Abstract
A new micromachined circulating polymerase chain reaction (PCR) chip is reported in this study. A novel liquid transportation mechanism utilizing a suction-type membrane and three microvalves were used to create a new microfluidic control module to rapidly transport the DNA samples and PCR reagents around three bio-reactors operating at three different temperatures. When operating at a membrane actuation frequency of 14.29 Hz and a pressure of 5 psi, the sample flow rate in the microfluidic control module can be as high as 18 μL/s. In addition, an array-type microheater was adopted to improve the temperature uniformity in the reaction chambers. Open-type reaction chambers were designed to facilitate temperature calibration. Experimental data from infrared images showed that the percentage of area inside the reaction chamber with a thermal variation of less than 1°C was over 90% for a denaturing temperature of 94°C. Three array-type heaters and temperature sensors were integrated into this new circulating PCR chip to modulate three specific operating temperatures for the denaturing, annealing, and extension steps of a PCR process. With this approach, the cycle numbers and reaction times of the three separate reaction steps can be individually adjusted. To verify the performance of this circulating PCR chip, a PCR process to amplify a detection gene (150 base pairs) associated with the hepatitis C virus was performed. Experimental results showed that DNA samples with concentrations ranging from 105 to 102copies/μL can be successfully amplified. Therefore, this new circulating PCR chip may provide a useful platform for genetic identification and molecular diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polymerase chain reaction (PCR) has been an enabling protocol for nucleic acid amplification (Mullis 1990; Mullis et al. 1986). It is presently a gold standard in the fields of genetic identification and molecular diagnosis (Saiki et al. 1985). For the detection of small traces of micro-organisms and pathogens in clinical specimens, the PCR procedure is widely used to amplify specific fragments of a detection gene through three specific temperatures with 25–45 complete thermal cycles. Typically, one complete thermal reaction cycle comprises a denaturing step (90–95°C) for separation of double-stranded DNA, an annealing step (50–65°C) for hybridization of primers, and a DNA extension step (70–75°C) to replicate the DNA targets. Based on the proper selection of specific primers, the concentration of a specific segment of double-stranded DNA can be theoretically doubled during each thermal cycle.
Bio-micro-electro-mechanical-system (Bio-MEMS) technology, which integrates domain knowledge from biology and microfabrication technologies, has been employed to develop a diverse range of micro devices and systems for automatic analysis of biomedical samples. Miniature bio-devices are capable of performing many critical functions, including sample pre-treatment, mixing, reaction, transportation, sorting, separation and detection, on a single substrate in an automated manner. Enabled by Bio-MEMS technology, these resulting miniature bio-devices may have several advantages over their large-scale counterparts such as a shorter assay time, lower sample/reagent consumption and a higher sensitivity, as well as a greater potential for integrating multiple functional modules onto a single chip to reduce size and power consumption. Among these miniature bio-devices, micro PCR chips have attracted considerable interest recently. Since the first demonstration of a functional PCR device, various types of these micro-devices have been presented in the literature (Northrup et al. 1993; Woolley et al. 1996; Poser et al. 1997; Anderson et al. 2000). In principle, micro PCR devices can be classified into two categories, namely stationary PCR chips (Northrup et al. 1995; Gulliksen et al. 2004; Liao et al. 2004) and flow-through PCR chips. In order to perform nucleic acid amplification utilizing the stationary PCR chips, the PCR mixture has to be stored in a stationary reaction chamber while a thermal control module regulates the thermal cycling inside the chamber. Compared with conventional large-scale PCR machines, the stationary PCR chip can perform faster thermal cycling with a heating rate of 10–20°C/s and a cooling rate of 2.5–5°C/s due to the small dimensions of the devices. However, it is still inevitable to modulate the heating and cooling cycles to amplify the DNA template inside the stationary reservoir, thus requiring a substantial operating time.
