Abstract
Detoxification of mercury (Hg) with selenium (Se) in the early postnatal period with regard to the expression of metallothionein protein (MT), essential element status, and lipid peroxidation level in tissues has not been studied. Seven-day-old Wistar pups were orally pretreated with Se [6 μmol Na2SeO3/kg body weight (b.w.)] for 3 days and then cotreated with Hg (6 μmol HgCl2/kg b.w.) for the following 4 days. This group (Se + Hg) was compared to the groups treated with Hg, Se, or vehicle (control). Compared to the Hg-group, Se + Hg-group exhibited lower renal MT expression, reduced accumulation of Hg, Cu and Zn, and reduced excretion of Se, Hg and Zn in urine. In the liver, MT was stimulated by Se treatment in both, Se and Se + Hg-group. Hepatic and brain levels of the endogenous essential elements Cu, Fe, Mg, and Zn remained unchanged in all of the studied groups. Brain Hg levels and oxidation of lipids measured as thiobarbituric acid reactive substances were diminished in Se + Hg-group of pups compared to the Hg-group. This study suggests that Se pretreatment can help reduce Hg in the tissues of suckling rats, simultaneously preventing impairment of essential element levels in the kidneys and their excessive excretion via urine. Also, Se was shown to prevent oxidative damage of lipids in the brain, which is particularly susceptible to Hg during the early postnatal period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg), depending on its chemical form, has shown toxicity in a number of organ systems including the renal, cardiovascular, gastrointestinal, hepatobiliary and neurological system (ATSDR 1999; Clarkson and Magos 2006; Bridges and Zalups 2010). Exposure to Hg in the early postnatal period has been recognized as an important public health issue because of the pronounced sensitivity of this period in life. A newborn organism is much more susceptible to toxic health effects compared to adults. Also, the exposure pathways are different and besides the usual (food and drink) intake, oral exploration and hand-to-mouth activity adds significantly to Hg intake (WHO 1986; U.S. Environmental Protection Agency, EPA 2002; Grandjean et al. 2008). Among the most significant channels of exposure to the inorganic form of Hg (I-Hg) for children are ingestion of batteries, homeopathic remedies, skin bleaching creams and soaps although children are most commonly exposed to the organic form of Hg, methylmercury (MeHg), via food (U.S. Environmental Protection Agency, EPA 2007). In industrial China, I-Hg is a more common pollutant in the environment than other forms of Hg (Feng et al. 2004). It is now known that I-Hg can be produced in the human body via slow demethylation of MeHg in the liver and brain, as well as via fast oxidation of elemental Hg in blood and tissues (ATSDR 1999; Clarkson and Magos 2006; Khan and Wang 2009). Both Clarkson (1997) and Khan and Wang (2009) proposed I-Hg as the possible proximate toxic form of Hg upon exposure to elemental Hg or demethylation of MeHg, respectively. Thus, research with the I-Hg is needed to clarify the mechanism of toxic action in the organism (Feng et al. 2004).
One of the toxic effects of Hg is the impairment of essential element [Fe, Cu, Zn, selenium (Se), Ca] homeostasis (Telišman 1995; Peraza et al. 1998). Hg (Cd and Cu, too), as higher affinity metal compared to Zn, was shown to replace Zn from the metal–metallothionein (MT) complex (reviewed in Sabolić et al. 2010). Feng et al. (2004) reported the imbalance of micro-nutritional elements in rats’ brain after in utero and lactational exposure to I-Hg. Vice versa, essential elements in excess appear to be protective against Hg toxicity (Chowdhury and Chandra 1987). The most studied interaction of Hg with an essential element is the one with Se (Watanabe 2002). Many studies have shown that supplementation with selenite prevents Hg toxicity (reviewed by Khan and Wang 2009; Rooney 2007; Ralston and Raymond 2010; Yang et al. 2008; Falnoga and Tušek-Žnidarič 2007; Luque-Garcia et al. 2013) but the exact mechanism is still unknown. Generally, Hg, and more rarely Se, content was measured in various tissues of adult (Magos and Webb 1976; Abdulla and Chmielnicka 1990; Nielsen and Andersen 1991; Agarwal and Behari 2007; Su et al. 2008; Agarwal et al. 2010) and young suckling animals (Orct et al. 2009) exposed to Hg and Se, but without exploring the effect on other endogenous essential elements, especially in young animals. Chmielnicka et al. (1986) found in adult rats that Se lowered Hg-induced renal deposition and urinary excretion of Cu and partly Zn in acute single dose experiment. Peixoto et al. (2008) investigated the effect of Zn-supplementation on Hg-intoxicated suckling rat pups and subsequent essential element changes. In these studies, exposure was parenteral, even though oral treatment would be of greater interest when it comes to human exposure.
MT, a protein of low molecular weight (Mr ~6 kDa), is inducible by various metals (Zn, Cu, Cd, Hg, Ag, Au, As) and reactive oxygen species (ROS), among other stimuli. Following binding to MT, otherwise reactive metal ion is stored inactive. Although it is a clearly confirmed fact in adult animals, induction of MT by metals in young animals has not been well studied (Zalups 2000; Sabolić et al. 2010), and the thus far published results are conflicting. Peixoto et al. (2007) confirmed induction of MT by ZnCl2, but not by HgCl2 in the kidney of 13-day-old rats. Also Brambila et al. (2002) failed to see induction of MT in the kidney of 1, 7, 14 and 21-day-old rats exposed to Hgº vapor in utero. Cherian et al. (1987) noted induction of MT by ZnSO4, but not by CdCl2 in newborn rats’ liver, while Fernandez et al. (2007) measured an up-regulation of MT-1 gene expression in the mice embryo after single CdCl2 injection to mice mothers.
