwide have elected to switch to a primary TSH test strategy. This test strategy has the advantage of detecting primary CH and subclinical hypothyroidism and at a lower recall rate. Programs considering switching to a primary TSH test strategy need to develop age-related TSH cutoffs to maintain an acceptable recall rate. Both test strategies have the potential to detect infants with CH characterized by “delayed TSH rise,” but only if they collect a routine or discretionary second specimen, now recommended in low-birth-weight and acutely ill infants. Lastly, a lower TSH cutoff appears to be one of the explanations for the recently described increased incidence of CH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital hypothyroidism (CH) is one of the most common preventable causes of mental retardation. Most infants with CH do not have obvious clinical manifestations of hypothyroidism at birth. This is likely the result of some residual neonatal thyroid function, as might be seen with thyroid hypoplasia, an ectopic gland, or mild dyshormonogenesis. In addition, with hypothyroidism, adaptation occurs in the target organ enzyme, type 2 deiodinase, which increases conversion of the prohormone thyroxine (T4) to the biologically active triiodothyronine (T3) (Ruiz de Ona et al. 1988). In addition, some maternal thyroid hormone crosses to the fetus prior to birth and may offer some temporary protection, even in newborns with complete absence of thyroid hormone production (Vulsma et al., 1989). The majority of CH cases are not familial, so it is not possible to identify a population of high-risk pregnant women who might deliver an infant with CH. For these reasons, as technology allowed adaptation of precise immunoassays for T4 and thyroid stimulating hormone (TSH) to the small quantity of blood in newborn screening specimens, screening for CH was added to existing programs beginning in the mid-1970s. Early detection and treatment of such infants has been successful as evidenced by near-normal neurocognitive outcome in the majority of infants with CH.
This article briefly reviews the development and evolution of screening programs implemented to detect infants with CH. The main purpose of this article is to provide an update of the advantages and disadvantages of the two main screening strategies: primary T4 vs. primary TSH testing. In addition, the update discusses the benefit of a routine or discretionary second specimen collected in target populations of newborns and finishes with a discussion of the likely role that lower TSH cutoffs play in the reported increased incidence of CH.
History and evolution of newborn screening test strategies for CH
The first reports of screening newborn populations for CH came from North America and were published in the mid-1970s. Dussault et al., employing a primary T4 test strategy, detected 7 infants with CH out of 47,000 newborns screened in Quebec (Dussault et al. 1975). Klein and Foley (1975), employing a TSH test strategy, detected 5 newborns with CH out of 32,000 newborns in Pittsburgh, PA, USA. Established screening programs around the world then began to add tests to detect infants with CH. Initially, most programs in North America, some in Europe, and those in Australia, New Zealand, and Israel employed a primary T4 test, with a follow-up TSH measurement in infants with a T4 below a selected cutoff, typically <10th percentile (Fisher et al, 1979, LaFranchi et al., 1979). Other programs in Europe and Japan employed a primary TSH test strategy (Illig et al, 1977; Delange et al., 1977, Miyai et al.,1978). These programs reported an incidence of CH of approximately 1:4,000 newborns.
With experience, programs confirmed that both screening test strategies detected a similar birth prevalence of infants with primary congenital hypothyroidism. However, differences between the two screening test strategies began to emerge, with reports that detection of other types of thyroid disorders, e.g. hypopituitary hypothyroidism, subclinical hypothyroidism, and cases characterized by “delayed TSH rise” was more likely with one or the other test strategy. In addition, differences between the recall rates were reported between the two test approaches, although the cutoffs chosen by individual programs also had a significant impact on the recall rate. With experience, programs began to evaluate their screening strategy, and some modified their testing approach based on decisions to focus on detection of only primary CH vs. detection of primary CH and other thyroid disorders and their judgment of acceptable recall rates. In addition, improvements in T4 and TSH assay sensitivity and accuracy in dried blood spot (DBS) specimens influenced test strategy decisions.