Alternatively, the flow-through PCR chips utilize three separate heating blocks to maintain three specific temperatures while the samples are flowing (Manz et al. 1992). Nucleic acids can be amplified as the DNA sample continuously flows through these three blocks, typically along microfluidic channels, during each thermal cycle without substantial heating and cooling periods. Therefore, the entire time required to perform a PCR procedure can be minimized. The entire PCR period depends on the sample flow rate and also the time needed for the sample to reach a thermal equilibrium at each step. Several microfluidic devices which perform a flow-through PCR procedure have been reported in literature. For example, a DNA sample moving through three capillaries at different temperatures to carry out DNA amplification was reported (Nakano et al. 1994). Similarly, a flow-through PCR chip using a serpentine channel across three thermostable copper blocks was reported (Kopp et al. 1998). However, it is possible for the melted single-strand DNA samples to directly form double-strand DNA again without amplification due to a smooth temperature gradient inside these devices. In order to solve this problem, spiral-channels (Hashimoto et al. 2004) and oscillatory-flow-based straight-channels (Chen et al. 2004) were adopted to perform the flow-through PCR procedure. Compared to the PCR chip with the spiral-channels, the oscillatory-flow-based PCR chip allows for the adjustment of cycle numbers with a rapid temperature transition. Another type of flow-through PCR chip is usually referred to as convective PCR chips, in which the DNA sample shuttles vertically between the two heating zones for denaturing and annealing, which performs the nucleic acid amplification in a cylindrical chamber (Krishnan et al. 2002).
For most flow-through PCR chips reported in the literature, it is challenging to precisely control the liquid flow (Belgrader et al. 2003; Fukuba et al. 2004; Sun et al. 2002). Recently, PCR chips integrated with the automatic delivery system were reported to replace the external driving components. Pneumatic microfluidic devices constructed from multilayer membranes to deliver the microfluids were integrated into the bio-devices (Unger et al. 2000; Koch et al. 1998; Laser et al. 2004). Compared with other fluid transportation mechanisms such as hydrodynamic-, magnetohydrodynamic-, and electrokinetic-driven microdevices, the pneumatic-driven microdevices are simple to fabricate and control, can be integrated with other functional devices, and good for optical detection (Huang et al. 2006). For instance, DNA amplification utilizing a flow-through PCR chip integrated with multiple-membrane activation was reported by the current research group (Wang et al. 2007). However, bubble formation is still a serious issue in the enclosed reservoir which adversely affects PCR performance. In addition, a dead volume of about 8% was observed. Difficulty in temperature calibration prior to use was also reported due to its closed-chamber design.
In this study, a new liquid transport mechanism is proposed to rapidly drive the sample flow through three individual reaction chambers operating at three specific temperatures to carry out the nucleic acid amplification. The new microfluidic control module comprising a suction-type membrane and three pneumatically-driven microvalves can drive the DNA sample through these reaction chambers with a sample flow rate of 18 μL/s without bubble formation and significant dead volume. In addition, the cycle numbers and the reaction time for the denaturing, annealing, and extension steps can be randomly adjusted by the microfluidic control module. Array-type microheaters with a self-compensation function (Hsieh et al. 2007) are adopted to improve the thermal uniformity inside the PCR chamber. Open-type reaction chambers are designed to facilitate temperature calibration. The development of the new circulating PCR chip integrated with the suction-type liquid transportation mechanism may provide a useful platform for nucleic acid amplification.
