Abstract
The purpose of this study was using a developed microfluidic chip to prepare size-controlled monodisperse chitosan microparticles encapsulating ampicillin. Our strategy is that a chitosan aqueous solution (the disperse phase) is fed into the microfluidic chip equipped with a cross-junction microchannel, and is sheared by the viscous oil flows (the continuous phase) to form monodisperse semi-product, chitosan emulsions. These fine emulsions are then gelled into stability upon gelation by injection of copper sulfate solution at the terminal microchannel of the microfluidic chip, and finally the uniform chitosan microparticles are formed in an efficient manner. The proposed chip is fabricated by a CO2 laser machine on a conventional poly methyl methacrylate (PMMA) substrate. This microfluidic chip has four inlet ports, one cross-channel and one outlet port. We have demonstrated that one can control the size of chitosan microparticles from 100 to 800 μm in diameter (with a variation less than 5%) by altering the relative sheath/sample flow rate ratio. Experimental data showed that when given a steady continuous phase (oil flow), the emulsion size increases with the increase in average velocity of the dispersed phase flow (sample flow). In addition, the release of the model drug (ampicillin) from these microspheres is proved to be once-daily for clinical application. We also revealed that appropriate particle sizes for different release patterns are predictable, enabling better applications of chitosan as a drug carrier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chitosan is currently gaining a great deal of attention for medical and pharmaceutical applications (Sinha et al., 2004; Dodane and Vilivalam, 1998; Paul and Sharma, 2000). Chitosan is a linear polysaccharide obtained by chemical deacetylation of chitin which is the structural component in the exoskeleton of crustaceans (shrimp, crabs, etc.) and the cell wall of fungi (Aiba, 1992). Due to its biocompatibility and biodegradability, and it can improve efficacy of wound healing, reduce toxicity, and improve uptake, the chitosan particles have been used in the development of drug delivery systems in recent years (Guliyeva et al., 2006; Ko et al., 2002; Agnihotri et al., 2004).
Several methodologies for the production of chitosan particles have been described in the literature, including coacervation (precipitation), spray-drying (atomization), emulsion techniques, and others (He et al., 1999; van der Lubben et al., 2001; Kawashima et al., 1998; Young et al., 1999; Mironov et al., 2005; Lee et al., 2006). However, the resulting size and size distribution of the microparticles cannot be easily controlled by these methods. Control of the particle size and the size distribution is important for controlled-release drug delivery, because they influence the clearance rate from the body and ultimately determines the drug dosage (Berklan et al., 2001). Basically, an ideal particle size could provide the optimal release rate and route of administration.
Many researchers have attempted to make smaller particles, but less attention has been paid to make monodisperse particles (Lee et al., 2006; Berklan et al., 2001; You et al., 2001; Sugiura et al., 2005; Iwamoto et al., 2002). Recently, Nakajima et al. developed a novel microfluidic device that utilized a silicon micro-nozzle array to produce Ca-alginate beads (Sugiura et al., 2005) and gelatin beads (Iwamoto et al., 2002) with the variation within 15%. In contrast, studies of monodisperse chitosan particles are not as numerous. Hence, it has become imperative to develop a reproducible method for generating chitosan particles with a uniform particle size.
Microfluidic chip (containing cross-junction microchannel) emulsification is a novel technique for preparing water-in-oil (w/o) and oil-in-water (o/w) emulsions (Kawakatsu et al., 1997; Tan et al., 2004, 2006; Cristini et al., 2004; Jahn et al., 2004). The mechanism of this type of microfluidic chips in droplet-volume control was well studied recently (Kawakatsu et al., 1997; Tan et al., 2004). Until now, no attention has been paid to apply microfluidic chip to control the performance of uniform chitosan microspheres. Based on the outstanding performance of the microfluidic chip, we utilize it in this work for pharmaceutics (e.g. chitosan microparticles generation).
The aim of this study is to investigate and compare the size of the chitosan microparticles obtained by a different ratio of flow rate in the side inlet channels to that in the center inlet channel. The developed microfluidic chip is easy to fabricate and set-up, and is easily programmed to generate a large set of uniform chitosan microparticles.
2 Experimental
2.1 Materials
Chitosan (low molecular weight), ampicillin and polyvinyl alcohol (PVA) 88–89% hydrolyzed are purchased from Sigma (Sigma Chemical Co., St. Louis, MO). Distilled water (DI water) is filtered by 0.22 nm syringe filter (Millipore Inc., Clifton, NJ) before used in the preparation process. All other reagents are commercially available and of the highest grade.