Most screening programs in North America, Europe, and Australia have switched from a primary T4 to a primary TSH test approach. This is partly due to the judgment that the major focus of newborn screening is to detect infants with primary CH. It has also been facilitated by improved sensitivity of DBS TSH assays, which allows better separation of affected from normal infants and thus a lower recall rate. In the United States, for example, the change from 1998 to 2008 in primary screening test strategy for the 50 states plus the District of Columbia and Puerto Rico/Virgin Islands is as follows (Table 1) (National Newborn Screening and Genetics Resource Center, NNSGRC, 2009).
Advantages and disadvantages of newborn screening test strategies
As programs gained experience in screening large newborn populations, differences between the two major test strategies in detection of other types of thyroid disorders and in the recall rate were recognized. Some programs employing a primary T4 test with follow-up of cases with a T4 below an absolute cutoff (e.g., <40 nmol/L) or with persistently low screening T4 levels (TSH levels normal), either by routine collection of a second DBS specimen or discretionary testing on target newborn populations, reported detection of cases of hypopituitary hypothyroidism (Hanna et al, 1986). The incidence of hypopituitary hypothyroidism was reported as being 1:25,000–1:100,000, much lower than primary CH. Another thyroid disorder discovered by routine or discretionary second testing was characterized by delayed TSH rise. Such infants typically had a low T4 level but normal TSH value on the first screening test, whereas subsequent DBS testing showed a persistently low T4 and elevated TSH level. Delayed TSH rise was reported to occur in approximately 1:18,000 newborns, most of whom were born preterm, with low-birthweight, or were acutely ill full-term infants (Mandel et al. 2000). Infants with hypopituitary hypothyroidism and delayed TSH rise would not be detected by programs employing a primary TSH test strategy with a single specimen collection. On the other hand, primary TSH testing has the potential to detect infants with milder primary hypothyroidism—in particular, those with a normal T4 and mildly elevated TSH level, or so-called subclinical hypothyroidism. The frequency of detection of milder CH cases is directly related to the TSH cutoff selected by the screening program (Corbetta et al. 2009). Corbetta et al. examined the incidence of CH in the Lombardy region of Italy at the higher TSH cutoff used historically compared with the current lower TSH cutoff. By lowering the screening TSH cutoff from 20 mU/L to 10–12 mU/L (whole blood), the incidence of CH increased from 1:2,654 to 1:1,446. The frequency of gland in situ, a milder form of CH, increased from 44% with the higher cutoff to 68% using the lower cutoff.
As each program began to consider the primary test strategy and cutoff levels, another major factor to consider was the recall rate. In general, recall rates were reported to be 3 to 5 times higher in programs employing a primary T4-follow-up TSH test approach vs. a primary TSH test strategy (NNSGRC 2009).
The advantages and disadvantages of each newborn screening test strategy are summarized in Table 2. All screening test strategies detect infants with permanent primary CH with a similar incidence: approximately 1:3,000. Programs employing a primary T4-follow-up TSH test approach also have the potential to detect infants with hypopituitary hypothyroidism and delayed TSH rise, but most report a higher recall rate to do this. On the other hand, programs employing a primary TSH test strategy have the potential to detect cases of mild subclinical hypothyroidism, and they report a lower recall rate. If primary TSH test programs elect to collect a second routine or discretionary DBS specimen, they also have the potential to detect infants with delayed TSH rise (just reported by Wiley et al. 2009). Rather than make this tradeoff between the type of thyroid disorder detected and recall rate, some programs have elected a simultaneous T4 and TSH test strategy. As noted above, 9 state screening programs in the United States employ simultaneous T4 and TSH testing. Interestingly, the rate of detection of CH in these 9 states in 2008 was 1:2,520 vs. an overall United States incidence of 1:2,343 (NNSGRC 2009).