2 Materials and methods
2.1 Design
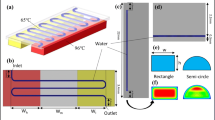
In order to automatically drive the sample flow, a new design for a liquid transportation mechanism is adopted and integrated into the circulating PCR chip. As shown in Fig. 1(a), the circulating PCR chip is comprised of a microfluidic control module and three thermal control modules. The microfluidic control module consists of a suction-type membrane, three microvalves and three open-type reaction chambers. The thermal control module located underneath the reaction chamber is composed of heating and temperature-sensing resistors for modulation to a specific reaction temperature. The microfluidic control module is used to drive the sample flow through the three reaction chambers to perform the denaturing, annealing, and extension steps for a PCR protocol, respectively. The suction-type polydimethylsiloxane (PDMS) membrane is first deflected by compressed air. When the compressed air is released, the PDMS membrane is restored back to its original position, thus generating a suction force to drive a certain amount of sample liquid from one reservoir to another one. In order to increase the flow rate and to avoid the formation of gas bubbles and dead volumes as well, a pressure of 5 psi is applied to deflect the membrane completely. In addition to the suction-type PDMS membrane, three pneumatically-driven microvalves are also integrated into the microfluidic control module. The pneumatic and liquid microchannels are formed on the upper and bottom PDMS layers, respectively. The slide glass is used to form the close liquid microchannels. Air tubing is directly connected to the pneumatic microchannels. When the pneumatic microchannels are injected with compressed air, the suction-type membrane and microvalves on the bottom PDMS layer are activated to drive the fluid flow through the three reaction regions and the liquid microchannel. The microvalves only open when the sample flow is driven from one reaction chamber to another one. Note that these valves are normally closed during the denaturing, annealing, and extension steps. When the PDMS membrane is operated continuously, the total volume of sample fluid can be moved from one reservoir to another one, thus performing an entire PCR process. A cross-sectional view of the suction-type liquid transportation mechanism utilizing the suction-type membrane and the microvalves is schematically shown in Fig. 1(b). Note that seven steps in total are involved for one liquid pumping process.
The circulating PCR chip is comprised of a microfluidic control module and a thermal control module to perform nucleic acid amplification. (a) Schematic illustration of the microfluidic control module comprising of a suction-type membrane, three pneumatically-driven microvalves, and three open-type reaction chambers. (b) Cross-sectional view of the liquid transport utilizing the suction-type membrane and the microvalves, (c) Exploded view of the circulating PCR chip comprising three layers
In addition to the microfluidic control module, a thermal control module comprising three array-type heating and temperature-sensing resistors is placed underneath the reaction chambers to provide three specific reaction temperatures for the denaturing, annealing, and extension steps of a PCR procedure (Fig. 1(c)). Array-type heating resistors that form a two-dimensional (2D), symmetrical, self-compensated heater are used to enhance the thermal uniformity in the reaction region (Hsieh et al. 2008). Platinum (Pt) is used for heating and temperature-sensing resistors to simplify the fabrication procedure. The Pt temperature-sensing resistors are placed nearby the array-type heating resistors to provide feedback signals for temperature control.
2.2 Fabrication process
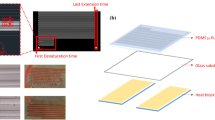
The circulating PCR chip was fabricated using micro-electro-mechanical-systems (MEMS) technology. Detailed information about the microfabrication process can be found in our previous work (Wang et al. 2007). Briefly, the circulating PCR chip was made of a soda-lime glass layer (300 × 400 × 1.1 mm3, G-Tech Optoelectronics Corp., Taiwan) and two PDMS (Sil-More Industrial Ltd., Sylgard 184A and Sylgard 184B) layers. The thermal control and microfluidic control modules were fabricated by two different fabrication processes. For the thermal control module, the Pt-based, array-type heating and temperature-sensing resistors were fabricated on the soda-lime glass. A positive photoresist (AZ 4620, Clariant Inc., Switzerland) was first spin-coated and patterned on the cleaned glass substrate. After a deposition of a titanium (Ti) layer with a thickness of 15 nm as an adhesion layer, a Pt layer with a thickness of 80 nm was deposited by an electro-beam evaporation process. Array-type heating and temperature-sensing resistors were then patterned with a standard lift-off process. The resistance of the Pt-based heating and temperature sensing resistors were measured to be 13.2 and 193.5 Ω, respectively. The electrical leads were formed with a 0.15-μm-thick gold (Au) layer fabricated by a similar fabrication process. A scanning electron microscope (SEM) image of the array-type heating and temperature-sensing resistors is shown in Fig. 2(a). The resistance of the array-type heating and temperature-sensing resistors fabricated utilizing an evaporating process is not stable. Therefore, the resistors must be sintered to lower contact resistance prior to use. The glass substrate with array-type heating and temperature-sensing resistors was then covered with a 50-μm-thick glass slide (Marienfeld Corp., Germany) by using ultraviolet (UV) sensitive glue to form an electrical isolation layer to avoid electrolysis during fluidic operation.
