Abstract
Silver nanoparticles are known to have antimicrobial properties and have been used extensively in medicine, although the mechanism(s) of action have not yet been clearly established. In the present study, the findings suggest a novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli, namely, the induction of a bacterial apoptosis-like response. We propose a possible mechanism for the bacterial apoptosis-like response that includes the following: accumulation of reactive oxygen species (ROS) (detected with H2DCFDA staining), increased intracellular calcium levels (detected with Fura-2 AM), phosphatidylserine exposure in the outer membrane (detected with Annexin V) which is the hallmarks of early apoptosis, disruption of the membrane potential [detected with DiBAC4(3)], activation of a bacterial caspase-like protein (detected by FITC-VAD-FMK staining) and DNA degradation (detected with TUNEL assay) which is the hallmarks of late apoptosis in bacterial cells treated with silver nanoparticles. We also performed RecA expression assay with western blotting and observed activation of SOS response to repair the damaged DNA. To summarize, silver nanoparticles are involved in the apoptosis-like response in E. coli and the novel mechanisms which were identified in this study, suggest that silver nanoparticles may be an effective antimicrobial agent with far lower propensity for inducing microbial resistance than antibiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious diseases caused by microorganisms have become increasingly difficult to treat because of increases in microbial resistance to multiple different antibiotics (Furuya and Lowy 2006). With the rapid development of resistance mechanisms in microorganisms which are often one step ahead of conventional antibiotics and the persistent requirement for effective therapeutic agents at low cost, many researchers have attempted to develop new antimicrobial agents that cost-effectively avoid such resistance. Such requirements have highlighted Ag-based antiseptics that may have broad-spectrum activity against both gram-positive and gram-negative bacteria and are much less likely to induce microbial resistance than conventional antibiotics (Jones et al. 2004).
The medicinal properties of silver have been known since at least the nineteenth century (Klasen 2000), and silver ion and silver-based compounds have been used in many antimicrobial applications, including dental work (Herrera et al. 2001), the coating of medical devices (Bosetti et al. 2002), and burn wounds (Atiyeh et al. 2007). However, the use of silver ions has one major drawback: they are easily inactivated by complexation and precipitation, and because of this, the use of silver ions has been limited. Silver nanoparticles (nano-Ag) can be a valuable alternative to ionic silver as they have no charge, are of a small size, have a high surface area to volume ratio, and have unique chemical and physical properties (Sintubin et al. 2009), all of which improves their biocompatibility. Nanoparticles modified for improved efficiency can be used in numerous physical, biological, and pharmaceutical applications. Due to the useful features of nano-Ag, it has become well known as an antibacterial agent, and many studies have reported that nano-Ag has powerful antimicrobial activity, mediated by the inhibition of bacterial cell division finally leading to cell death through destroying bacterial membranous structure (Li et al. 2010). Based on previous report, we hypothesized that nano-Ag might possess a broader mode of action including membrane damage and the results presented here suggest the possibility of a bacterial apoptosis-like response as a dual bactericidal effect.
Apoptosis in eukaryotes is genetically regulated and executed, and apoptotic cells exhibit several well-known characteristics, such as externalization of phosphatidylserine, distinct DNA fragmentation (Taylor et al. 2008). This form of programmed cell death is required for the elimination of harmful cells and the regulation of homeostasis in multicellular organisms (Nagata 1997; Majno and Joris 1995). Although apoptosis is generally associated with eukaryotic cells, the phenomenon has been also described in prokaryotic cells (Engelberg-Kulka et al. 2006). We investigated whether nano-Ag could induce an apoptosis-like response in Escherichia coli, a widely used experimental bacterial strain due to its presence on the body surface of mammals and its ability to cause infections in mammals.
We performed several experiments to confirm that the nano-Ag-induced bacterial cell death showed apoptotic features, and we observed accumulation of ROS, intracellular calcium levels, the presence of phosphatidylserine in the outer leaflet of the plasma membrane, dissipation of the plasma membrane potential, the activation of a bacterial caspase-like protein and DNA fragmentation. Additionally, we assessed the expressions of RecA which involved in bacterial SOS response to repair damaged DNA.