To gain a better understanding of advantages and disadvantages of these screening test strategies, the next sections examine the various thyroid disorders. This includes selected reports of incidence and screening and confirmatory serum thyroid test results. For comparison, results for each thyroid disorder are compared with primary CH.
Hypopituitary hypothyroidism
Although not the primary objective of newborn screening, some programs report detection of infants with hypopituitary hypothyroidism (also known as central hypothyroidism or secondary hypothyroidism). In general, these programs employ a primary T4-follow-up TSH or simultaneous T4 and TSH test strategy. The reported incidence of hypopituitary hypothyroidism varies from 1:16,000 to 1:100,000. The 2008 summary of all programs in the United States reported 38 cases in 2,114,913 newborns screened, an incidence of 1:55,656 (NNSGRC 2009). Programs that detect hypopituitary hypothyroidism in infants do so by follow-up with a DBS T4 below an absolute cutoff, e.g., <40 nmol/L, or by collecting a routine second DBS specimen.
On screening, infants with hypopituitary hypothyroidism have persistently low T4 levels but normal TSH values. In contrast to detecting a near-majority of primary hypothyroidism cases, it seems likely that those programs reporting infants with hypopituitary hypothyroidism do not detect all cases of congenital TSH deficiency. In a report from Oregon in the United States, of 19 known cases over a 10-year period (an incidence of 1:19,000), 8 were detected by newborn screening, whereas 11 were not detected by screening tests. Rather, these 11 were diagnosed after presentation of clinical features consistent with congenital hypopituitarism, such as hypoglycemia, prolonged jaundice, and, in boys, micropenis and undescended testes (Hanna et al. 1986).
A Dutch screening program using the T4/thyroxine-binding globulin (TBG) ratio to detect central hypothyroidism reported an incidence of 1:16,404, likely closer to the true incidence of congenital TSH deficiency (Lanting et al. 2005). Using this unique screening test strategy, the Dutch investigators calculated the cost of detecting each case of primary hypothyroidism to be US $6,353, whereas the incremental cost to detect each case of central hypothyroidism was US $11,206. They concluded that detecting central hypothyroidism by their laboratory approach was cost effective (Lanting et al. 2005).
A novel approach to detecting central hypothyroidism was presented at the 7th International and Latin American Congress for Inborn Errors of Metabolism and Neonatal Screening. This screening program from Sapporo, Japan, measures both TSH and free T4 on DBS specimens (Fukushi et al. 2009). This group reported detection of 11 infants with central hypothyroidism out of 183,290 infants screened, an incidence of 1:16,663, which is very close to the incidence reported by the Dutch.
In general, infants with hypopituitary hypothyroidism appear to have the same degree of hypothyroidism as infants with primary hypothyroidism. A comparison of DBS and serum thyroid function tests in cases with primary and hypopituitary hypothyroidism detected by the Northwest U.S. Regional Screening Program over a 20-year period is presented in Table 3. Mean T4 on the first and second DBS test was slightly higher in infants with hypopituitary hypothyroidism, but this difference narrowed in the serum T4 levels: 41.6 nmol/L vs. 55.6 nmol/L, respectively (LaFranchi et al. 1985; Hunter et al. 1998).
Delayed TSH rise
Infants with so-called delayed TSH rise typically have a low T4 level but normal TSH value on the 1st newborn screening test, whereas subsequent testing shows a persistently low T4 and elevated TSH level. These cases are most likely to be detected by a primary T4-follow-up TSH test strategy that carries out follow-up testing on infants with persistently low T4 levels, but potentially, they also could be detected by programs that employ a primary TSH test if the program collects a routine second DBS specimen for testing. Preterm, low-birthweight, and acutely ill term babies make up the majority of delayed TSH rise cases. Thus, either primary T4 or primary TSH test programs that collect a second “discretionary” DBS in targeted populations, such as preterm, low-birthweight, or acutely ill newborns, will detect these cases. Mandel et al. reported the incidence of delayed TSH rise by birthweight in infants born in Massachusetts, USA, over a 4-year period (Mandel et al. 2000) (see Table 4). The overall incidence of delayed TSH rise was 1:18,412, but in infants with a birthweight between 1,501-2,499 g, the incidence was 1:4,225, whereas in infants with a birthweight <1,500 g, the incidence was 1:324. Of all CH cases in babies <2,500 g, 40% had delayed TSH rise and were not detected by the first test. The TSH elevation was detected on average at 30 days of life, with a range of 11–176 days.