SEM images of (a) the array-type heating and temperature-sensing resistors, (b) the SU-8 50 template for a microvalve and a reaction chamber, (c) the replicated PDMS structures from the SU-8 50 template. Photographs of (d) an assembled circulating PCR chip, (e) the cross-sectional view of the micro circulating PCR chips, and (f) the complete system including a microcontroller, an air compressor, a regulator and a micro circulating PCR chip. The dimensions of the PCR chip are measured to be 28 mm in width and 40 mm in length, respectively
The microfluidic control module was fabricated by using the SU-8 50 template and a PDMS replication process. The templates of the microfluidic control module were made from thick negative photoresist, (SU-8 50, MicroChem Corp., MA, USA). The 200-μm-thick negative photoresist was individually spin-coated on two silicon substrates to form the upper layer of the pneumatic components and the lower layer of the microfluidic channel. After a soft-baking process, a standard lithography process with an exposure dose equal to 750 mJ/cm2 was then performed and followed by a post exposure bake process. The well-defined SU-8 50 template was obtained after the development process by immersing the exposed substrates in a developer solution (MicroChem Corp., MA, USA) under ultrasonic agitation. PDMS structures with inverse images were then replicated from the SU-8 50 template by thoroughly mixing the PDMS prepolymer and curing agent (Sylgard 184A/B, Sil-More Industrial Ltd., USA) in a ratio of 10:1 by weight and spinning them onto the SU-8 50 template. After curing at 120°C for 4 h, the cured PDMS layers were then peeled off mechanically. Then the suction-type membrane, microvalves and three reaction chambers were formed when the double-layer PDMS structures were bonded together by using an oxygen plasma treatment. The suction-type microfluidic chip and a cover glass substrate with a thickness of 100 μm were finally treated with oxygen plasma again and bonded together to form the assembled microfluidic chip. SEM images of the SU-8 50 template and the corresponding PDMS microvalve and suction-type membrane are shown in Fig. 2(b) and (c). The dimensions of the space under the suction-type membrane are 5 mm in diameter and 150 μm in depth. Figure 2(d) and (e) show photographs of the assembled circulating PCR chip and the cross-sectional view of the micro circulating PCR chips. The dimensions of the PCR chip are measured to be 28 mm in width and 40 mm in length, respectively.
2.3 Experimental setup
Three array-type heating and temperature-sensing resistors can regulate three specific reaction temperatures for the denaturing, annealing, and extension steps of a PCR procedure. Three heaters inside three reaction controller are individually controlled. As mentioned previously, the open-type reaction chambers were adopted to facilitate the temperature calibration prior to use. It was performed by inserting a thermocouple (model-3003, DER EE Inc., Taiwan) into the reaction chamber. After temperature calibration, the suction-type membrane and the three microvalves were all deflected by supplying compressed air at 5 psi (see Fig. 1(b-1)). Then a 20-μL DNA sample was pipetted inside the open-type denaturing chamber. In addition, 20-μL of mineral oil was used to cover the DNA sample to prevent sample evaporation.
In this study, a PDMS suction-type membrane and three microvalves were used to rapidly drive the sample through the three reaction chambers. The membranes were deflected in sequence as shown in Fig. 1(b) by using an air compressor (MDR2–1A/11, JUN-AIR Inc., Denmark) connected to electromagnetic valves (EMVs, S070M-5BG-32, SMC Inc., Japan), and a microcontroller (ATMEGA8535, ATMEL Corp., USA). Figure 2(f) shows a picture of the complete system including a microcontroller, an air compressor, a regulator and a micro circulating PCR chip for DNA amplification. After completing the pre-denaturing and denaturing step, the sample was driven from the denaturing chamber to the annealing chamber. Then the microfluidic control module can drive the sample flow into the extension chamber for DNA replication. The DNA sample was step-wise driven through the three reaction chambers with the required thermal cycles to complete a PCR procedure.