Materials and methods
Preparation of nano-Ag and bacterial strains
Solid silver (100 g) was dissolved in 100 mL of 100 % nitric acid at 90 °C, and 1 L of distilled water was added. By adding sodium chloride to the silver solution, the Ag ions were precipitated, and they clustered together to form monodispersed nanoparticles in an aqueous medium. The sizes and morphology of the nano-Ag particles were examined by TEM (H-7600; Hitachi Ltd, Tokyo, Japan). The results showed that nano-Ag was spherical and that its average size was 3 nm (data not shown). The final concentration of colloidal silver was 60,000 ppm. (ICP Spectrophotometer, Optima 7300DV; PerkinElmer, Waltham, MA, USA). This solution was diluted and then used to investigate antimicrobial effects.
Escherichia coli (ATCC 25922) cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Before use, the bacteria were stored in 30 % glycerol and frozen at −70 °C. The bacterial cells were grown in Luria–Bertani (LB) broth (Difco Laboratories, Detroit, MI, USA) with aeration at 37 °C. Cell growth was monitored by measuring the optical density at 620 nm with a microtiter ELISA Reader (Molecular Devices Emax; Sunnyvale, CA, USA).
Determination of MIC and time-kill kinetic analysis
Susceptibility tests with nano-Ag and norfloxacin were performed in 96-well microtitre plates using a standard twofold broth microdilution method of the antibacterial agents in LB broth, following Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI 2005). E. coli were grown in LB medium and aliquots of 100 μL of the bacterial cells were then seeded in the wells of a 96-well microtitre plate at a density of 1 × 106 cells/mL; 10 μL each of the serially diluted solutions of the compounds was then added to the bacterial cells. The MIC was defined as the lowest concentration of drug inhibiting bisible growth after overnight incubaction at 37 °C (Hwang et al. 2012). The minimum inhibitory concentrations (MIC) of nano-Ag is 2 μg/mL and that of norfloxacin is 0.125 μg/mL (data not shown).
E. coli cells (1 × 106 cells/mL of LB) were incubated with nano-Ag or norfloxacin at their respective MICs. Culture samples were collected after 0, 2, 4, 6, and 8 h of incubation and spread onto LB agar plates. Colony-forming units were counted after incubation at 37 °C for 24 h, and percent survival was determined relative to the control.
ROS accumulation assay
ROS accumulation was measured with the fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular probes, Eugene, OR, USA) (Jakubowski et al. 2000). E. coli cells (1 × 106 cells/mL of LB) were treated with nano-Ag or norfloxacin at their respective MICs for 2 h at 37 °C. After incubation, the cells were washed with PBS, stained with 10 μM H2DCFDA, and analyzed with a FACSCalibur flow cytometer (Becton–Dickinson, San Jose, CA, USA).
Fluorometric measurement of intracellular calcium levels
Cytosolic calcium levels were measured with fura-2 acetoxymethyl ester (AM) (Molecular Probes) (Arduino et al. 2009). E. coli cells (1 × 106 cells/mL of LB) were incubated with nano-Ag or norfloxacin at their respective MICs for 2 h at 37 °C. Next, the cells were washed twice in Krebs buffer (132 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 6 mM glucose, 10 mM HEPES, 10 mM NaHCO3, and 1 mM CaCl2, pH 7.4) and loaded with 5 μM fura-2 AM supplemented with 0.01 % Pluronic F-127 (Molecular Probes) and 1 % BSA in Krebs buffer for 30 min at 37 °C. Pluronic F-127 was used to help disperse AM esters of the fluorescent ion indicator. Next, the loaded cells were washed three times in calcium-free Krebs buffer and analyzed with a spectrofluorophotometer (Shimadzu RF-5301PC; Shimadzu, Japan).
Detection of the hallmarks of early apoptosis
E. coli cells were stained with Annexin V-FITC and propidium iodide (PI) (Madeo et al. 1997) by using the FITC-Annexin V apoptosis detection kit (BD Pharmingen, San Diego, CA, USA). E. coli cells were incubated with nano-Ag or norfloxacin at their respective MICs for 2 h at 37 °C, and then incubated for 20 min in Annexin V binding buffer containing 5 μL/mL Annexin V-FITC and 1 μL/mL PI. Cells were analyzed with a FACSCalibur flow cytometer.