Essential elements are needed in the active sites of some endogenous antioxidant enzymes that cleave ROS and protect cells from oxidative damage. Ercal et al. (2001) pointed out a few indirect mechanisms of Hg-induced oxidative damage: depletion of glutathione (GSH) which results with the accumulation of ROS in the cell; inhibition of mitochondrial oxidative phosphorylation suggested in renal tubular damage; alteration of Ca (activates hydrolytic enzymes) homeostasis; and effect on the activity of catalase, Cu–Zn superoxide dismutase, GSH peroxidase, and xanthine oxidase. It was reported that exposure to Hg results in oxidative stress followed by lipid peroxidation (reviewed in Telišman 1995; Peraza et al. 1998; Zalups 2000; Ercal et al. 2001; Ralston and Raymond 2010; Romero et al. 2014; Agha et al. 2014). The impact of Se supplementation on Hg-induced lipid peroxidation has been studied only in adult animals (Farina et al. 2003; Perottoni et al. 2004a, b; Agarwal and Behari 2007; Su et al. 2008; Brandão et al. 2009; Agarwal et al. 2010; Agha et al. 2014).
The present work was designed to study the effect of oral Se supplementation of Hg-intoxicated suckling rats on MT, endogenous essential elements and the level of lipid peroxidation in kidney, liver, brain, and urine of rats in the sensitive early postnatal period.
Materials and methods
Animals
Before the beginning of this experiment, 20 female Wistar rats bred at the Laboratory Animal Unit of the Institute for Medical Research and Occupational Health, Zagreb, Croatia were mated with males in a 3:1 ratio. After birth, the number of pups was reduced to eight in the respective litter. Four females with deliveries on the same day and 40 pups of both sexes were used in this experiment. The animals were maintained in a 12 h light/dark cycle at room temperature of 21 ± 1 °C and constant humidity of 40 %. Each litter was kept in an individual polycarbonate cage (26.5 × 20.7 × 14.0 cm) with a stainless steel lid. The cages were cleaned and pine shaving bedding was changed daily. Mother rats were given a normal rat diet (Mucedola, Italy) and deionised water ad libitum throughout the experiment. All research procedures were carried out in accordance with the National Animal Welfare Act, and approved by the Institute’s Ethics Committee and the Croatian Ministry of Agriculture, Forestry, and Water Management.
Experimental design
The experiment started at 7 days of age, as it was proposed that rats at this age may be more comparable to humans at birth (Miller 1983). Eight pups (four male and four female) were randomly assigned to each of the four litters (with one mother rat in each) on postnatal day 2 (PND 2; day of birth = PND 0). Two pups were taken from each litter in order to form one of the four experimental groups with eight animals per group:
-
(1)
Control group distilled water orally for 7 days,
-
(2)
Se-group oral doses of 6 μmol Na2SeO3/kg body weight (b.w.)/day for 7 days,
-
(3)
Hg-group oral doses of 6 μmol HgCl2/kg b.w./day for 4 days,
-
(4)
Se + Hg-group oral doses of 6 μmol Na2SeO3/kg b.w./day for 7 days + 6 μmol of HgCl2/kg b.w./day for 4 days.
Water, Se, and/or Hg solutions were administered to pups using an artificial feeding method introduced by Kostial et al. (1971). Every morning before the first administration, each pup was weighed. The daily dose was freshly prepared and administered in two portions (at 9:00 a.m. and 2:00 p.m.) with an automatic pipette (25 μl), four drops a day in total. In-between administrations, all pups were returned to their lactating mother rats and allowed to suckle. Pups always received Se 15 min before Hg (Se + Hg-group). Animals pretreated with Se (Se- and Se + Hg-group) received sodium selenite (p.a., Sigma-Aldrich, USA) at a daily dose of 0.5 mg Se/kg b.w. for seven consecutive days (PNDs 7–13). This procedure is called pretreatment because the Se + Hg pups had been receiving Se for 4 days before they were cotreated with Hg for the following 4 days. The daily dose of Se was calculated to achieve an equimolar ratio to the daily dose of Hg (Hg:Se, 1:1). Hg was administered as HgCl2 (p.a., Kemika, Croatia) at a daily dose of 1.3 mg Hg/kg b.w. for four consecutive days (PND 10–13). The same Hg dose was given to the Hg alone and the Se + Hg-group. The daily dose of Hg administered to each animal was 5 % of the acute LD50 oral dose found previously for HgCl2 (35 mg/kg b.w.) in suckling rats (Kostial et al. 1978). The daily dose of Hg applied here caused no apparent adverse health effects in suckling rats (Orct et al. 2009) and was lower than the one from the study of Nielsen and Andersen (1991) where no renal necrosis was found (oral dose 25 μmol HgCl2/kg b.w.) in mice.
On PND 14, 24 h after the last treatment, pups were anaesthetised (Narketan 0.8 ml/kg b.w. + Xylapan 0.6 ml/kg b.w., i.p., Vetoquinol AG, Switzerland), and dissected in the same sequence for each animal.
