Abstract
A novel and simple method for size control of self-assembled nanoparticles is suggested in this paper. Polymeric nanoparticles were prepared from amphiphilic chitosan derivatives fluorescein isothiocyanate (FITC)-conjugated glycol chitosans (FGCs). The attachment of hydrophobic FITC onto hydrophilic glycol chitosan induced the amphiphilic conjugate to form self-assembled nanoparticles in aqueous media, depending on degree of substitution. The size of self-assembled nanoparticles was controlled by a novel emulsion/solvent evaporation method. Adding a small amount of an immiscible solvent with water (chloroform) to FGC nanoparticle suspensions in aqueous media followed by ultrasonification and solvent evaporation led to partial dissociation and subsequent reformation of nanoparticles. The evaporation of chloroform facilitated the hydrophobic association, which resulted in more dense and hardened hydrophobic cores. The size of nanoparticles was closely related with the FGC concentration in the emulsion. The mean diameters of self-assembled nanoparticles were 150–500 nm at the FGC concentrations of 0.3–2.5 mg/ml. Higher FGC concentration resulted in larger particles. The polydispersity factors (μ 2/Γ 2) of the reformed nanoparticles were fairly low (0.001–0.094), indicating narrow size distribution. The FGC nanoparticles were stable in phosphate-buffered saline at 37°C up to 20 days. Lactose was a good excipient for maintaining the structural integrity of nanoparticles during freeze-drying. Without lactose, the freeze-dried nanoparticles were not homogeneously redispersed in aqueous media. However, the freeze-dried nanoparticles with lactose were spontaneously redispersed in aqueous milieu with their own sizes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymeric amphiphiles with an appropriate hydrophilic/hydrophobic balance spontaneously form self-assembled nanoparticles in an aqueous environment as one of colloidal systems because of the formation to interfacial-free energy-minimized structure. Polymeric self-assembled nanoparticles have been widely used as drug carriers in the field of biotechnology [1–5]. They have a unique core–shell structure. The hydrophobic core serves as a microreservoir for hydrophobic drugs and acts as a depot to release the drugs for a certain period of time, while the hydrophilic shell reduces their interaction with plasma proteins and increases their half-lives in blood circulation.
Well-controlled polymeric nanoparticles could be a useful carrier in the fields of drug delivery systems. The size control of polymeric nanoparticles is of great importance because the size has a significant effect on their pharmacokinetics at the whole body. For example, the tissue distribution of nanoparticles and their passive targeting to tumor tissues by the enhanced permeability and retention (EPR) effect were largely dependent on their size [6–8]. The size of self-assembled nanoparticles may differ according to many factors such as the molecular weight (MW) of a main polymeric constituent, the kind of substitutents, the hydrophilic/hydrophobic balance, etc. [7, 9, 10]. Our group has reported a series of polymeric self-assembled nanoparticles for drug and gene delivery [5, 7, 10–13]. In the previous works, it was quite difficult to obtain polymeric nanoparticles with a desirable size for a specific use. Size control is an actual challenge in nanoparticulate drug delivery systems.
Here, we propose a novel emulsion/solvent evaporation method to control the size of self-assembled nanoparticles. Hydrophobic fluorescein isothiocyanate (FITC) was conjugated to hydrophilic glycol chitosan (GC) for self-assembly in aqueous milieu. The hydrophobic/hydrophilic balance of the self-assembled nanoparticles was controlled to be stable in aqueous media. The size of nanoparticles was manipulated from 150 to 500 nm in a mean diameter by a novel emulsion/solvent evaporation method. The nanoparticles were characterized by dynamic light scattering and transmission electron microscopy (TEM). In addition, lactose anhydrous was suggested as an excellent excipient for maintaining the nanoparticulate structure in this paper.
Materials and methods
Materials
Glycol chitosan (GC, MW=250 kDa, degree of deacetylation=88%) was purchased from Sigma (St. Louis, MO). GC was dissolved in distilled water, filtered to remove insoluble impurities, and dialyzed against distilled water. Fluorescein isothiocyanate, chloroform, and lactose anhydrous were purchased from Sigma and used without further purification. All other chemicals were of analytical grade and were used as received.
Depolymerization of glycol chitosan
Two grams of GC was dissolved in 150 ml of 4 M hydrochloric acid, which was placed in a preheated water bath at 50°C for 5–10 h. The reaction was stopped by adding 4 M NaOH and then dialyzed for 2 days against distilled water. The dialysate was freeze-dried.