Membrane depolarization assay
To analyze membrane disturbance after nano-Ag or norfloxacin treatment, the treated E. coli cells (1 × 106 cells/mL of LB) were harvested and suspended in PBS. After incubation with nano-Ag or norfloxacin at their respective MIC for 2 h at 37 °C, the cells were harvested by centrifugation and resuspended in 1 mL of PBS. The cells were then treated with 5 μg of bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)] (Molecular Probes) (Bos et al. 2012). Flow cytometric analysis was performed with a FACSCalibur flow cytometer.
Analysis of the intracellular levels of bacterial caspase-like proteins
Activated proteins in E. coli that can act as caspase substrate proteins were measured with the CaspACE FITC-VAD-FMK In Situ Marker (Promega, Fitchburg, WI, USA). Cells (1 × 106 cells/mL of LB) were treated with nano-Ag or norfloxacin for 2 h at 37 °C. The treated cells were washed in PBS, suspended in 200 μL of staining solution containing 10 μM CaspACE FITC-VAD-FMK In Situ Marker, and incubated at room temperature in the dark for 30 min. The cells were then washed and resuspended in PBS. The activation of bacterial proteins that had affinity for the caspase substrate peptide was analyzed with a FACSCalibur flow cytometer (Madeo et al. 2002).
Detection of the hallmarks of late apoptosis and activation of DNA damage repair system
DNA strand breaks in E. coli were detected by using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (Phillips et al. 2003). Cells (1 × 106 cells/mL of LB) treated with nano-Ag or norfloxacin for 6 h were washed in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4), permeabilized in permeabilization solution (0.1 % Triton X-100 and 0.1 % sodium citrate) for 2 min on ice, and washed again with PBS. The DNA fragments were labeled for 1 h at 37 °C with an in situ cell death detection kit thatuses FITC-conjugated deoxyuridine triphosphate (FITC-dUTP). The stained cells were observed with a fluorescence microscope (Nikon Eclipse Ti–S; Nikon, Japan), and fluorescence was measured with a FACSCalibur flow cytometer.
Isolation of RecA and western blot analysis were performed as follows: E. coli cells (1 × 106 cells/mL of LB) were cultured in LB medium at 37 °C, collected by centrifugation at 300×g, and washed twice with PBS. These cells were treated with nano-Ag or norfloxacin for 6 h at 37 °C. The treated cells were lysed by sonication and then centrifuged at 12,000×g for 20 min to remove intact cells and any cell debris. The supernatant was collected, and proteins were precipitated with trichloroacetic acid (TCA) (Sanchez 2001). The washed TCA-precipitated proteins were used to assay for RecA by western blotting. The protein content of the precipitate was estimated with a spectrophotometer (NanoVue Plus; GE Healthcare, UK). Each sample, equivalent to 10 μg of protein, was resolved using 7.5 % SDS-PAGE. Separated proteins were transferred to a nitrocellulose membrane and analyzed by western blotting with a rabbit polyclonal anti-RecAantibody (Abcam, UK). A horseradish peroxidase-conjugated goat anti-rabbit IgG (Biovision, Milpitas, CA, USA) was used as the secondary antibody, and an enhanced-chemiluminescence substrate was used to detect RecA (Dwyer et al. 2012).
Results
Nano-Ag has antibacterial activity and promotes increased intracellular ROS levels in E. coli
A time-kill assay for E. coli was performed to determine the antibacterial activity of nano-Ag. To test for a antibacterial effect, norfloxacin was used as a positive control. Norfloxacin is a synthetic antibacterial agent and a broad-spectrum antibiotic that is active against both gram-positive and gram-negative bacteria. Norfloxacin induces accumulation of ROS, highly destructive molecules that appear to oxidize DNA and lethal double-strand DNA breaks caused by incomplete repair can contribute to cell death in bacteria (Kohanski et al. 2007; Foti et al. 2012). The assay results showed that nano-Ag has antibacterial activity. About 99 % of E. coli cells incubated with nano-Ag at the MIC died after 6 h whereas 99 % of the bacterial cells incubated with norfloxacin at the MIC died after 4 h (Fig. 1a).