Sampling and analysis of elements
Blood was collected from the heart in BD Vacutainer® tubes with lithium heparin anticoagulant. Plasma was obtained by centrifugation of the collected blood samples (3000 rpm, for 15 min). Urine was collected directly from urinary bladder by a syringe. Animals were killed by bleeding from the abdominal aorta. Their liver, kidneys, and brain were removed, weighed, and rinsed with cold deionised water. Liver and brain were cut in half. The first part of the liver, brain, the right kidney, whole blood, and plasma were stored at −20 °C until element analysis. The other half of the liver and brain tissues, and the left kidney were homogenised on ice in 1.15 % KCl buffer in a Potter–Elvehjem homogeniser (Cole and Palmer, USA) with a Teflon pestle, and used immediately to measure lipid peroxidation. Aliquots of the liver and kidney homogenates were stored at −20 °C until used for MT immunoblotting. Hg and essential elements (Cu, Fe, Mg, Se, Zn) mass fractions were analysed from the same thawed tissue samples (right kidney, part of liver, part of brain) digested in an UltraCLAVE IV microwave digestion system (Milestone, Sorisole, Italy) after adding 2 ml of concentrated HNO3, 65 % s.p. grade (Merck, Germany) in quartz vessels with Teflon caps. Whole blood, plasma and urine analyses were carried out without sample digestion. Total Hg was analyzed by total decomposition, amalgamation, atomic absorption spectrometry (TDA-AAS) in an Hg analyzer (AMA 254, Leco, USA). Se and Cu were analyzed by electrothermal AAS with Mg and Pd as a matrix modifier and Zeeman background correction (AAnalyst 600, Perkin Elmer Instruments, USA). For Fe, Mg and Zn quantification, the digested samples were aspirated into the air-acetylene flame of a Varian AA-375 atomic absorption spectrometer (Mulgrave, Victoria, Australia). The reliability of analytical methods was evaluated using standard reference materials: bovine liver 1577b (NIST, USA), horse kidney H8 (IAEA, Austria), Seronorm™ Trace Elements Whole Blood L-2 (Sero AS, Norway), Seronorm™ Trace Elements Serum L-2 (Sero AS, Norway), and Seronorm™ Trace Elements Urine (Sero AS, Norway). The results of our analyses were within ±10 % of the certified values.
The results of element mass fractions in organs are expressed as micrograms per gram of wet tissue weight (μg/g w.w.). Hg and Se in whole blood and plasma are expressed as μg/l and in urine as μg/mg creatinine.
Metallothionein determination
Sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) and Western blotting was used for MT quantification in rat pups’ liver and kidney tissues. Crude tissue homogenates (20 % w/v) were rehomogenized through 25 gauge needle, diluted 1:1 in buffer (300 mM mannitol, 5 mM ethylene glycol tetraacetic acid, 12 mM Tris/HCl, pH 7.4) and centrifuged (Eppendorf Centrifuge 5417 R, Eppendorf AG, Hamburg, Germany) at 5000×g at 4 °C for 15 min. The pellet was discarded. Proteins were measured in the supernatant by the dye-binding assay (Bradford 1976). Before SDS-PAGE, the supernatant was mixed with sample buffer (1 % SDS, 12 % v/v glycerol, 30 mM Tris/HCl, pH 6.8) containing reducing agent (5 % 2-mercaptoethanol), and denatured by heating at 95 °C for 5 min. The heat-treated proteins (60 μg protein/lane) were then separated through 18 % SDS-PAGE MiniProtean III system and wet-transferred using mini trans-blot electrophoretic transfer cell (Bio-Rad Laboratories, Hercules, CA, USA) to Immobilon membrane (PVDF, Millipore, Bedford, MA, USA). Following transfer, the Immobilon membrane was incubated in 1 % glutaraldehyde for 60 min to enhance MT retention in the membrane (Mizzen et al. 1996), washed several times with water and preincubated with the blotting buffer (0.15 M NaCl, 1 % Triton-X-100, 20 mM Tris/HCl, pH 7.4) with 5 % nonfat dry milk, at room temperature for 60 min to block nonspecific binding. The membrane was then incubated overnight at 4 °C in the blotting buffer (0.15 M NaCl, 1 % Triton-X-100, 20 mM Tris/HCl, pH 7.4) with a monoclonal mouse antibody against the horse MT (clone E9, DAKO, Carpinteria, CA, USA; 1:500), which recognizes a highly conserved domain common to MT-I and MT-II of many species, including rats. The membrane was then washed with several changes of blotting buffer, incubated for 60 min in the same buffer that contained secondary antibody (0.1 μg/ml, alkaline phosphatase-labeled goat anti-mouse IgG; GAMAP; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), washed again, and stained for alkaline phosphatase activity using the 5-bromo-4-chloro-3-indolyl phosphate in conjunction with nitro blue tetrazolium as indicators of the protein bands.
Relative density of each protein band was evaluated by densitometry using the Quantity One 1-D Analysis Software (Bio-Rad Laboratories, Inc.). An area of the MT-related strongest protein band in the control protein band of the respective blot was marked, and an equal area was applied to all other relevant bands in the same blot. The area density of each band was expressed in arbitrary units, relative to the strongest band density (=1 arbitrary unit) in the corresponding control samples. The immunoblotting figures are representative from findings in six control and six treated rats per experimental group. The numeric data are expressed as mean ± SEM.