Detection of infants with delayed TSH rise was also presented by Wiley et al. at the 7th International and Latin American Congress for Inborn Errors of Metabolism and Neonatal Screening. This group from Sydney, Australia, employs primary TSH testing. By virtue of collection of a 2nd DBS specimen at 1 month of age in very low birthweight (VLBW) infants (<1,500 gm), 21 cases with delayed TSH rise out of 301,000 infants screened were detected—an incidence of 1:14,333 (Wiley et al. 2009).
Cases of delayed TSH rise appear to have a slightly milder degree of hypothyroidism compared with other infants with primary CH at diagnosis. DBS and a serum thyroid function test results in cases with primary hypothyroidism detected on the first specimen are compared with infants with delayed TSH rise detected on subsequent testing in the Northwest U.S. Regional Screening Program over a 20-year period (Table 5). The mean DBS T4 in delayed TSH rise cases was low—82.4 nmol/L—though higher than babies detected on the first specimen—49.2 nmol/L. On the second DBS specimen, in delayed TSH rise cases, mean T4 fell to 58.3 nmol/L, with mean TSH rising to 117 mU/L. There is no published information on whether hypothyroidism is transient or permanent in infants with delayed TSH rise, but based on its high incidence in preterm infants, we suspect many cases will be transient.
Subclinical (mild) hypothyroidism
Subclinical hypothyroidism is defined by a normal serum T4 and elevated TSH level. As these cases are mild, TSH elevation is typically in the 10–25 mU/L range. Subclinical hypothyroidism will only be detected by programs employing a primary TSH test strategy. Even then, depending on the program’s TSH cutoff, some mild cases may not be detected. To my knowledge, there are no reports of a series of cases with subclinical hypothyroidism detected by newborn screening programs. The Northwest Regional Screening Program did report findings in 9 cases of mild primary hypothyroidism detected out of 1,747,805 newborns over a 20-year period (Hunter et al. 1998). These 9 infants had DBS TSH levels <25 mU/L on both the routine first and second screening test (by definition). Based on persistently low DBS T4 levels, these infants underwent serum testing to confirm a diagnosis of CH. Diagnostic serum testing showed a T4 of 69.1 nmol/L, which is not much higher than the T4 level of 41.6 nmol/L in infants detected with more typical CH. The mean serum TSH in mild CH cases, however, was 17.8 mU/L—much lower than the mean TSH of 138 mU/L in the more typical CH cases. Two of the 9 cases had Down syndrome.
Switching to a primary TSH test strategy: program considerations
As described earlier, with increasing TSH assay sensitivity in the DBS specimen and with a goal of reducing recall rates, several screening programs have switched to a primary TSH test strategy. Before making this change, programs need to consider the range of age of specimen collection in their newborn population. There is a rise in TSH levels after birth; serum TSH rises from cord blood levels of 1–20 mU/L to peak around 60–80 mU/L 30 min after delivery. TSH levels then fall over the next few days, and by a week of life are in the 1–8 mU/L range typical of early infancy. Programs in which the majority of first DBS specimens are collected after 48 h of age typically have a single DBS TSH cutoff and have an acceptable recall rate. However, programs in which a significant proportion of newborns has the first DBS specimen collected before 48 h or even before 24 h of age need to take into account these dramatic changes in TSH if they are to avoid an unacceptable recall rate. Programs with a primary T4-follow-up TSH test strategy and a significant proportion of specimens collected before 48 h of age also need to develop age-related TSH cutoffs.