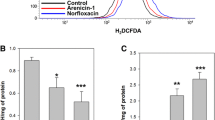
Antibacterial effect of nano-Ag and ROS accumulation by nano-Ag in E. coli. a Time-kill kinetics of 2 μg/mL nano-Ag and 0.125 μg/mL norfloxacin in E. coli. About 99 % of E. coli cells incubated with nano-Ag at the MIC died after 6 h whereas 99 % of the bacterial cells incubated with norfloxacin at the MIC died after 4 h. All the experiments were performed twice. b E. coli cells were treated with 2 μg/mL nano-Ag and 0.125 μg/mL norfloxacin for 2 h at 37 °C, with 10 μM H2DCFDA dye. The cells stained were measured by FACSCalibur flow cytometer
To examine the mechanism underlying the apoptosis-like responses, we first analyzed ROS production, which is known as participant in silver-induced bacterial cell death (Dwyer et al. 2012; Morones-Ramirez et al. 2013). The intracellular ROS level was measured with H2DCFDA, a fluorescent probe oxidized by ROS (Jakubowski et al. 2000). The population of the cells increased to 46.12 and 58.70 % after treatment with nano-Ag and norfloxacin, respectively, as compared to 19.79 % in untreated cells indicating increased ROS production (Fig. 1b).
Nano-Ag plays a role in modulating intracellular calcium levels and induces phosphatidylserine externalization in E. coli
In eukaryotic cells, calcium signaling plays a clear and prominent role in the regulation of cellular processes, including cell death (Yoon et al. 2012). To determine whether calcium signaling is involved in the cell death of E. coli, intracellular calcium levels were measured with the membrane-permeable derivative of the ratiometric calcium indicator, fura-2 AM (Zherebitskaya et al. 2012). Free calcium levels increased in cells treated with nano-Ag or norfloxacin (Fig. 2a), indicating that calcium signaling is involved in the bacterial cell death in E. coli.
Nano-Ag significantly increased cytosolic calcium levels in E. coli and induced phosphatidylserine exposure on outer leaflets of membrane. a Relative intracellular calcium levels visualized with fura 2-AM using a spectrofluorophotometer. b Phosphatidylserine externalization of E. coli plasma membranes detected by Annexin V-FITC fluorescence. (a) Untreated control cells, (b) cells treated with 2 μg/mL nano-Ag, (c) cells treated with 0.125 μg/mL norfloxacin for 2 h at 37 °C
We also detected early apoptotic effects in nano-Ag-treated cells; therefore, we confirmed that bacterial cell death caused by nano-Ag exhibits early apoptotic features. Phosphatidylserine is a phospholipid located in the inner leaflet of the plasma membrane that becomes exposed on the outer leaflet during early stages of apoptosis in response to particular calcium-dependent stimuli (van den Eijnde et al. 1998). With a double staining method using Annexin V-FITC, which has high affinity for outer leaflet phosphatidylserine, and PI, which is an intact membrane impermeable dye, apoptotic cells can be distinguished from late apoptosis and necrotic cells (Rieger AM et al. Rieger et al. 2011). Early apoptotic cells can only be stained with Annexin V-FITC, whereas late apoptotic and necrotic cells stain with both PI and Annexin V-FITC. Our results showed that the cell population in the lower right (LR) quadrant, which indicates the proportion of early apoptotic cells (Annexin V positive/PI negative), increased to 21.06 % following nano-Ag treatment, and to 27.31 % following norfloxacin treatment for 2 h (Fig. 2b). Therefore, the bacterial cell death caused by nano-Ag exhibits features of early apoptosis.