Conjugation of FITC to GC
Glycol chitosan (0.5 g) was dissolved in 100 ml bicarbonate buffer (0.1 M Na2CO3, 0.1 M NaHCO3, pH 9.5). FITC was added to the GC solution and stirred for 24 h at room temperature. The solution was dialyzed for 3 days against 50% (v/v) of water/ethanol mixture and then freeze-dried. The FITC content in FITC–GC conjugate (FGC) was determined by a fluorometer (ISS K2, Champaign, IL), based on the standard curve obtained from FITC alone. The excitation and emission wavelengths were 495 and 520 nm, respectively.
Preparation of self-assembled nanoparticles by a novel emulsion/solvent evaporation method
Fluorescein glycol chitosans were suspended in a phosphate-buffered saline (PBS, pH 7.4) at different concentrations (0.3–2.5 mg/ml) under gentle shaking for 6 h. A small amount of chloroform was added to the FGC suspension, which spontaneously formed an emulsion, and then it was sonicated five times using a probe-type sonicator (Sigma Ultrasonic Processor, GEX-600) at 90 W for 2 min each, in which the pulse was turned off for 1 s with an interval of 5 s. Residual chloroform was removed using a rotary evaporator at 50°C for 30 s under reduced pressure. The nanoparticle suspension was passed through a syringe filter (pore size 0.8 μm, Millipore, Billerica, MA). Lactose anhydrous was added to the nanoparticle suspensions. The amounts of lactose anhydrous were 10–50 equivalent to FGC weight. The nanoparticle suspensions containing lactose were then freeze-dried.
Gel permeation chromatography
The MW of GC was determined by a gel permeation chromatography (GPC) Waters GPC 410 system (Milford, MA) equipped with three ultrahydrogel (120, 250, and 500) after calibration with standard pullulan samples (Shodex Standard p-82, SHOWA DENKO, Tokyo, Japan) at a flow rate of 0.6 ml/min in distilled water/dimethylformamide (DMF) (v/v=9.5/0.5 containing NaOH 0.05 mol/l). Every sample was filtered by a syringe filter (pore size 0.45 μm, Millipore).
Dynamic light scattering
To determine the particle size and size distribution of self-assembled nanoparticles, dynamic light scattering measurement was performed using a helium ion laser system (Spectra Physics Laser Model 127-35, Mountain View, CA), which was operated at 633 nm and 37±1°C. The scattered light was measured at an angle of 90° and was collected with a Brookhaven BI-9000AT autocorrelator (Holtsville, NY). The concentration of self-assembled nanoparticles was varied from 0.3 to 2.5 mg/ml. The hydrodynamic diameter of nanoparticles was calculated by the Stokes–Einstein equation. The polydispersity factor, represented as μ 2/Γ 2, was evaluated from the cumulant method, where μ 2 is the second cumulant of the decay function, and Γ is the average characteristic line width.
Transmission electron microscopy
The morphology of nanoparticles was observed by transmission electron microscopy (TEM, Philips CM 30, Eindhoven, Netherlands) operated at an accelerating voltage of 200 kV. A sample solution in water was dropped onto a copper grid coated with carbon, taped with a filter paper to remove surface water, and air-dried for 5 min. The nanoparticles deposited on the grid were then negatively stained by 2 wt% uranyl acetate solution.
Results
Self-assembly of FGC in aqueous milieu
Fluorescein glycol chitosan was synthesized by conjugation of hydrophobic FITC to a hydrophilic GC backbone [7, 14]. The isothiocyanate group of FITC easily reacted with the primary amine group of GC in a bicarbonate buffer solution (pH 9.5). FGCs with different amounts of FITC were prepared by simply changing the feed ratio of FITC to GC. The characteristics of FGC, including degree of substitution (DS), were listed in Table 1. The FGCs with low FITC contents below 2 wt% were water-soluble and did not form self-assembled nanoparticles, whereas those with high FITC contents above 5 wt% were not homogeneously dispersed in PBS because of immoderate hydrophobicity. That is, the FGC formed self-assembled nanoparticles in the range of 2.0–5.0 wt% of FITC contents. The size of self-assembled nanoparticles was dependent on DS. As DS increased from 1.4 to 2.5, the hydrodynamic diameter was reduced from 380 to 280 nm. All FGC nanoparticles had a broad size distribution, the polydispersity factor, μ 2/Γ 2, from 0.084 to 0.431.