Lipid peroxidation measurement
Lipid peroxidation was measured in tissue homogenate aliquots as the amount of malondialdehyde (MDA), a decomposition product of polyunsaturated fatty acid hydroperoxides, formed in the thiobarbituric acid reaction as described by Ohkawa et al. (1979). Thiobarbituric acid reactive substances (TBARSs) were quantified by comparing absorption on a spectrophotometer Cecil 9000 series (Cecil Instruments, Cambridge, UK) at 532 nm to the standard curve of MDA equivalent generated by hydrolysis of 1,1,3,3 tetramethoxypropane. TBARS data were expressed as nmol/g w.w.
Statistical analysis
Results are presented as mean ± SEM. Statistical analysis was performed using Statistica for Windows software, version 12.0 (StatSoft, Inc., Tulsa, USA) after log-transformation of elementary data. When data showed equal variance (Bartlett) and followed a normal distribution (Shapiro–Wilk), one-way analysis of variance with post hoc analysis (Tukey’s HSD test) was used to determine significant differences between the groups. In other cases, when the criteria for parametric methods were not fulfilled, the effect of treatment was assessed using the Kruskal–Wallis test with different subsets identified by the Mann–Whitney U-test. Differences were considered significant at P ≤ 0.05.
Results
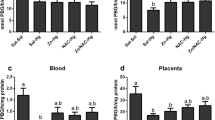
Whole b.w. gain during the experimental period (PND 7–14) and organ weight at the last day of experiment (PND 14) did not differ significantly between the four studied groups (Table 1). Exposure to Hg elevated Hg content in all of the studied compartments (P < 0.001), most pronouncedly in the kidney (Table 2). Se supplementation before and during exposure to Hg reduced Hg in whole blood, plasma, brain, kidney, and urine (P < 0.001). Supplementation with Se (Se-group) significantly elevated Se content in plasma, urine (P < 0.001) and tissues (P < 0.001) of suckling rats (Table 3). However, when pups were supplemented with Se and exposed to Hg, except in urine where Se level decreased, the level of Se remained unchanged in plasma, liver and kidney of suckling pups compared to the ones administered Se alone. Unlike in the kidney and urine, exposure to Se or/and Hg did not cause disturbances in the brain and hepatic levels of essential elements (Cu, Fe, Mg and Zn; Fig. 1). Hg exposure caused an increase in renal Cu (P < 0.001) and Zn (P < 0.001) compared to control pups, while in the group administered both Se and Hg, this effect was diminished. We also noticed higher excretion of Cu (P < 0.01), Mg (P < 0.01) and Zn (P < 0.01) in the urine of pups exposed to Hg compared to control pups. This Hg-induced impairment in excretion of Cu, Mg and Zn was diminished in Se + Hg-group, but the observed decrease in comparison to the Hg group was statistically significant only for Zn (P < 0.05). Pups exposed to Se and Hg had higher renal Mg than the control (P < 0.01) and Hg exposed animals (P < 0.05).
The effect of Hg or/and Se oral exposure on Cu (a), Fe (b), Mg (c) and Zn (d) levels in brain, liver, kidneys and urine of suckling rats (mean + SEM). Exposures are described in “Materials and methods” section. *P < 0.05 versus control group, #P < 0.05 versus Hg-group
The immunoblotting studies with anti-MT antibody exhibited in the kidney and liver homogenates two major protein bands with Mr of ~12 kDa (possibly MT dimer) and ~18 kDa (possibly MT trimer; Fig. 2). Since the monomeric protein has Mr of 6–7 kDa we assume that these higher Mr bands are a consequence of protein aggregation due to well-known self oligomerization property of MT (Haase and Maret 2008) in spite of the presence of reducing agent (2-mercaptoethanol). Generally, higher MT expression was observed in the liver compared to kidney (data not shown), which is in accordance with previous findings by Mehra and Bremner (1984). The density data of combined (12 + 18 kDa) protein bands revealed that exposure to Hg strongly enhanced MT expression in the kidney (P < 0.001), but not in the liver of treated pups compared to control rats. Supplementation with Se (Se + Hg-group) strongly reduced the Hg-stimulated renal MT expression (P < 0.001), but not entirely to the level of control animals (control vs. Se + Hg: P < 0.01). Immunoblotting of the liver samples showed a significant heterogeneity in the protein band density in all experimental groups, but there was an obvious trend of MT upregulation related to Se supplementation (Se-group vs. Se + Hg-group), while HgCl2 alone had no effect on MT expression compared to control pups.
The effect of Hg or/and Se oral exposure on metallothionein levels in kidneys and liver of suckling rats, as shown in Western blots of tissue homogenates. Exposures and experimental details are described in “Materials and methods” section. Each blot is representative for the findings in six control and six treated rats per experimental group. Bars represent mean ± SEM (n = 6 in each bar) of the combined density of 12 and 18 kDa protein bands. *P < 0.05 versus control group, #P < 0.05 versus Hg-group
Lipid peroxidation was enhanced in the brain (P < 0.01) and liver (P < 0.01) of Hg-exposed rats and supplementation with Se managed to significantly diminish this effect only in the brain (P < 0.01), although the same trend was obvious also in the liver of pups (Fig. 3). Significant changes in lipid peroxidation were noticed in the liver and kidney of Se-treated pups compared to control group (P < 0.01).