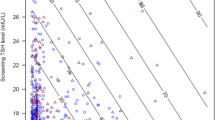
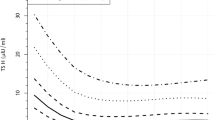
An example of the impact of early first DBS specimen collection and resulting TSH cutoffs is presented from the Oregon Screening Program in Figs. 1 and 2. Figure 1 shows the cumulative specimen collection by age in Oregon newborns from 2009 (unpublished). In Oregon, USA, 21.3% of infants have their first DBS test collected in the 1st 24 h of life, with another 69.5% collected between 24 and 48 h of life. Thus, 90.8% have their first DBS specimen collected in the 1st 48 h of life. This is the result of the trend toward early hospital discharge of newborns in the United States. Figure 2 shows the age-related DBS TSH cutoff for the Oregon program. The cutoff is set at the mean + 3 standard deviations (SD) for each age category. It falls from 103 mU/L (serum) for specimens collected between 0 and 11 h of age to 40 mU/L for those collected between 12 and 23 h, then to 32 mU/L for those collected between 24 and 48 h, and then 30 mU/L for those collected between 49 and 96 h.
Collection of a routine 2nd DBS specimen or a discretionary 2nd specimen in targeted populations
Several screening programs collect two routine DBS specimens and test both for CH. Typically, the 1st specimen is collected around 2–5 days of age, whereas the 2nd specimen is collected at 1–3 weeks of age. The rationale behind collecting two routine specimens varies from program to program. For example, in Oregon, collecting two routine specimens was initiated in the 1960s when there was concern that some babies with phenylketonuria and inadequate protein intake might be missed on the first specimen. When screening for CH was added in 1975, both specimens underwent testing for hypothyroidism. After the first 10 years’ experience, the Northwest Regional Screening Program reported that 10% of CH cases had normal thyroid function on the first specimen and were detected only by testing on the second specimen (LaFranchi et al. 1985). Other screening programs that collect two routine specimens reported similar findings (Maniatis et al. 2006). Infants detected on the second DBS specimen appear to have a milder form of hypothyroidism. A comparison of DBS and serum thyroid function tests in cases detected by the routine first and second specimen in the Northwest U.S. Regional Screening Program over a 10-year period are presented in Table 6. In infants detected on the 2nd routine specimen, DBS T4 levels were higher and TSH levels lower on the 1st specimen, but by the 2nd specimen, both thyroid function tests were similar to those in infants detected on the 1st specimen. Serum testing again showed a somewhat milder hypothyroidism.
With reports that a discrete percentage of infants with CH have normal results on the 1st DBS test, and with reports of infants with delayed TSH rise, several screening programs elected to undertake discretionary collection of a 2nd specimen in infant populations likely at higher risk to be missed on the 1st DBS test. Preterm or otherwise low-birth-weight infants make up the largest category of such at-risk infants. The next largest group is acutely ill term infants or those with other perinatal complications (see Table 7 for a list of such at-risk groups). In addition, one pair of same-sex twins with CH may be missed on the 1st DBS test, owing to twin-to-twin transfusion from the normal to the hypothyroid twin, temporarily raising the low T4 level. Approximately 25% of infants born in the United States now undergo a routine or discretionary 2nd newborn screening test (NNSGRC 2009).