Fifty microliters of water was used to measure the sample flow rate of the suction-type membrane pump at various operating frequencies (at a constant air pressure of 5 psi). The time required to deliver 50 μL of water was measured and used to calculate the flow rate. In this study, three measurements were performed to get an average flow rate. The 5-psi air pressure was found to be the minimum pressure required to deflect the membrane completely.
The temperature fields of the three reaction regions were measured by using infrared (IR) thermography (TVS-200N, Nippon Avinics Co Ltd. Japan) with a 29-μm spatial resolution. The accuracy of the temperature measurement is ±1°C.
2.4 Virus strain and PCR reagent
In this study, the performance of the circulating PCR chip was verified by amplifying a detection gene associated with Hepatitis C virus (HCV) with a length of 150 base-pairs (bp). A forward primer (5′-GTT GAT CCA AGA AAG GAC CCG GTC-3′), and a reverse primer (5′-GAG GAA CTA CTG TCT TCA CG-3′) were used (Chien et al. 2006). The PCR mixture has the following composition: 7.6 μL of ddH2O; 2.4 μL of 25 mM Mg2+; 2 μL of 2.5 μM of forward primer and 2 μL of 5.0 μM of reverse primer; 2 μL of enzyme mix (Roche, USA), and 4 μL of HCV DNA template with different initial concentrations. Therefore the total PCR sample volume is 20 μL.
The thermal cycling for HCV amplification was set as follows: 10 min for pre-denaturing at 94°C; 30 s for denaturing at 94°C, 30 s for annealing at 53°C, 40 s for extension at 72°C, and finally a post-extension at 72°C for 4 min. In order to avoid evaporation of the DNA samples and reagents, 20 μL of mineral oil (Sigma, USA) were added into the three reaction chambers, respectively. When the thermal reaction was finished, the DNA sample and mineral oil were pipetted from the reaction chamber. Finally, the PCR products were analyzed by using a 2% agarose gel (UR-AGA001, UniRegion, USA) electrophoresis separation stained by ethidium bromide (Sigma Chemical, USA) and then visualized under UV light.
3 Results and discussion
In this study, a suction-type membrane was used to transport a DNA sample. A 5-psi pressure was applied to assure a full deflection of the suction-type membrane. The duration between each step of the membrane deflections (as shown in Fig. 1(b)), which is controlled by the operating frequency of the EMV, is the major parameter for the flow rate. Note that one complete suction motion contains seven membrane steps to generate the suction pumping action. The duration of each step is defined as a step time, which can be precisely adjusted by EMVs. Therefore, an operating frequency of the microfluidic control module is defined as the reciprocal of a complete suction motion time (seven steps). Figure 3 shows the relationship between the sample flow rate and the operating frequency of the microfluidic control module at a compressed air pressure of 5 psi. It is clearly seen that the sample flow rate increases with the increasing operating frequency. A sample flow rate as high as 18 μL/s can be achieved at a maximum operating frequency of 14.3 Hz, which was limited by the experimental setup.
The relationship between the sample flow rate and the operating frequency of the microfluidic control module at a compressed air pressure of 5 psi. An increase in the operating frequency increases the sample flow rate. The sample flow rate can be as high as 18 μL/s at an operating frequency of 14.3 Hz
The remnants of the DNA sample entrained by the dead volume of the reaction chamber affect the efficiency of the DNA amplification. The residual mixture can be degraded by sustained exposure to high temperatures in the denaturing reaction chamber. Nonspecific annealing reactions in the annealing reaction chamber can also be observed. Therefore, the elimination of dead volume is crucial to the improvement in DNA amplification efficiency. In this study, the regions in/between the microvalves and the suction-type membrane are the main cause for the formation of the dead volume. To address this problem, 5 psi of compressed air was used such that the membranes can be deflected completely. After 1.2 sec of liquid transport by using the suction-type membrane pumps (included the 20 μL PCR sample and a little mineral oil), most of the DNA sample has been transported to the next reaction chamber. The dead volume is estimated to be 1 μL at an applied pressure of 2-psi, which is equal to 5% of the total DNA sample (20 μL). By increasing the pressure to 5 psi, the dead volume can be reduced to about 0.5 μL, which is equal to 2.5% of the total DNA sample. Figure 4 shows a series of photographs to demonstrate that the DNA sample can flow through three reaction chambers by using the microfluidic control module. The new suction-type membrane and three microvalves can rapidly transport the PCR mixture without significant dead volume (about 2.5%). In order to shorten the time needed for a PCR procedure, the flow rate can be increased by raising the operating frequency of the microfluidic control module. Moreover, the number of thermal cycles and the reaction time for the denaturing, annealing, and extension steps can be randomly adjusted. Note that in practical application, a small amount of mineral oil was restored in the reaction chamber on purpose to avoid formation of air bubbles.