The effect of Hg or/and Se oral exposure on the level of lipid peroxidation (TBARS) in the brain, liver and kidneys of suckling rats (mean + SEM). Exposures are described in “Materials and methods” section. *P < 0.05 versus control group, #P < 0.05 versus Hg-group
Discussion
A number of studies on experimental animals have confirmed that Se supplementation decreases I-Hg toxicity, but knowledge on the effects on MT, endogenous essential elements, their excretion and lipid peroxidation, especially in young animals during oral exposure, is lacking. Young animals, especially those on a milk diet (suckling animals) are due to age specificities and enhanced absorption of toxic metals a good model for studying toxic metal exposure in this sensitive life period.
The doses of inorganic Hg and Se to which pups were exposed in this study did not cause adverse health effects in view of decreased b.w. or organ weight. However, Hg and Se administered to pups did cause significant redistribution of Hg, whose levels are usually highest in the kidneys and largely bound to MT. Western blot of renal samples confirmed stimulated expression of MT that correlated with elevated Hg concentration in Hg-group. Also, following Hg redistribution in Se + Hg-group, immunoblots revealed lower MT expression in the renal tissue. According to the available literature, this is the first report of Hg-stimulated renal MT expression in neonatal rat. Peixoto et al. (2007) did not find enhanced MT expression in the liver or kidneys of rat pups of similar age receiving HgCl2 (s.c.) in even higher dose than in our study. Also Brambila et al. (2002) failed to see an induction of MT in renal tissue of neonatal rats exposed in utero to Hgº vapor.
Our findings of delayed transport of Hg from the blood of Se + Hg-group and higher affinity for storage in the liver than in the kidneys of suckling rats compared to Hg-group are in agreement with previous findings in adult animals (Chmielnicka et al. 1986; reviewed in Yang et al. 2008; Khan and Wang 2009) and with our earlier experiments with suckling pups (Orct et al. 2009). Fast formation of water soluble mercuric selenide–selenoprotein P complex in the blood markedly reduces transport of Hg to renal cells but is proven to appear in the hepatocytes after 24 h where it might undergo proteolysis and form inert Hg–Se complex with a very slow elimination time (reviewed in Yang et al. 2008). Unlike the situation in brain and kidneys, where Se supplementation lowered Hg content, Se did not change Hg accumulation in the liver of our pups compared to the Hg-group, as also found in our earlier study with Se:Hg 1:1 ratio (Orct et al. 2009). In that study, Se supplementation in higher Se:Hg ratios decreased the hepatic Hg content by 2.8 (ratio 2:1) and 3.9 times (ratio 3:1) compared to Hg-treated pups, but also increased the liver weight. Because of the dual nature of Se and elevated liver weight in Se + Hg-group (ratio 3:1), indicating possible toxic effects of Se (Orct et al. 2009), we further investigated only the equimolar ratio following the “primum non nocere” demand for supplements/remedies. On the contrary, Su et al. (2008) in their chronic exposure study reported an increase in hepatic and blood Hg and no change in renal Hg in the Se + Hg-group of orally treated adult rats. In both the chronic (Agarwal and Behari 2007) and acute parenterally exposed rats (Agarwal et al. 2010), Hg was reported to increase in liver and kidneys when Se was coadministered. In the latter studies, the authors exposed rats to lower Se doses compared to the Hg dose, unlike in our experiment where the doses were equimolar, which could partly explain the discrepancy between the results. The phenomena that increased Se intake changes I-Hg distribution between the kidney and liver was confirmed earlier by Nielsen and Andersen (1991). Unlike in the kidneys, Western blot of the hepatic tissues indicated Se, not Hg as a stimulator of MT synthesis. Similar results were found by Iwai et al. (1988) in adult mice injected with sodium selenite. In the Se-treated animals they also noted elevated Zn concentrations and suggested perturbation of Zn homeostasis as a mechanism for stimulation of MT synthesis. Latter findings, reviewed by Maret (2000), confirmed Se compounds as oxidants of MT in vivo, which may act to release Zn from MT, and possibly stimulate thionein synthesis via the Zn-mediated mechanism.
The fact that Se supplementation of Hg-exposed pups causes reduction in excretion of Hg was published in our previous study (Orct et al. 2009), and confirmed here. Magos and Webb (1976) and Chmielnicka et al. (1986) reported the same findings in adult rats—reduction of urinary and faecal Hg mass fraction in the Se + Hg-group. A detail left unexplored in our previous study (Orct et al. 2009) concerned the final Se concentrations in the urine of Se + Hg-group compared to the Se-group. Here we revealed that urinary Se dropped when Se-supplemented pups were exposed to Hg, as also reported by Magos and Webb (1976) for adults, thereby sharing the faith of Hg due to the formation of the aforementioned mercuric selenide–selenoprotein P complex. Similar results in young animals are not available in other studies. Our study showed that Se supplementation of suckling rats before and during exposure to Hg can reduce Hg accumulation in the kidney and brain and MT expression in the kidney, but also reduces the excretion of both Se and Hg. At the same time, supplemented Se is deposited to tissues irrespective of the presence of Hg and can benefit the respective cells with its antioxidative potential.