Reports of higher incidence of congenital hypothyroidism: potential explanations
Pilot screening programs developed in the 1970 s reported an incidence of CH of approximately 1:4,000 (Fisher et al. 1979). Records of all screening programs in the United States show an increasing trend in the incidence—from 1:4,094 in 1987 to 1:2,273 in 2002 (NNSGRC 2009) (see Fig. 3). This apparent increase in incidence was further explored by Harris and Pass from the State of New York (Harris and Pass, 2007), who reported that over the same 15-year time period (1987–2002), the incidence of CH in New York increased from 1:3,078 to 1:1,458. Although the investigators could not identify a single cause for this increase, they examined several factors, each of which were likely contributors. The incidence of CH was higher in infants in certain racial and ethnic groups, and the birth rate in these groups had increased from 1987 to 2002. For example, the incidence in Hispanic infants vs. non-Hispanic white infants was 1:1,559 vs. 1:1,815; over the period 1989–2000, the number of Hispanic births increased 53% vs. 6% in non-Hispanic whites. An even higher incidence was reported in Asian infants: 1:1,016. During the same 11-year period, the number of Asian births increased 37%. Harris and Pass also reported an increased incidence in low-birth-weight infants and infants born to younger mothers. The incidence of CH by birthweight was >2,500 g = 1:1,843; 1,500–2,500 g = 1:851, and <1,500 g = 1:396. Over the 1987–2000 time period, there was a 23% increase in the number of infants born <1,500 g and a 16% increase in the number of infants born 1,500–2,500 g compared with only a 6% increase in infants >2,500 g. It is likely that some of these low-birth-weight babies will not have permanent hypothyroidism, although Harris and Pass did not have any follow-up data to address this point (Harris and Pass 2007).
The state of New York employs a primary T4-follow-up TSH test strategy. Second specimens are collected only on certain infants: if the T4 is >2 SD below the mean and the TSH is <20 mU/L, or if the T4 is <10th percentile and the TSH is 20–30 mU/L (serum). Harris and Pass (2007) stated that this test protocol has been unchanged since 1978, so they did not think a change in test cutoffs played a role in the increased CH incidence (though methodologies and instrumentation had changed).
As noted above, other screening programs report that changes in cutoffs affect detection rate. One recent example is from the region of Lombardy (Corbetta et al. 2009). Between 1999 and 2005, the incidence of CH using a TSH cutoff of 12 mU/L (DBS) was 1:1,816, whereas if the cutoff was 10 mU/L, the incidence rose to 1:1,154. Using a virtual DBS cutoff of 20 mU/L over the same time period resulted in an incidence of 1:2,654. The percent of cases classified as gland in situ was 44% with the higher cutoff and 68% with the lower cutoff. Gland in situ was found more commonly in low-birth-weight babies. Follow-up after 3–5 years showed that 22% of gland in situ cases were transient and now had normal thyroid function, whereas another 44% had mild hypothyroidism defined as a serum TSH between 5.0 and 9.9 mU/L (Corbetta et al. 2009). Thus, a lower TSH cutoff can be an explanation for an increase in the incidence of CH, and many of these cases do appear to represent transient or mild hypothyroidism.
Another recent example of the effect of lowering the TSH cutoff was presented by Chiesa et al. from Argentina at the 7th International and Latin American Congress for Inborn Errors of Metabolism and Neonatal Screening. Prior to December 2002, their TSH cutoff was 15 mU/L (whole blood), whereas in 2003, the cutoff was lowered to 10 mU/L. With the drop in the TSH cutoff, the incidence of CH increased from 1:2,904 to 1:2,412. The percentage of cases with athyreosis and ectopic glands decreased, whereas the percentage of eutopic glands increased (Chiesa et al. 2009).
It is open to debate as to whether cases of mild CH detected by lower TSH cutoffs are in fact at risk for cognitive impairment. As pointed out by the experience from the Lombardy region, some of these cases will have transient CH. In a follow-up study from Germany of 61 children with either transient CH or transient hyperthyrotropinemia, only three children showed persistent problems, whereas the remainder had normal thyroid function and physical growth at 6–14 years of age (Kohler et al. 1996). A Danish study compared psychometric outcome in children detected by a retrospective TSH test carried out at 5 years of age on their neonatal DBS sample (Alm et al. 1984). Approximately half of the children had been diagnosed by clinical manifestations of hypothyroidism and started on treatment at a median age of 5 months. Their average intelligence quotient (IQ) was 87. Of the untreated group, approximately one half were now euthyroid, with a mean IQ of 107, whereas the other half were hypothyroid but had a mean IQ of 100. These results appear to show that undetected cases of mild CH have mild cognitive impairment. More information on follow-up of children with mild or transient CH detected by programs with a lowered TSH cutoff will help current programs decide whether or not to adjust their TSH cutoff.