Nano-Ag dissipates the plasma membrane potential (ΔΨp) and induces the expression of a bacterial caspase-like protein
To determine whether intracellular calcium ion overload can affect the bacterial plasma membrane, membrane depolarization analysis was performed with DiBAC4(3), which is sensitive to cell membrane potential and can permeabilize depolarized cells (Liao et al. 1999). As shown in Fig. 3b, the population of fluorescent nano-Ag-treated cells (at the MIC) increased by 89.88 % compared to untreated cells, and the fluorescent norfloxacin-treated cell population (at the MIC) increased by 98.76 %, indicating membrane potential disturbance. This indicates that the antibacterial effect of nano-Ag is related to the dissipation of ΔΨp.
DiBAC4(3) staining for detection of plasma membrane potential (ΔΨp) changes in E. coli a and caspase-like protein activation in E. coli after treatment of nano-Ag or norfloxacin for 2 h at 37 °C, with 10 μΜ CaspACE FITC-VAD-FMK In Situ Marker b. (a) Untreated control cells, (b) cells treated with 2 μg/mL nano-Ag, (c) cells treated with 0.125 μg/mL norfloxacin
Caspases (cysteine-dependent aspartate-specific proteases) are considered to be the central enzymes that initiate the apoptotic cascade in eukaryotes (Thornberry and Lazebnik 1998). Although bacterial orthologs of caspases based on DNA sequence similarity have yet to be found (Vercammen et al. 2007), functional orthologs in E. coli have been detected (Halliwell and Aruoma 1991), and we used VAD-FMK, a FITC-conjugated peptide pan-caspase inhibitor, to investigate bacterial caspase-like protein expression. VAD-FMK is transported into cells and binds to the active site of the caspase. The fluorescence in nano-Ag-treated cells was higher than that in untreated cells, indicating active expression of proteins that specifically bind to the caspase substrate. Flow cytometric analysis showed that 37.03 % of cells treated with nano-Ag and 47.88 % of cells treated with norfloxacin were fluorescent following incubation with VAD-FMK (Fig. 3b). These data provide evidence of the presence of bacterial caspase-like proteins in E. coli and indicate that this protein (or proteins) plays a role in bacterial apoptosis-like response (Dwyer et al. 2012).
Nano-Ag-induced bacterial cell death exhibits features of late apoptosis and activation of DNA repair system
We also observed features associated with late apoptosis in cells undergoing nano-Ag-induced bacterial cell death, by using a method for observing DNA fragmentation (TUNEL assay), which is a widely used method for observing DNA degradation (Labat-Moleur et al. 1998). We used FITC-conjugated dUTP to label free 3′ hydroxyl termini with modified nucleotides, catalyzed by terminal deoxynucleotidyl transferase, and then measured the fluorescent cell population with flow cytometry. In addition, cells with apoptotic phenotypes (TUNEL-positive) were observed with a fluorescence microscope. The population of fluorescent E. coli cells increased to 55.23 and 61.52 % after treatment with nano-Ag and norfloxacin, respectively, for 6 h; fluorescent E. coli cells comprised 27.52 % of the untreated cells (Fig. 4a). We also observed green fluorescence and filamentation of cells treated with nano-Ag or norfloxacin (Fig. 4B) (Dwyer et al. 2012).
Detection of DNA fragmentation and activation of SOS response indirectly in E. coli cells treated with nano-Ag or norfloxacin. a DNA fragmentation assay with TUNEL fluorescence. b Observation of TUNEL fluorescence under fluorescence microscopy. (a) Untreated control cells, (b) cells treated with 2 μg/mL nano-Ag, and (c) cells treated with 0.125 μg/mL norfloxacin for 6 h at 37 °C. c RecA which is related with SOS response, expression analysis by western blot. E. coli cells were treated with 2 μg/mL nano-Ag or 0.125 μg/mL norfloxacin. Samples were taken 6 h after treatment. (Relative pixel density)
Additionally, we performed western blotting to confirm that nano-Ag caused an increase in the intracellular concentration of RecA, that is involved in the bacterial SOS response (Maul and Sutton 2005). As shown in Fig. 4c, blots from cells treated with nano-Ag or norfloxacin at their respective MICs showed RecA bands that were thicker than the corresponding band in untreated cells. We therefore assume that nano-Ag-induced bacterial cell death involves the damages in DNA and that DNA repair system is also activated by RecA protein.