The most prominent changes in essential elements in our study were found in the kidneys of Hg- and/or Se-exposed pups. Significant increase of renal Cu and Zn after oral HgCl2 exposure is in agreement with reports in adult animals (Bogden et al. 1980; Chmielnicka et al. 1986; Liu et al. 1992) and suckling pups (only renal Cu, not Zn; Peixoto et al. 2008). Our report of Hg-induced excretion of Cu and Zn in urine of suckling rats is unique, and correlates with findings in adult animals (Chmielnicka et al. 1986; Liu et al. 1992). It is known from the literature that HgCl2 stimulates MT synthesis in kidneys, and not in the liver of adult animals (Zalups 2000). The same was confirmed in this study with suckling rats. Due to the strong binding and sequestration affinity of MT for Cu, Zn, Cd and Hg, increase of Hg-stimulated MT content might results with more binding sites for Cu and Zn and consequently with their higher retention in the kidney, as proposed earlier (Bogden et al. 1980; Chmielnicka et al. 1986; Liu et al. 1992; Peixoto et al. 2008). Abdulla and Chmielnicka (1990) reported that MT induced by metals contains Zn and/or Cu together with toxic metals. The more prominent increase in renal Cu than Zn concentrations observed in our pups after exposure to HgCl2 could be the consequence of physiological differences in Cu/Zn domination in MT, while Cu is more abundant in renal MT and Zn in hepatic MT (Abdulla and Chmielnicka 1990). One of the most original findings of this study is the effect of Se on the above reported Hg-induced changes in essential metals of suckling rats; we found that Se pretreatment modified HgCl2-induced increase in renal Zn and Cu by lowering them to the levels of the control group. Although reduced, the renal MT levels in Se + Hg- versus Hg-group did not drop to control levels as seen for the content of essential elements Cu and Zn, but rather followed the trend of Hg levels. This might be a consequence of well known higher affinity of Hg to bind MT compared to Zn/Cu (Zalups 2000). In addition, excretion of Zn (and Cu, but not significantly) in urine completely followed the pattern of renal Zn and Cu levels in the experimental groups. Exogenous Se influences the shift of Hg, otherwise bound to MT, to form a high molecular weight complex with Se and selenoprotein P, thought to be a precursor of inert HgSe (s) (Khan and Wang 2009). Once bound to an inert complex, Hg would not be able to exert biological effects as a free ion (e.g., induction of MT) and influence the essential elements in tissue. Opposite to our Mg quantification data and results from Bogden et al. (1980), Peixoto et al. (2008) reported a Hg-induced decrease in renal, but not hepatic Mg concentrations in suckling rats. We noticed an impairment of Mg metabolism only when we supplemented Hg-exposed pups with Se. Although Mg was elevated in the Se + Hg-group in all three tissues compared to other experimental groups, the difference was significant only in the kidney and we cannot propose the underlying mechanism. On the other hand, this study is the first to report that Hg exposure to suckling pups induced magnesuria, also seen before in adult animals (Liu et al. 1991) as a result of the nephrotoxic effect of HgCl2. Iron was the only element that showed no impairment of metabolism in any tissue, and excretion in any experimental group of pups. Neither Hg nor Se influenced endogenous essential elements in the liver and brain tissue of our test animals. Similar findings were reported by Bogden et al. (1980) and Liu et al. (1992), whereas Peixoto et al. (2008) found elevated liver Zn and Fe concentrations after exposure to I-Hg. Our study showed that Se supplementation was able to prevent renal accumulation of endogenous Cu and Zn mediated by increased MT levels, and to prevent excretion of Zn and, less distinctly, of Cu and Mg, via urine in Hg-exposed suckling rats.
This study is also the first to report the effect of Se on the TBARS level, as a marker of lipid peroxidation in Hg-exposed suckling rats. The protective effect of Se was confirmed only in the brain tissue of Se + Hg-group compared to the Hg-only group. The proposed mechanisms of protection imply that Se, as a constitutive part of selenoproteins, either scavenges ROS (which induce lipid oxidation in cells and tissues) issued by Hg-exposure, or prevents creation of ROS by complexing Hg ions into inert compounds as HgSe (s). Results from the available literature are numerous, but varying. Organic Se prevented hepatic and renal I-Hg-induced lipid peroxidation in chronic (Su et al. 2008), and subchronic (Brandão et al. 2009) experiments in vivo. Perottoni et al. (2004b) reported that organic Se in vitro prevented I-Hg-induced lipid peroxidation, while in acute in vivo experiment there was no such effect. Inorganic Se was reported to diminish I-Hg-induced lipid peroxidation in serum of subchronically (Agha et al. 2014) and in kidneys of acutely exposed rats, but failed to exert the same effects in vitro (Perottoni et al. 2004a), in acutely exposed mice (Farina et al. 2003), or in subchronically exposed rats (Agarwal and Behari 2007). In the acute study performed by Agarwal et al. (2010), pretreatment and posttreatment with inorganic Se restored lipid peroxidation in the kidneys and brain of adult rats exposed to I-Hg. Regarding the level of TBARS, we can conclude that Se supplementation before and during exposure to Hg can prevent the oxidation of lipids in the brain, but not liver and kidneys of suckling rats.