Even though the three reaction chambers are kept at specific temperatures, the pumped DNA samples still require a finite time to reach thermal equilibrium after they are transported from the other chambers. In this study, mineral oil was used to avoid the formation of gas bubbles and sample evaporation. It also helps the pumped DNA sample to reach thermal equilibrium within a shorter period of time since most of the mineral oil (about 17 μL) still remains in the original reaction chamber and is kept at the appropriate set temperature at all times. Experimental data show that it takes about 2 s for the 20 μL PCR mixture to reach thermal equilibrium.
The temperature distributions in the reaction chamber can affect the efficiency of a PCR procedure. The non-uniformity of the temperature field can induce a thermal gradient to affect all three reactions, especially the formation of non-specific hybridization during the annealing reaction. To improve the uniformity of the temperature distribution inside the reaction chamber, array-type heating resistors with 2D, symmetrical, self-compensation function were adopted in the circulating PCR chip (Hsieh et al. 2008). In this study, three array-type heating and temperature-sensing resistors were continuously supplied with driving currents to modulate three reaction temperatures of the denaturing, annealing, and extension steps. Three temperature-sensing resistors placed in the middle of the array-type heating resistors were used to sense and feedback the precise temperature signals to the microcontroller. Figure 5 shows an IR image of the temperature fields for the three reaction temperatures. Note that these images were taken without the liquid stored in the reaction chamber. The IR images just measured the surface temperature of the cover glass. After injecting the PCR mixture into the open reaction chamber, a high thermal uniformity was observed. The thermal uniformity had a variation of less than 1–2°C, which was reported to be precise enough for most PCR processes (Hsieh et al. 2008). For the PCR thermal cycling of HCV (150 bps), the denaturing, annealing, and extension reaction temperatures are 94°C, 53°C, and 72°C, respectively. In the denaturing reaction region (94°C), the percentage of the area with a thermal variation less than 2°C was measured to be about 98%. Ninety percent of the reaction region has a variation of less than 1°C. The array-type heating resistors can provide a 100% uniform area with a thermal variation of less than 1°C or 2°C at a set annealing temperature of 53°C. Similarly, the percentages with a thermal variation of less than 1°C and 2°C in the extension reaction region at 72°C are 95% and 100%, respectively. The IR image also shows that the three individual reaction regions can be independently isolated without significant thermal cross-talk.
Infrared image of the temperature distributions inside the three reaction chambers. Three array-type heating and temperature-sensing resistors are used to modulate the three reaction temperatures for the denaturing, annealing, and extension steps for a PCR procedure. This indicates that the microheaters are able to create a uniform temperature distribution within the reaction chambers, marked as black dotted circles in the image
A 150-bp gene for detection of HCV was amplified to confirm the performance of the circulating PCR chip. The entire period for the PCR procedure is about 60 minutes including the time for pre-denaturing, 40 thermal reaction cycles, and a post-extension reaction. Compared with a conventional PCR machine (PCR Sprint Thermal Cycler, Thermo Electron Corporation, MA, USA), the time for a PCR procedure performed utilizing the circulating PCR chip is only 60% of the traditional time required (100 min).