Discussion
Nano-Ag has already been shown to be an effective biocide against many species of bacteria, and many studies have attempted to elucidate the mechanisms by which nano-Ag exerts this antimicrobial activity (Marambio-Jones and Hoek 2010). Although several possibility of antibacterial properties by nano-Ag has been reported (Li et al. 2010; Rai et al. 2009), the antibacterial mechanism of nano-Ag is incompletely understood (Morones et al. 2005). Therefore, we suggest that a bacterial apoptosis-like response underlies the antibacterial mechanism.
Several lines of evidence indicate that the bacterial cell death caused by nano-Ag resembles apoptosis in eukaryotes, including the externalization of phosphatidylserine and DNA fragmentation. Phosphatidylserine is known to have an important role in the regulation of apoptosis in response to particular calcium-dependent stimuli (van den Eijnde et al. 1998). The normal distribution of this lipid on the inner leaflet of the membrane bilayer is then disrupted because of stimulation of enzymes such as flippases or scramblase, which can move phosphatidylserine in both directions across the membrane, and inhibition of aminophospholipid translocases, which returns the lipid to the inner side of the membrane. E. coli cells treated with nano-Ag became Annexin V+/PI−, indicating that phosphatidylserine was exposed on the outer surface of the plasma membrane. As the membrane active property of nano-Ag has been reported (Li et al. 2010), Annexin V+/PI+ indicative cells also detected. However, the population of cells with early apoptotic features was outstanding at lower concentration of nano-Ag than that of Li et al’s experiment. DNA degradation can be feature of late apoptosis. In the TUNEL assay, DNA fragmentation can be observed as typical features of late apoptosis (Kyrylkova et al. 2012). Increased fluorescence intensity indicated that nano-Ag can act as an antibacterial agent by inducing an apoptosis-like response. We also observed cell filamentation, a marker of cell cycle arrest in E. coli that is generally mediated by SulA under stress conditions; this phenomenon is evidence of induction of the SOS response, the repair system in E. coli (Fonville et al. 2010). However, in the presence of nano-Ag, this repair system was ultimately ineffective, and the cells eventually died due to extreme damage, as evidenced by the apoptosis effects.
We assumed that the process of apoptosis-like response in E. coli is accompanied by generation of ROS. Bacterial cells produce ROS from intracellular metabolites under normal conditions, and the damage that these can cause is prevented by antioxidant machinery. However, when the cells are placed under stress conditions, ROS accumulated and consequently overloaded the antioxidant system (Costa and Moradas-Ferreira 2001). The superfluity of ROS, such as hydrogen peroxide (H2O2), superoxide anion (O2 −), nitric oxide (NO), and hydroxyl radicals (·OH), is believed to have an effect on the several steps of the apoptosis cascade (Ludovico Ludovico et al. 2005). We observed changes in the ROS levels in E. coli after nano-Ag treatment with the ROS indicator H2DCFDA, observed as an increase in fluorescence. It seems that ROS is involved in the nano-Ag-induced bacterial apoptosis-like response by causing damage to cell components and triggering increases in the free calcium levels of the bacterial cells (Dwyer et al. 2012; Chakraborti et al. 1999).
Although the importance of calcium as a cell regulator is well established in eukaryotes, the role of calcium in prokaryotes is more elusive (Yoon et al. 2012). It has been found that calcium ions are involved in some biological activities, including maintenance of cell structure, motility, and transport, and a number of calcium-binding proteins have been isolated from several prokaryotic organisms, suggesting the possibility of calcium signal transduction in bacteria (Dominguez 2004). In the current study, intracellular calcium levels and membrane depolarization increased during the apoptosis-like response in E. coli. Phosphatidylserine externalization can also occur in this step because of calcium stimulation on enzymes that dissipates membrane arrangement (Verhoven et al. 1995). In eukaryotic cells, an increase in cytosolic calcium stimulates mitochondrial uptake, and excessive calcium uptake causes depolarization of the mitochondrial membrane and opens the permeability transition pore, leading to the release of apoptogenic factors (Kroemer and Reed 2000; Crompton 1999). As nano-Ag-treated E. coli cells exhibited a dramatic depolarization of the plasma membrane, we infer that nano-Ag exerts its activity on bacterial membranes and induces a calcium-related depolarization of bacterial cells. The similarities between the mitochondrial and bacterial responses are consistent with their shared evolutionary origin (Hakansson et al. 2011).