References
Abdulla M, Chmielnicka J (1990) New aspects on the distribution and metabolism of essential trace elements after dietary exposure to toxic metals. Biol Trace Elem Res 23:25–53
Agarwal R, Behari JR (2007) Effect of selenium pretreatment in chronic mercury intoxication in rats. Bull Environ Contam Toxicol 79:306–310. doi:10.1007/s00128-007-9226-3
Agarwal R, Raisuddin S, Tewari S, Goel SK, Raizada RB, Behari JR (2010) Evaluation of comparative effect of pre- and posttreatment of selenium on mercury-induced oxidative stress, histological alterations, and metallothionein mRNA expression in rats. J Biochem Mol Toxicol 24:123–135. doi:10.1002/jbt.20320
Agha FE, Youness ER, Selim MMH, Ahmed HH (2014) Nephroprotective potential of selenium and taurine against mercuric chloride induced nephropathy in rats. Ren Fail 36:704–716. doi:10.3109/0886022X.2014.890012
ATSDR (1999) Toxicological profile for mercury. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta. http://www.atsdr.cdc.gov/ToxProfiles/tp46.pdf. Accessed 15 Nov 2014
Bogden JD, Kemp FW, Troiano RA, Jortner BS, Timpone C, Giuliani D (1980) Effect of mercuric chloride and methylmercury chloride exposure on tissue concentrations of six essential minerals. Environ Res 21:350–359
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brambila E, Liu J, Morgan DL, Beliles RP, Waalkes MP (2002) Effect of mercury vapor exposure on metallothionein and glutathione S-transferase gene expression in the kidney of nonpregnant, pregnant, and neonatal rats. J Toxicol Environ Health A 65:1273–1288. doi:10.1080/00984100290071405
Brandão R, Borges LP, Nogueira CW (2009) Concomitant administration of sodium 2,3-dimercapto-1-propanesulphonate (DMPS) and diphenyl diselenide reduces effectiveness of DMPS in restoring damage induced by mercuric chloride in mice. Food Chem Toxicol 47:1771–1778. doi:10.1016/j.fct.2009.04.035
Bridges CC, Zalups RK (2010) Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B 13:385–410. doi:10.1080/10937401003673750
Cherian MG, Templeton DM, Gallant KR, Banerjee D (1987) Biosynthesis and metabolism of metallothionein in rat during perinatal development. Exp Suppl 52:499–505
Chmielnicka J, Brzeźnicka E, Sniady A (1986) Kidney concentrations and urinary excretion of mercury, zinc and copper following the administration of mercuric chloride and sodium selenite to rats. Arch Toxicol 59:16–20
Chowdhury BA, Chandra RK (1987) Biological and health implications of toxic heavy metal and essential trace element interactions. Prog Food Nutr Sci 11:55–113
Clarkson TW (1997) The toxicology of mercury. Crit Rev Clin Lab Sci 34:369–403
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Ercal N, Gure-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress Part I: mechanisms involved in metal induced oxidative damage. Curr Top Med Chem 1:529–539
Falnoga I, Tušek-Žnidarič M (2007) Selenium–mercury interactions in man and animals. Biol Trace Elem Res 119:212–220. doi:10.1007/s12011-007-8009-3
Farina M, Brandão R, de Lara FS, Pagliosa LB, Soares FA, Souza DO, Rocha JB (2003) Profile of nonprotein thiols, lipid peroxidation and delta-aminolevulinate dehydratase activity in mouse kidney and liver in response to acute exposure to mercuric chloride and sodium selenite. Toxicology 184:179–187
Feng W, Wang M, Li B, Liu J, Chai Z, Zhao J, Deng G (2004) Mercury and trace element distribution in organic tissues and regional brain of fetal rat after in utero and weaning exposure to low dose of inorganic mercury. Toxicol Lett 152:223–234. doi:10.1016/j.toxlet.2004.05.001
Fernandez EL, Dencker L, Tallkvist J (2007) Expression of ZnT-1 (Slc30a1) and MT-1 (Mt1) in the conceptus of cadmium treated mice. Reprod Toxicol 24:353–358. doi:10.1016/j.reprotox.2007.06.006
Grandjean P et al (2008) The Faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol 102:73–75. doi:10.1111/j.1742-7843.2007.00114.x
Haase H, Maret W (2008) Partial oxidation and oxidative polymerization of metallothionein. Electrophoresis 29:4165–4176
Iwai N, Watanabe C, Suzuki T, Suzuki KT, Tohyama C (1988) Metallothionein induction by sodium selenite at two different ambient temperatures in mice. Arch Toxicol 62:447–451
Khan MA, Wang F (2009) Mercury–selenium compounds and their toxicological significance: toward a molecular understanding of the mercury–selenium antagonism. Environ Toxicol Chem 28:1567–1577. doi:10.1897/08-375.1
Kostial K, Šimonović I, Pišonić M (1971) Lead absorption from the intestine in newborn rats. Nature 233:564
Kostial K, Kello D, Jugo S, Rabar I, Maljković T (1978) Influence of age on metal metabolism and toxicity. Environ Health Perspect 25:81–86
Liu X, Jin T, Nordberg GF (1991) Increased urinary calcium and magnesium excretion in rats injected with mercuric chloride. Pharmacol Toxicol 68:254–259
Liu X, Nordberg GF, Jin T (1992) Increased urinary excretion of zinc and copper by mercuric chloride injection in rats. Biometals 5:17–22
Luque-Garcia JL, Cabezas-Sanchez P, Anunciação DS, Camara C (2013) Analytical and bioanalytical approaches to unravel the selenium–mercury antagonism: a review. Anal Chim Acta 801:1–13. doi:10.1016/j.aca.2013.08.043