The 20 μL of PCR products were extracted from the open reaction chambers after finishing the PCR procedure. Then 5 μL of the PCR product was separated by gel electrophoresis in a 2% agarose gel with 110 V for 32 min. The initial concentrations of 150-bp HCV template ranging from 105 to 10 copies/μL were tested. Figure 6 shows the electropherograms of amplified PCR products with a variety of initial DNA concentration: 10, 102, 103, 104 and 105 copies/μL, respectively. Note that 45 cycles was performed for 10 copies/μL. For cases of 102, 103, 104 copies/μL, 40 cycles were performed. For 105 copies/μL, only 35 cycles were performed due to the fact that the DNA amplification decays after 35 cycles at this high concentration (Chien et al. 2006). The intensity of the fluorescence signals from a standard DNA marker (Yeastern Biotech Corp., Taiwan) and the amplified DNA products were compared to quantify the amplified DNA products. The concentration of the 100-bp DNA marker is 15 ng/μL and the injection volume of the marker is 5 μL. The detection limit of the initial concentration of the 150-bp HCV template was then experimental found to be 10 copies/μL.
Electropherograms of amplified PCR products from different initial concentrations of samples by using the circulating PCR chip. The 150-bp detection gene for HCV is used. The detection limit of the circulating PCR chip is found to be 10 copies/μL; L 100-bp DNA markers; C5 105 copies/μL; C4 104 copies/μL; C3 103 copies/μL; C2 102 copies/μL; C1 10 copies/μL
4 Conclusion
This study reports a new circulating PCR chip using a suction-type membrane and three microvalves to drive the sample flowing through three individual reaction chambers. The flow rate of the suction-type membrane can be as high as 18-μL/s using 5-psi of compressed air and an operating frequency of 14.3 Hz. In addition, three sets of temperature-sensing and array-type heating resistors with a self-compensation function were used to modulate three different specific reaction temperatures for a given PCR protocol with a uniform thermal distribution within the respective areas. This circulating PCR chip was fabricated by using MEMS techniques. An open-type reaction chamber was adopted in this study to facilitate the operation for temperature calibration and can be easily accessible for further optical detection if necessary. In order to investigate the performance of the circulating PCR chip, the amplification of a 150-bp HCV gene was performed. Experimental data showed that the detection limit was 10 copies/μL. This circulating PCR chip may provide a useful platform for automatic genetic detection and molecular diagnosis.
Abbreviations
- 2D:
-
Two-dimensional
- Au:
-
Gold Bio-MEMS Bio-micro-electro-mechanical-systems
- bp:
-
Base pair
- HCV:
-
Hepatitis C virus
- DNA:
-
Deoxyribonucleic acid
- EMV:
-
Electromagnetic valve
- IR:
-
Infrared
- PCR:
-
Polymerase chain reaction
- PDMS:
-
Polydimethylsiloxane
- Pt:
-
Platinum
- SEM:
-
Scanning electron microscope
- Ti:
-
Titanium
- UV:
-
Ultra-violet
References
R.C. Anderson, X. Su, G.J. Bogdan, J. Fenton, Nucleic Acids Res. 28(12) (2000). doi:10.1093/nar/28.12.e60
P. Belgrader, C.J. Elkin, S.B. Brown, S.N. Nasarabadi, R.G. Langlois, F.P. Milanovich et al., Anal. Chem. 75(14), 3446–3450 (2003). doi:10.1021/ac034062u