2.2 Principle
Our strategy is based on hydrodynamic-focusing on the forming of a series monodisperse of self-assembling sphere structures, the so-called water-in-oil (w/o) emulsions, in the cross-junction microchannel. These fine emulsions, consisting of chitosan, are then undergoing in situ ionotropic gelation by getting contact with copper ion (II) flow (be injected into terminal microchannel) to accomplish uniform chitosan microparticles in an efficient manner (Fig. 1). The mechanism of the chitosan emulsions transfer to the chitosan microparticles was that (i) after the chitosan emulsions be generated in the cross-junction microchannel, they precipitated spontaneously at the bottom of oil flow due to higher density than that of the oil, and (ii) therefore, chitosan emulsions could react with copper ion at the interface between oil flow and copper sulfate flow at the terminal microchannel. By this way, the emulsification of two immiscible liquids and in situ ionic gelation simultaneously occurred in the developed microfluidic chip, and this one shot operation performs the “Lab on a chip”.
2.3 Fabrication of a microfluidic chip
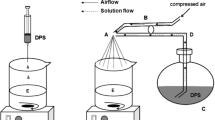
The developed microfluidic chip is laid out on a conventional poly methyl methacrylate (PMMA) substrate (length/width/depth: 270.0 mm/210.0 mm/1.5 mm) with a laser micromachining process using a CO2 laser machine (LaserPro Venus, GCC, Taiwan). Figure 2 shows the schematic drawing of the microfluidic chip. The chip (length/width/depth: 110.0 mm/60.0 mm/7.5 mm) consists of five layers (an expanded view is shown in Fig. 2(a)) which are, from top to bottom, the cover layer (upper laminate), the sample and oil inlets, the main layer (cross-junction channel and reagent inlet), the reagent-inlet channel layer and the bottom layer (lower laminate), respectively. These five layers are integrated by screws (tightened at 1–1.2 Nm) followed by thermal binding in an oven (OPO-45, CHENG SANG, Taiwan) at 110 ± 5°C for 90 min. This device is then naturally annealed to room temperature to produce the microfluidic chip. This chip has four inlet ports, one cross-channel, and one observation section, as shown in Fig. 2(b). The broadened channels (600 μm in width) near the outlet of the cross channel and observation chamber (1200 μm in width) are designed for slowing down the flow and enhance the analysis observation. This platform is low-cost, easy to fabricate, easy to set up, as well as easy to organize and program for generating chitosan microspheres.
2.4 Experimental procedure
First, set up the fluids of the center inlet, side inlet and reagent inlet channels with chitosan solution, oil and copper ion (II) solution, respectively. Generally speaking, the material to be encapsulated is mixed with the chitosan solution. Second, all the fluids are then injected through the chip inlets by syringe pumps (Kdscientific KDS230) programmed by a PC. In this work we focus a stream of chitosan solution at a cross-junction microchannel by two oil streams, enabling the construction of water-in-oil (w/o) chitosan emulsions along the microchannel axis. Finally, these emulsions then undergo ionic gelation by contact with a stream of copper ion (II) solution to produce chitosan microspheres. A commercial microscope is used to observe the experimental results. The image detection system consists of an optical microscope (BX60, Olympus, Japan) and a digital camera (DP70, Olympus, Japan). Figure 3 shows an overview of the experimental setup used. The diameter of each microsphere is measured and averaged. A total of 50 microspheres are measured to provide an average size.
2.5 In vitro drug release studies
The entrapment efficiency reversely derived from the ampicillin content in the supernatant of ampicillin is determined by spectrophotometry (Hitachi-U1800, Japan) at the wavelength of 203 nm. All samples were analyzed in triplicate. Ampicillin loaded chitosan microparticles are dispersed in transport buffer at pH 7.4, which is used to simulate physiological fluid. The buffer solution is kept in an orbital shaker at constant gentle shaking of 100 rpm at 37°C. At pre-determined time intervals, the suspensions are centrifuged at 12,000 rpm for 8 min. The precipitated particles are re-suspended in fresh buffer and placed back into the shaker. The supernatant containing released ampicillin is then analyzed by spectrophotometry to determine the percentage release of the ampicillin from the microparticles. Results were expressed as the means ± standard deviation (n = 3). The statistical difference was assumed to be significant when P < 0.05 by the two-sided Student t-test.
3 Results and discussion
3.1 Preparation of uniform ampicillin loaded chitosan emulsions
For the w/o chitosan emulsions generation, the pregel solution (which is prepared by mixing 25 mL of 16.6% (w/v) ampicillin and 5 mL of 3.0% (w/v) chitosan, 4 mPa s (cP), density 0.928) and sunflower seed oil (40 mPa s (cP), density 0.869, Uni-President Enterprises Corp., Taiwan) are employed as the water-phase fluid (the disperse phase) and oil-phase fluid (continuous phase), respectively. A water-soluble dye (red ink) is dissolved in the pregel solution for immediate (real-time) observation. As soon as the pregel solution (the disperse phase) is fed into with a syringe pump, it is sheared by two oil streams at the cross-junction channel, resulting in the instantaneous formation of chitosan emulsions containing ampicillin. Utilizing an inverted fluorescent microscope, the forming of the uniform emulsions in the compartment is characterized.