Magos L, Webb M (1976) Differences in distribution and excretion of selenium and cadmium or mercury after their simultaneous administration subcutaneously in equimolar doses. Arch Toxicol 36:63–69
Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130(5S Suppl):1455S–1458S
Mehra RK, Bremner I (1984) Metallothionein-I in the plasma and liver of neonatal rats. Biochem J 217:859–862
Miller RK (1983) Perinatal toxicology: its recognition and fundamentals. Am J Ind Med 4:205–244
Mizzen CA, Cartel NJ, Yu WH, Fraser PE, McLachlan DR (1996) Sensitive detection of metallothioneins-1, -2 and -3 in tissue homogenates by immunoblotting: a method for enhanced membrane transfer and retention. J Biochem Biophys Methods 32:77–83
Nielsen JB, Andersen O (1991) A comparison of the effects of sodium selenite and seleno-l-methionine on disposition of orally administered mercuric chloride. J Trace Elem Electrolytes Health Dis 5:245–250
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Orct T, Lazarus M, Jurasović J, Blanuša M, Piasek M, Kostial K (2009) Influence of selenium dose on mercury distribution and retention in suckling rats. J Appl Toxicol 29:585–589. doi:10.1002/jat.1444
Peixoto NC, Serafim MA, Flores EMM, Bebianno MJ, Pereira ME (2007) Metallothionein, zinc, and mercury levels in tissues of young rats exposed to zinc and subsequently to mercury. Life Sci 81:1264–1271. doi:10.1016/j.lfs.2007.08.038
Peixoto NC, Rocha LC, Moraes DP, Bebianno MJ, Dressler VL, Flores EM, Pereira ME (2008) Changes in levels of essential elements in suckling rats exposed to zinc and mercury. Chemosphere 72:1327–1332. doi:10.1016/j.chemosphere.2008.04.027
Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT (1998) Effects of micronutrients on metal toxicity. Environ Health Perspect 106(Suppl 1):203–216
Perottoni J, Lobato LP, Silveira A, Rocha JB, Emanuelli T (2004a) Effects of mercury and selenite on delta-aminolevulinate dehydratase activity and on selected oxidative stress parameters in rats. Environ Res 95:166–173. doi:10.1016/j.envres.2003.08.007
Perottoni J, Rodrigues OE, Paixão MW, Zeni G, Lobato LP, Braga AL, Rocha JB, Emanuelli T (2004b) Renal and hepatic ALA-D activity and selected oxidative stress parameters of rats exposed to inorganic mercury and organoselenium compounds. Food Chem Toxicol 42:17–28. doi:10.1016/j.fct.2003.08.002
Ralston NV, Raymond LJ (2010) Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 278:112–123. doi:10.1016/j.tox.2010.06.004
Romero A, Ramos E, de Los Ríos C, Egea J, Del Pino J, Reiter RJ (2014) A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56:343–370. doi:10.1111/jpi.12132
Rooney JP (2007) The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 234:145–156. doi:10.1016/j.tox.2007.02.016
Sabolić I, Breljak D, Škarica M, Herak-Kramberger CM (2010) Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 23:897–926. doi:10.1007/s10534-010-9351-z
Su L, Wang M, Yin ST, Wang HL, Chen L, Sun LG, Ruan DY (2008) The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicol Environ Saf 70:483–489. doi:10.1016/j.ecoenv.2007.05.018
Telišman S (1995) Interactions of essential and/or toxic metals and metalloid regarding interindividual differences in susceptibility to various toxicants and chronic diseases in man. Arh Hig Rada Toksikol 46:459–476
U.S. Environmental Protection Agency, EPA (2002) Child-specific exposure factors handbook. EPA/600/P-00/002B. National Center for Environmental Assessment, Washington, DC. http://www.epa.gov/ncea. Accessed 10 Dec 2014
U.S. Environmental Protection Agency, EPA (2007) Inorganic mercury. Toxicity and exposure assessment for children’s health (TEACH) chemical summary. http://www.epa.gov/teach/chem_summ/mercury_inorg_summary.pdf. Accessed 15 Nov 2014
Watanabe C (2002) Modification of mercury toxicity by selenium: practical importance? Tohoku J Exp Med 196:71–77
WHO (1986) Environmental Health Criteria 59: principles for evaluating health risks from chemicals during infancy and childhood: the need for a special approach. World Health Organization, Geneva
Yang D-Y, Chen Y-W, Gunn JM, Belzile N (2008) Selenium and mercury in organisms: interactions and mechanisms. Environ Rev 16:71–92. doi:10.1139/A08-001
Zalups RK (2000) Molecular interactions with mercury in the kidney. Pharmacol Rev 52:113–144
Acknowledgments
This work was supported by the Ministry of Science Education and Sports of the Republic of Croatia (Project Grants No. 022-0222148-2135 and 022-0222148-2146). The technical assistance of Mrs. Đurđa Breški, Marija Ciganović and Snježana Mataušić is gratefully acknowledged. The authors thank Mr. Makso Herman for language advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orct, T., Lazarus, M., Ljubojević, M. et al. Metallothionein, essential elements and lipid peroxidation in mercury-exposed suckling rats pretreated with selenium. Biometals 28, 701–712 (2015). https://doi.org/10.1007/s10534-015-9859-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9859-3