Z. Chen, S. Qian, W.R. Abrams, D. Malamud, H.H. Bau, Anal. Chem. 76(13), 3707–3715 (2004). doi:10.1021/ac049914k
J.H. Chien, D.S. Lee, Y.T. Cheng, S.H. Yeh, W.P. Chou, P.H. Chen, Opt. Commun. 266, 744–750 (2006). doi:10.1016/j.optcom.2006.05.043
T. Fukuba, T. Naganuma, T. Fuiji, Proceedings of the 2002 International Symposium, 101–105 (2004)
A. Gulliksen, L. Solli, F. Karlsen, H. Rogne, E. Hovig, T. Nordstrøm, R. Sirevåg, Anal. Chem. 76, 9–14 (2004). doi:10.1021/ac034779h
M. Hashimoto, P.C. Chen, M.W. Mitchell, D.E. Nikitopoulos, S.A. Soper, M.C. Murphy, Lab Chip 4(6), 638–345 (2004). doi:10.1039/b406860b
T.M. Hsieh, C.H. Luo, F.C. Huang, J.H. Wang, L.J. Chien, G.B. Lee, Sensors Actuators B. 130, 848–856 (2008)
F.C. Huang, C.S. Liao, G.B. Lee, Electrophoresis 27(16), 3297–3305 (2006). doi:10.1002/elps.200600458
C.W. Huang, S.B. Huang, G.B. Lee, J. Micromech. Microeng. 16, 2265–2272 (2006). doi:10.1088/0960-1317/16/11/003
M. Koch, N. Harris, A. Evans, N. White, A. Brunnschweiler, Sens. Actuators A. 70, 98–103 (1998). doi:10.1016/S0924-4247(98)00120-4
M.U. Kopp, A.J. de Mello, A. Manz, Science 280(5366), 1046–1048 (1998). doi:10.1126/science.280.5366.1046
M. Krishnan, V.M. Ugaz, M.A. Burn, Science 298(5594), 793 (2002). doi:10.1126/science.298.5594.793
D.J. Laser, J.G. Santiago, J. Micromech. Microeng. 14, R35–R64 (2004)
C.S. Liao, G.B. Lee, J.J. Wu, C.C. Chang, T.M. Hsieh, C.H. Luo, Biosens. Bioelectron. 20, 1341–1348 (2004). doi:10.1016/j.bios.2004.05.006
A. Manz, D.J. Harrison, E.M.J. Verpoorte, J.C. Fettinger, A. Paulus, H. Lüdi, H.M. Widmer, J. Chromatography. 593(1–2), 253–258 (1992)
K.B. Mullis, Sci Am. 262(4), 56–65 (1990)
K.B. Mullis, F. Faloona, S. Scharf, R. Saiki, G. Horn, H. Erlich, Cold Spring Harbor Symp. Quant. Biol. 51, 263–273 (1986)
H. Nakano, K. Matsuda, M. Yohda, T. Nagamune, I. Endo, T. Yamane, Biosci. Biotechnol. Biochem. 58(2), 349–352 (1994)
M.A. Northrup, M.T. Ching, R.M. White, R.T. Wltson, Transducer, Chicago, USA, 924–926 (1993)
M.A. Northrup, C. Gonzalez, D. Hadley, R.F. Hills, P. Landre, S. Lehew, R. Saiki, J.J. Shinsky, R. Watson, R. Watson Jr., Proceeding Transducers, 764–767 (1995)
S. Poser, T. Schulz, U. Dillner, V. Baier, J.M. Kőhler, D. Schimkat, Sens. Actuators A. 62, 672–675 (1997)
R.K. Saiki, S. Scharf, F. Faloona, K.B. Mullis, G.T. Horn, H.A. Erlich, N. Arnheim, Science. 230(4732), 1350–1354 (1985)
K. Sun, A. Yamaguchi, Y. Ishida, S. Matsuo, H. Misawa, Sens. Actuators B. 84, 283–289 (2002)
M.A. Unger, H.P. Chou, T. Thorsen, A. Scherer, R.Q. Stephen, Science 288(5463), 113–116 (2000). doi:10.1126/science.288.5463.113
C.H. Wang, Y.Y. Chen, C.S. Liao, T.M. Hsieh, C.H. Luo, J.J. Wu, H.H. Lee, G.B. Lee, J. Micromech. Microeng. 17(2), 367–375 (2007)
A.T. Woolley, D. Hadley, P. Landre, A.J. de Mello, R.A. Mathies, M.A. Northrup, Anal. Chem. 68(23), 4081–4086 (1996)
Acknowledgements
The authors gratefully acknowledge the financial supports provided by the National Science Council of Taiwan NSC 96-2622-E-002-001 and NSC 96-2120-M-006-008 for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chien, LJ., Wang, JH., Hsieh, TM. et al. A micro circulating PCR chip using a suction-type membrane for fluidic transport. Biomed Microdevices 11, 359–367 (2009). https://doi.org/10.1007/s10544-008-9242-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-008-9242-z