In the initial experiments, the flow rates of the sample fluid and the oil fluids were set to 0.05 mL/min and 0.7 mL/min, respectively. We found that the sample fluid was compressed by a shear force to an arrow shape (Fig. 4(a)) and then separated into emulsions of about 400 μm in diameter. In addition, the size distribution of the emulsions is quite uniform (400 ± 10 μm). The flow rates of the oil and the pregel solution are adjusted to control the degree of hydrodynamic focusing and the width of the center stream, resulting in the manipulation of chitosan emulsions. Therefore, we conclude that by using the microfluidic chip there is great potential in fabricating regular chitosan emulsions.
(a) Mono-dispersed chitosan emulsions are generated at the cross-junction with oil flow: 0.7 mL/min and sample flow: 0.05 mL/min. The size distribution of formed emulsions is quite uniform (400 ± 10 μm). The arrow-shaped flow indicates the direction of emulsion generation and scale bar is 500 μm. (b) Generated chitosan particles can be observed in the collection reservoir (scale bar 200 μm)
3.2 Formation of chitosan microspheres
The semi-products (chitosan emulsions) were formed in the continuous oil flow. The continuous oil flow could prevent these semi-products from fusing together, and could transport these monodisperse emulsions to contact with the copper ion fluid. By means of injecting 20% CuSO4 solution through the reagent inlet at the terminal channel, ampicillin loaded chitosan microparticles were formed after 20-min ionic gelation cross-linking in the collection reservoir (Fig. 4(b)). These chitosan particles are separated from the CuSO4 solution and oil by vacuum filtration. They are washed two times on a filter with 30 mL n-hexane/ether and with 10 mL 50 mM Tris-HCl buffer (pH 7.2). They were then subject to freeze-drying. After being dipped in liquid nitrogen, they were dried at −70°C under vacuum (0.1 mmHg) for 10 h and then vacuum-dried at room temperature for 1 h. We found the shapes of most chitosan microspheres remained spheroid after the gelation and the freeze-drying process. The obtained chitosan particles shrank after the freeze-drying process. For example, the chitosan particles which measured 200 μm in diameter before the freeze-drying process decreased in size to 2 μm in diameter, with a standard deviation of less than 15% after the freeze-drying process.
3.3 The influence of flow rate
To gain further understanding, the relationship between size and flow speed were studied. The emulsion size can be easily varied by changing the flow conditions in the microchannels. Figure 5 shows the relationship between the average flow rate of the phases and the emulsion size (diameter). When given a steady 0.1 mL/min continuous phase, the emulsion size increases with the increase in average velocity of the dispersed phase flow. In addition, when the oil flow was set to 0.4, 0.7, or 1.0 mL/min, the same tendency was observed. On the other hand, for a given fixed rate of dispersed phase flow, the emulsion size decreases as the average rate of the continuous phase increased.
The experiment data shown in Figs. 6(a) and (b) were chitosan emulsions with a narrow diameter distribution (standard deviation less than 5%) and uni-gap in the microchannel, demonstrating that fine and precise control can be obtained by using microfluidic interfaces to manipulate the assembly of microparticles. Figures 6(c) and (d) showed that when the rate of the oil flow increases, the gap distance of the emulsions decreases (at the same water flow rate). It is evident that the size and gap of the emulsions, generated in the cross-junction, are controllable and reproducible using the microfluidic technique.
3.4 In vitro drug release
Ampicillin, being a broad spectrum antibiotic, has a short biological half-life of 0.75–1.50 h. Recent research has been directed to design formulations for its sustained and controlled release to make the application of ampicillin more effective (Giunchedi et al., 1998; Anal and Stevens, 2005). Hence, it was used as the model drug to be encapsulated and to evaluate the drug release rate and in vitro antibacterial effects. The microparticles prepared by this method would be useful as sophisticated biomaterials due to their characteristic physical, chemical and biological properties; for example, as cell transplantation carriers, drug carriers and gene delivery carriers. We found that the release rate of ampicillin decreases with increases with the size of ampicillin-chitosan microparticles and achieve an objective to once daily dosing protocol for ampicillin (Fig. 7).