Size control of FGC nanoparticles by a novel emulsion/solvent evaporation method
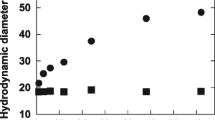
The size of self-assembled nanoparticles was manipulated by a novel emulsion/solvent evaporation method. The addition of a small amount of chloroform to an aqueous FGC nanoparticle suspension followed by ultrasonification and solvent evaporation led to partial dissociation of the nanoparticles and subsequent reformation of the self-assembled nanostructure. In all cases, a change in FGC concentration in the emulsion resulted in a change in particle size, as shown in Fig. 1. The mean diameters of the self-assembled nanoparticles prepared at the FGC concentrations of 0.3–2.5 mg/ml were 150–500 nm. Higher polymer concentration resulted in larger particles. The polydispersity factors (μ 2/Γ 2) of the reformed self-assembled nanoparticles, being estimated by cumulant method, were fairly low (0.001–0.094), indicating the narrow size distribution. TEM observation confirmed the size and size distribution of nanoparticles (Fig. 2).
Effect of FGC concentration on particle size. The nanoparticles were prepared by adding chloroform (10 μl) to aqueous FGC suspensions (1 ml) with different FGC concentrations. The error bar represents standard deviation (n=3). The FGC-X represents FITC-conjugated glycol chitosan whose degree of substitution is X
To investigate the effect of polymer MW on particle size, GC with different MWs was prepared by acid hydrolysis [9]. Increasing the acid degradation time decreased the MW of the resulting polymer. The commercially supplied GC with an MW of 250 kDa were degraded to 41 and 25 kDa, 5 and 10 h after acid hydrolysis, respectively. FITC was also conjugated to the depolymerized GCs for nanoparticle formation. While FITC-conjugated depolymerized GC with an MW of 41 kDa and DS of 1.4 (FDGC41-1.4) formed self-assembled nanoparticles in aqueous media, FDGC with the same DS but an MW of 25 kDa (FDGC25-1.4) did not form. The mean diameter of FDGC41-1.4 was around 210 nm before size control. The particle sizes after the emulsion/solvent evaporation process were 150–380 nm, depending on the FDGC41-1.4 concentration in the emulsion, as shown in Fig. 3.
To observe the effect of solvent amount on particle size, different amounts of chloroform were added to aqueous FGC nanoparticle suspensions. As shown in Fig. 4, it is apparent that the amount of chloroform in the experimental range did not significantly influence the size of self-assembled nanoparticles.
Lactose anhydrous as an excipient for maintaining nanoparticulate structure
Lactose, which has been widely used as an excipient, is a disaccharide naturally present in milk [15, 16]. Lactose anhydrous was used as a cryo-/lyoprotectant of FGC nanoparticles during freeze-drying. Without lactose, severe aggregation of nanoparticles occurred during freeze-drying, and the aggregates were not homogeneously redispersed in aqueous media. On the contrary, the freeze-dried nanoparticles with lactose were spontaneously redispersed in water with their own sizes, as shown in Figs. 5 and 6, indicating that lactose anhydrous can prevent aggregation of FGC nanoparticles during freeze-drying. To investigate the effect of the amount of lactose anhydrous, various amounts of lactose anhydrous were added to nanoparticle suspensions. Up to 20 equivalent of lactose anhydrous to FGC weight, the self-assembled nanoparticles were not immediately dispersed in water, as shown in Fig. 6. They needed at least 24 h for complete dispersion. Over 30 equivalent of lactose anhydrous to FGC weight, the FGC nanoparticles were immediately dispersed.
Lactose anhydrous (LA) as a cryo-/lyoprotectant of FGC nanoparticles during freeze-drying. The white bars represent the mean diameters of FGC-1.4 nanoparticles in PBS just after chloroform evaporation. The black bars represent the mean diameters of FGC-1.4 nanoparticles redispersed in PBS after lyophilization of aqueous FGC-1.4 suspensions containing 50 equivalent of lactose anhydrous to FGC-1.4 weight
Effect of the amount of lactose anhydrous (LA) on the colloidal stability of FGC-1.4 nanoparticles. The black bars represent the mean diameters of FGC-1.4 nanoparticles in PBS just after chloroform evaporation. The gray bars represent the mean diameters of FGC-1.4 nanoparticles redispersed in PBS after lyophilization of aqueous FGC-1.4 suspensions containing different amounts of lactose anhydrous (*, needed at least 24 h for homogenous redispersion)
Discussion
The changes in proposed structure during the emulsion/solvent evaporation process are schematically represented in Fig. 7. The amphiphilic chitosan derivatives spontaneously form self-assembled nanoparticles in aqueous media. However, their size distribution was fairly broad. By adding the organic solvent chloroform, the nanoparticles are partly dissociated and subsequently reassociated because chloroform dissolves the hydrophobic multicores of nanoparticles. Through successive ultrasonification and solvent evaporation, the nanoparticles are reformed with new hydrophobic multicores which are more dense and packed.