Summary and conclusions
Congenital hypothyroidism is a newborn screening success story. Whereas screening programs have been in place for more than 30 years, the optimal test strategy continues to undergo evaluation. Most programs in the United States and around the world continue to prefer a primary T4-follow-up TSH test strategy. This approach has the advantage of detecting not only primary CH but has the potential to detect cases of hypopituitary hypothyroidism. These latter cases are typically detected either by follow-up with a DBS T4 below an absolute cutoff or by collecting a second routine or discretionary DBS specimen demonstrating persistently low T4 levels, which increases the recall rate for this test approach. Several programs in the United States and many around the world have elected to switch to a primary TSH test strategy. This approach has the advantage of detecting not only primary CH but has the potential to detect cases of subclinical hypothyroidism. Primary TSH testing typically has a lower recall rate than primary T4-follow-up TSH testing programs. Programs considering a switch to primary TSH test strategy will need to develop age-related TSH cutoffs if a significant proportion of newborns undergo specimen collection <48 h of age, in order to maintain an acceptable recall rate. Both screening test approaches have the potential to detect infants with primary CH characterized by delayed TSH rise but only if the programs collect a second routine or discretionary DBS specimen. Many programs are now incorporating discretionary second-specimen collection in preterm/low-birth-weight or acutely ill term/normal-weight infants. Lastly, there has been a trend of increasing incidence of CH in the United States over the last 20 years. Analysis from the state of New York shows this to be primarily a result of demographic changes. However, a report from the region of Lombardy shows the powerful effect of lowering the TSH cutoff from 20 mU/L to 10–12 mU/L (whole blood), which led to a doubling of the incidence. On follow-up at age 3–5 years, approximately half of the additional cases represented mild CH (TSH 5–9.9 mU/L), whereas a quarter of the additional cases were transient. It is unclear whether these mild or transient cases are at risk for significant cognitive impairment.
Abbreviations
- CH:
-
Congenital hypothyroidism
- DBS:
-
Dried blood spot
- NNSGRC:
-
National Newborn Screening and Genetics Resource Center
- SCH:
-
Subclinical hypothyroidism
- T3:
-
Triiodothyronine
- T4:
-
Thyroxine
- TBG:
-
Thyroxine-binding globulin
- TSH:
-
Thyroid stimulating hormone
References
Alm J, Hagenfeldt L, Larsson A, Lundberg K (1984) Incidence of congenital hypothyroidism: retrospective study of neonatal laboratory screening versus clinical symptoms as indicators leading to diagnosis. Br Med J 289:1171–1175
Chiesa A, Prieto L, Papendieck P, Gilligan G, Niremberg M, Gruneiro-Papendieck L (2009) Characterization of thyroid disorders found by primary congenital hypothyroidism (CH) neonatal screening: Something is changing? Revi Invest Clin 61(Suppl 1):30
Corbetta C, Webert G, Cortinovis F et al (2009) A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol 71:739–745
Delange F, Camus M, Winkler M, Dodion J, Ermans AM (1977) Serum thyrotropin Determination on day 5 of life as screening procedure for congenital hypothyroidism. Arch Dis Child 52:89–96
Dussault JH, Coulombe P, Laberge C et al (1975) Preliminary report on a mass screening program for neonatal hypothyroidism. J Pediatr 86:670–674
Fisher DA, Dussault JH, Foley TP Jr et al (1979) Screening for congenital hypothyroidism: Results of screening one million North American infants. J Pediatr 94:700–705
Fukushi M, Fujikura K, Hanai J, Yano K, Tamima T, Fujieda K (2009) Neonatal screening for congenital hypothyroidism by measurement of TSH and free T4. Rev Invest Clin 61(Suppl 1):30
Hanna CE, Krainz PL, Skeels MR et al (1986) Detection of congenital hypopituitary hypothyroidism: ten-year experience in the Northwest Regional Screening Program. J Pediatr 109:959–964
Harris KB, Pass KA (2007) Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab 91:268–277
Hunter MK, Mandel SH, Sesser DE, et al. (1998) Follow-up of newborns with low thyroxine and non-elevated thyroid-stimulating hormone-screening concentrations: Results of the 20-year experience in the Northwest Regional Newborn Screening Program. 132:70–74.