4 Conclusion
Controlled fabrication of chitosan microparticles is a prerequisite for the controlled release of drugs. This paper demonstrates using a microfluidic device to generate uniform micro chitosan droplets and gel in copper ion solution afterward, and also showing ampicillin encapsulation in chitosan microparticles. The developed microfluidic chip has the advantages of active control of droplet diameter (ranging from 100 to 800 μm with a variation less than 5%), simple and low cost process, and high throughput. Experimental data showed that the release profiles of the model drug (ampicillin) from these chitosan microspheres are proved to be once-daily for clinical applications. It has turned out to be one of the most efficient methods for the creation of uniform chitosan microspheres. The approach in manipulation of chitosan microspheres will provide many potential usages for pharmaceutical applications.
References
S.A. Agnihotri, N.N. Mallikarjuna, and T.M. Aminabhavi, J. Contr. Release 100, 5–28 (2004).
S. Aiba, Int. J. Biol. Macromol. 14, 225–228 (1992).
A.K. Anal and W.F. Stevens, Int. J. Pharm. 290, 45–54 (2005).
C. Berkland, K.K. Kim, and D.W. Pack, J. Contr. Release 73, 59–74 (2001).
V. Cristini and Y.C. Tan, Lab on a Chip 4, 257–264 (2004).
V. Dodane and V.D. Vilivalam, Pharm. Sci. Technol. Today 1, 246–253 (1998).
P. Giunchedi, I. Genta, B. Conti, R.A.A. Muzzarelli, and U. Conte, Biomaterials 19, 157–161 (1998).
U. Guliyeva, F. Oner, S. Ozsoy, and R. Haziroglu, Euro. J. Pharm. Biopharm. 62, 17–25 (2006).
P. He, S.S. Davis, and L. Illum, Int. J. Pharm. 187, 53–65 (1999).
S. Iwamoto, K. Nakagawa, S. Sugiura, and M. Nakajima, AAPS Pharm. Sci. Tech. 3, 25 (2002).
A. Jahn, W.N. Vreeland, M. Gaitan, and L.E. Locascio, J. Am. Chem. Soc. 126, 2674–2675 (2004).
T. Kawakatsu, Y. Kikuchi, and M. Nakajima, J. Am. Oil Chem. Soc. 74, 317–321 (1997).
Y. Kawashima, H. Yamamoto, H. Takeuchi, T. Hino, and T. Niwa, Euro. J. Pharm. Biopharm. 45, 41–48 (1998).
J.A. Ko, H.J. Park, S.J. Hwang, J.B. Park, and J.S. Lee, Int. J. Pharm. 249, 165–174 (2002).
M. Lee, Y.W. Cho, J.H. Park, H. Chung, S.Y. Jeong, K. Choi, D.H. Moon, S.Y. Kim, I.S. Kim, and I.C. Kwon, Coll. Polym. Sci. 284, 506–512 (2006).
I.M. van der Lubben, J.C. Verhoef, A.C. van Aelst, G. Borchard, and H.E. Junginger, Biomaterials 22, 687–694 (2001).
A.V. Mironov, O.L. Vedenina, G.A. Vikhoreva, N.R. Kil’deeva, and A.I. Albulov, Fib. Chem. 37, 22–25 (2005).
W. Paul and C.P. Sharma, STP Pharma Sci. 10, 5–22 (2000).
V.R. Sinha, A.K. Singla, S. Wadhawan, R. Kaushik, R. Kumria, K. Bansal, and S. Dhawan, Int. J. Pharm. 274, 1–33 (2004).
S. Sugiura, T. Oda, Y. Izumida, Y. Aoyagi, M. Satake, A. Ochiai, N. Ohkohchi, and M. Nakajima, Biomaterials 26, 3327–3331 (2005).
Y.C. Tan, J.S. Fisher, A.I. Lee, V. Cristini, and A.P. Lee, Lab on a Chip 4, 292–298 (2004).
Y.C. Tan, V. Cristini, and A.P. Lee, Sens. Actu. B-Chem. 114, 350–356 (2006).
J.O. You, S.B. Park, H.Y. Park, S. Haam, C.H. Chung, and W. S. Kim, J. Microencapsul. 18, 521–532 (2001).
T.J. Young, K.P. Johnston, K. Mishima, and H. Tanaka, J. Pharm. Sci. 88, 640–650 (1999).
Acknowledgments
This work was supported by a grant from the National Science Council of Taiwan, R.O.C. (NSC-94-2320-B-214-005, NSC 95-2320-B-214-002-MY2) and Industrial Technology Research Institute. We are indebted to Prof. Eng-Chi Wang, Kaohsiung Medical University, Taiwan, for encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, CH., Huang, KS. & Chang, JY. Manufacturing monodisperse chitosan microparticles containing ampicillin using a microchannel chip. Biomed Microdevices 9, 253–259 (2007). https://doi.org/10.1007/s10544-006-9029-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-006-9029-z