It seems that bacterial protein(s) activate in the procedure of bacterial apoptosis-like response inducing protease and nuclease activation in E. coli, like as caspase (Dwyer et al. 2012). The presence of bacterial caspase orthologs, based on their DNA sequences, should be identified. However, to date, only functional orthologs have been detected (Halliwell and Aruoma 1991). In this study, bacterial proteins that have affinity to caspase inhibitor VAD-FMK were found to be activated after treatment with nano-Ag, shown by an increase in intracellular fluorescence. This unknown caspase-like protein seems to vitalize nuclease in bacterial cell, like as DNA fragmentation factor, DFF40 in eukaryotes (Widlak et al. 2000). This can make the bacterial cells suffer severe DNA damages and SOS response is operated by RecA proteins. RecA is generally thought to have a co-protease function in the autocatalytic cleavage of the LexA repressor, which inhibits the expression of bacterial repair system-related genes (Kamenšek et al. 2010). The active form of RecA aids in the cleavage of LexA, resulting in the induction of the SOS response in stressful conditions. During repair the damaged DNA, SulA stops cell division by binding to FtsZ, and this causes filamentation as we observed in TUNEL assay. Several studies also depict this phenomenon in nano-Ag-induced bacterial cell death and support DNA active property of nano-Ag inducing SOS response (Li et al. 2010; Rai et al. 2009). Dwyer et al. (2012) suggested the possibility of a relationship between RecA and caspase and assumed that RecA can act as a major regulator in both SOS repair system and apoptosis-like response in bacteria. On this inspection, further studies are needed to determine the hypothesis.
In the present study, we examined many apoptotic features of nano-Ag-treated bacterial cells death, including phosphatidylserine externalization (indicative of early apoptosis) and DNA damage (indicative of late apoptosis). We also observed intracellular accumulation of ROS, increased calcium levels, and membrane depolarization, which are all indicative of membrane disturbances, and we observed the activation of a bacterial caspase-like protein related to the apoptosis cascade. Based on the evidence obtained in the current study, we suggest that nano-Ag has a novel antibacterial mechanism of action, namely, the induction of a bacterial apoptosis-like response, and therefore has the potential to be a useful antimicrobial therapeutic agent.
References
Arduino DM, Esteves AR, Domingues AF, Pereira CM, Cardoso SM, Oliveira CR (2009) ER-mediated stress induces mitochondrial-dependent caspase activation in NT2 neuron-like cells. BMB Rep 42:719–724
Atiyeh BS, Costaqliola M, Hayek SN, Dibo SA (2007) Effect of silver on burn wound infection control and healing: review of the literature. Burns 33:139–148
Bos J, Yakhnina AA, Gitai Z (2012) BapE DNA endonuclease induces an apoptotic-like response to DNA damage in Caulobacter. Proc Natl Acad Sci USA 109:18096–18101
Bosetti M, Massè A, Tobin E, Cannas M (2002) Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials 23:887–892
Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S (1999) Oxidant, mitochondria and calcium: an overview. Cell Signal 11:77–85
Costa V, Moradas-Ferreira P (2001) Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into aging, apoptosis and disease. Mol Aspects Med 22:217–246
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249
Dominguez DC (2004) Calcium signaling in bacteria. Mol Microbiol 54:291–297
Dwyer DJ, Camacho DM, Kohanski MA, Callura JM, Collins JJ (2012) Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell 46:561–572
Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R (2006) Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet 2:e135
Fonville NC, Bates D, Hastings PJ, Hanawalt PC, Rosenberg SM (2010) Role of RecA and the SOS response in thymineless death in Escherichia coli. PLoS Genet 6:e1000865
Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC (2012) Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336:315–319
Furuya EY, Lowy FD (2006) Antimicrobial-resistant bacteria in the community setting. Nat Rev Microbiol 4:36–45
Hakansson AP, Roche-Hakansson H, Mossberg AK, Svanborg C (2011) Apoptosis-like death in bacteria induced HAMLET, a human milk lipid-protein complex. PLoS ONE 6:e17717