The solubility difference between GC and FITC to chloroform is quite great, which induced the severe core–shell phase separation. Chloroform forced the hydrophobic FITC to gather more closely, and subsequent evaporation of chloroform from the cores facilitated the hydrophobic association of FITC, which resulted in more dense and hardened hydrophobic cores. The size distribution of reassembled nanoparticles was outstandingly narrow. The size of reassembled nanoparticles showed the direct relationship with the FGC concentration in the emulsion. Increases in FGC concentration corresponded to increases in particle size, which means easy handling of particle size. Furthermore, the core-dense nanoparticles by the novel method showed excellent colloidal stability in PBS. The nanoparticles maintained their own sizes in PBS at 37°C and pH 7.4 up to 20 days.
The second important finding is lactose as a cryo-/lyoprotectant for maintaining the nanoparticulate structure during freeze-drying. The quality of drug formulations depends not only on active components but also the performance of excipients. Lactose performed important and specific functions to guarantee the colloidal stability of self-assembled nanoparticles. When lactose was added to nanoparticle suspensions in PBS, the hydrogen bonding between lactose and nanoparticles prevented the interparticle interaction between nanoparticles. A large amount of lactose surrounded each nanoparticle separately, which resulted in immediate redispersion of freeze-dried lactose/nanoparticle complexes. The hydrodynamic diameter of the redispersed nanoparticles was the same as the original one. It is expected that lactose may be a useful excipient to guarantee the immediate colloidal redipersion in other nanoparticulate systems.
Conclusions
The size of self-assembled nanoparticles was tuned by controlling the polymer concentration during the emulsion/solvent evaporation process. By the novel method, the nanoparticles with mean diameters of 150–500 nm and narrow size distribution were reformed. The novel method induced a more efficient packing of hydrophobic cores, which resulted in higher colloidal stability of self-assembled nanoparticles. Lactose was an excellent excipient to help nanoparticles maintain their colloidal nanostructure.
References
Barratt G (2003) Cell Mol Life Sci 60:21
Kataoka K, Harada A, Nagasaki Y (2001) Adv Drug Deliv Rev 47:113
Janes KA, Calvo P, Alonso MJ (2001) Adv Drug Deliv Rev 47:83
Mu L, Seow PH, Ang SN, Feng SS (2004) Colloid Polym Sci 283:58
Lee KY, Kim JH, Kwon IC, Jeong SY (2000) Colloid Polym Sci 278:1216
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK (1998) Proc Natl Acad Sci U S A 95:4607
Son YJ, Jang JS, Cho YW, Chung H, Park RW, Kwon IC, Kim IS, Park JY, Seo SB, Park CR, Jeong SY (2003) J Control Release 91:135
Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK (1995) Cancer Res 55:3752
Wang W, McConaghy AM, Tetley L, Uchegbu IF (2001) Langmuir 17:631
Kwon S, Park JH, Chung H, Kwon IC, Jeong SY (2003) Langmuir 19:10188
Park JH, Kwon S, Nam JO, Park RW, Chung H, Seo SB, Kim IS, Kwon IC, Jeong SY (2004) J Control Release 95:579
Lee KY, Jo WH, Kwon IC, Kim YH, Jeong SY (1998) Macromolecules 31:378
Kim YH, Gihm SH, Park CR, Lee KY, Kim TW, Kwon IC, Chung H, Jeong SY (2001) Bioconjug Chem 12:932
Onishi H, Machida Y (1999) Biomaterials 20:175
Pifferi G, Santoro P, Pedrani M (1999) Farmaco 54:1
Jain R, Railkar AS, Malick AW, Rhodes CT, Shah NH (1998) Eur J Pharm Biopharm 46:177
Acknowledgements
This study was supported by the Ministry of Science and Technology (Grant No. M10414030002-05N1403-00220 and M10414030002-05N1403-00240) and the Advanced Medical Technology Cluster, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, M., Cho, Y.W., Park, J.H. et al. Size control of self-assembled nanoparticles by an emulsion/solvent evaporation method. Colloid Polym Sci 284, 506–512 (2006). https://doi.org/10.1007/s00396-005-1413-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-005-1413-3