Illig R, Torresani T, Sobradillo B (1977) Early detection of neonatal hypothyroidism by serial TSH determination in dried blood. Helv Paediatr Acta 32:289–297
Klein AG, Foley TP Jr (1975) Letter: Screening for hypothyroidism. J Pediatr 87:667–668
Kohler B, Schnabel D, Biebermann H, Gruters A (1996) Transient congenital hypothyroidism and hyperthyrotropinemia: normal thyroid function and physical development at the ages of 6-14 years. J Clin Endocrinol Metab 81:1563–1567
LaFranchi SH, Murphey WH, Foley TP Jr (1979) Neonatal hypothyroidism detected by the Northwest Regional Screening Program. Pediatrics 63:180–191
LaFranchi SH, Hanna CE, Krainz PL, Skeels MR, Miyahira RS, Sesser DE (1985) Screening for congenital hypothyroidism with specimen collection at two time periods: Results of the Northwest Regional Screening Program. Pediatrics 76:734–740
Lanting CI, van Tijn DA, Loeber JG, Vulsma T, de Vijlder JJ, Verkerk PH (2005) Clinical effectiveness and cost-effectiveness of the use of the thyroxine/thyroxine-binding globulin ratio to detect congenital hypothyroidism of thyroidal and central origin in a neonatal screening program. Pediatrics 116(1):168–73
Mandel SJ, Hermos RJ, Larson CA, Prigozhin AB, Rojas DA, Mitchell ML (2000) Atypical Hypothyroidism and the very low birthweight infant. Thyroid 8:693–695
Maniatis AK, Taylor L, Letson GW, Bloch CA, Kappy MS, Zeitler P (2006) Congenital Hypothyroidism and the second newborn metabolic screening in Colorado, USA. J Pediatr Endocrinol Metab 19:31–38
Miyai K, Oura T, Kawashima M, et al. (1978) A new method of paired thyrotropin assay as a screening test for neonatal hypothyroidism. J Clin Endocrinol Metab 47:1028-1033
National Newborn Screening and Genetics Resource Center (NNSGRC), 2009 National Newborn Screening Information System (http://genes-r-us.uthscsa.edu).
Ruiz de Ona C, Obregon MJ, Escobar del Rey F, Morreale de Escobar G (1988) Developmental changes in rat brain 5’-deiodinase and thyroid hormones during the fetal period: the effects of fetal hypothyroidism and maternal thyroid hormones. Pediatr Res 24:588–594
Vulsma T, Gons MH, deVijlder JJM (1989) Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med (321):13–16
Wiley V, Bijarnia S, Wikcken B (2009) Screening for hypothyroidism in very low birth weight babies. Rev Invest Clin 61(Suppl 1):31
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Rodney Pollitt
References to electronic databases:
OMIM #218700, U.S. National Newborn Screening and Genetics Resource Center: http://genes-r-us.uthscsa.edu, United Kingdom UCL Institute of Child Health: http://www.ich.ucl.ac.uk/clinical_information/clinical_guidelines/cmg_guideline_00079
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
LaFranchi, S.H. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis 33 (Suppl 2), 225–233 (2010). https://doi.org/10.1007/s10545-010-9062-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9062-1