Halliwell B, Aruoma OI (1991) DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett 281:9–19
Herrera M, Carrion P, Baca P, Liebana J, Castillo A (2001) In vitro antibacterial activity of glass-ionomer cements. J Clin Pediatr Dent 104:141–148
Hwang IS, Hwang JH, Choi H, Kim KJ, Lee DG (2012) Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol 61:1719–1726
Jakubowski W, Biliński T, Bartosz G (2000) Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic Biol Med 28:659–664
Jones SA, Bowler PG, Walker M, Parsons D (2004) Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair Regen 12:288–294
Kamenšek S, Podlesek Z, Gillor O, Zgur-Bertok D (2010) Genes regulated by the Escherichia coli SOS repressor LexA exhibit heterogeneous expression. BMC Microbiol 10:283
Klasen HJ (2000) Historical review of the use of silver in the treatment of burns. I Early uses. Burns 33:117–138
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C (2012) Detection of apoptosis by TUNEL assay. Methods Mol Biol 887:41–47
Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A (1998) TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem 46:327–334
Li WR, Xie XB, Shi QS, Zeng HY, Ou-Yang YS, Chen YB (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85:1115–1122
Liao RS, Rennie RP, Talbot JA (1999) Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob Agents Chemother 43:1034–1041
Ludovico P, Madeo F, Silva MT (2005) Yeast programmed cell death: an intricate puzzle. IUBMB Life 57:129–135
Madeo F, Fröhlich E, Fröhlich KU (1997) A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol 139:729–734
Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Fröhlich KU (2002) A caspase-related protease regulates apoptosis in yeast. Mol Cell 9:911–917
Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146:3–15
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Maul RW, Sutton MD (2005) Roles of the Escherichia coli RecA protein and the global SOS response in effecting DNA polymerase selection in vivo. J Bacteriol 187:7607–7618
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ (2013) Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med 4:190ra81
Nagata A (1997) Apoptosis by death factor. Cell 88:355–365
Phillips AJ, Sudbery I, Ramsdale M (2003) Apoptosis induced by environmental stresses and amphothericin B in Candida albicans. Proc Natl Acad Sci USA 100:14327–14332
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of microbials. Biotechnol Adv 27:76–83
Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR (2011) Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp 50:2597
Sanchez L (2001) TCA protein precipitation. Protocols on line. http://www.its.caltech.edu/~bjorker/Protocols/TCA_ppt_protocol.pdf. Accessed 5 Aug 2014
Sintubin L, De Windt W, Dick J, Mast J, van der Ha D, Verstraete W, Boon N (2009) Lactic acid bacteria as a reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol 84:741–749
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
van den Eijnde SM, Boshart L, Baehrecke EH, De Zeeuw CI, Reutelingsperger CP, Vermeij-Keers C (1998) Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis 3:9–16
Vercammen D, Declercq W, Vandenabeele P, Van Breusegem F (2007) Are metacaspases caspases? J Cell Biol 179:375–380
Verhoven B, Schlegel RA, Williamson P (1995) Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 182:1597–1601
Widlak P, Li P, Wang X, Garrard WT (2000) Cleavage preferences of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease) on naked DNA and chromatin substrates. J Biol Chem 275:8226–8232
Yoon MJ, Kim EH, Kwon TK, Park SA, Choi KS (2012) Simultaneous mitochondrial Ca2+ overload and proteasomal inhibition are responsible for the induction of paraptosis in malignant breast cancer cells. Cancer Lett 324:197–209
Zherebitskaya E, Schapansky J, Akude E, Smith DR, Van der Ploeg R, Solovyova N, Verkhratsky A, Fernyhough P (2012) Sensory neurons derived from diabetic rats have diminished internal Ca2+ stores linked to impaired re-uptake by the endoplasmic reticulum. ASN Neuro 4:e00072
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ008158), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, W., Kim, KJ. & Lee, D.G. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli . Biometals 27, 1191–1201 (2014). https://doi.org/10.1007/s10534-014-9782-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9782-z