Abstract
Biotically-mediated weathering helps to shape Earth’s surface. For example, plants expend carbon (C) to mobilize nutrients in forms whose relative abundances vary with depth. It thus is likely that trees’ nutrient acquisition strategies—their investment in rooting systems and exudates—may function differently following disturbance-induced changes in depth of rooting zones and soil nutrient stocks. These changes may persist across centuries. We test the hypothesis that plant C allocation for nutrient acquisition is depth dependent as a function of rooting system development and relative abundances of organic vs. mineral nutrient stocks. We further posit that patterns of belowground C allocation to nutrient acquisition reveal anthropogenic signatures through many decades of forest regeneration. To test this idea, we examined fine root abundances and rooting system C in organic acid exudates and exo-enzymes in tandem with depth distributions of organically- and mineral-bound P stocks. Our design permitted us to estimate C tradeoffs between organic vs. mineral nutrient benefits in paired forests with many similar aboveground traits but different ages: post-agricultural mixed-pine forests and older reference hardwoods. Fine roots were more abundant throughout the upper 2 m in reference forest soils than in regenerating stands. Rooting systems in all forests exhibited depth-dependent C allocations to nutrient acquisition reflecting relative abundances of organic vs. mineral bound P stocks. Further, organic vs. mineral stocks underwent redistribution with historic land use, producing distinct ecosystem nutritional economies. In reference forests, rooting systems are allocating C to relatively deep fine roots and low-C exudation strategies that can increase mobility of mineral-bound P stocks. Regenerating forests exhibit relatively shallower fine root distributions and more diverse exudation strategies reflecting more variable nutrient stocks. We observed these disparities in rooting systems’ depth and nutritional mechanisms even though the regenerating forests have attained aboveground biomass stocks similar to those in reference hardwood forests. These distinctions offer plausible belowground mechanisms for observations of continued C sink strength in relatively old forests, and have implications for soil C fates and soil development on timescales relevant to human lifetimes. As such, depth-dependent nutrient returns on plant C investments represent a subtle but consequential signal of the Anthropocene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is growing appreciation of the diverse roles that tree roots play as soil architects even deep within soil profiles (Richter et al. 2007; Roering et al. 2010; Brantley et al. 2017) and as mediators of unexpectedly large material and energy fluxes at the ecosystem scale (Yin et al. 2014; Finzi et al. 2015; Mayer et al. 2018). Tree roots influence hydrologic flow paths (Bond et al. 2008; Band et al. 2014; Fan et al. 2017), mine soil profiles for nutrients from soil minerals (Lucas 2001; Jobbagy and Jackson 2004; Soper et al. 2018) and organic matter (Adams and Pate 1992; van Vuuren et al. 1996; Aber and Melillo 2001; Janzen 2006; Newmann 2007), can both slow and accelerate erosion (Brantley et al. 2012; Pawlik 2013), and redistribute nutrients in ways that influence metrics of mineralogy (Austin et al. 2018) and soil development (Brantley et al. 2012, 2017). They also are responsible for pumping fixed carbon (C) into the subsoil via root-respired CO2 (Nadelhoffer and Raich 1992; Lambers et al. 2000; Ryan and Law 2005; Billings et al. 2018; Cherkinsky et al. 2018), the CO2 respired by microbes dependent on them (Schneckenberger et al. 2008; Keiluweit et al. 2015), root and microbial C exudates (Grayston et al. 1996; Lynch and Ho 2005; Thorley et al. 2015), and the construction of roots themselves (Nadelhoffer and Raich 1992; Jackson et al. 1997). Although years of research highlight the millennia over which roots have been helping to construct Earth’s skin (Berner 1992; Kelly et al. 1998; Berner 2003), deep soil processes are a historically understudied trait (Richter and Billings 2015) and deep roots likely have a disproportionately strong influence over ecosystem functions (Maeght et al 2013; Pierret et al. 2016). Indeed, deep root activities have recently earned trees the designation of “builders and plumbers of Earth’s Critical Zone” (Brantley et al. 2017).
Human-driven alterations in aboveground biotic communities can result in persistently altered biogeochemical signals beneath the surface (Ellis 2011; Waters et al. 2016). We focus on two specific, common phenomena relevant to root behavior. First, deeply rooted, long-lived plants have been replaced with more shallowly rooted ecosystems in many regions, especially where annual, row-crop agriculture is a dominant land use (Canadell et al. 1996; Fan et al. 2016). However, where agricultural lands are abandoned, regenerating forests are common; this is especially true in the northern hemisphere (World Bank 2015). Despite aboveground shifts toward earlier successional species, regenerating systems can still exhibit many of the qualities of older forests with minimal human imprint (Eaton and Lawrence 2006; Martin et al. 2013; Billings et al. 2018); for example, relatively young forests can achieve similar hydrologic functioning as much older forests after just 20 years (Jipp et al. 1998). However, root networks require many decades or centuries to establish structures comparable in depth and complexity to much older forests (Dupouey et al. 2002; Knops and Bradley 2009; Devine et al. 2011; Yuan & Chen, 2012; Pierret et al. 2016; Rodina et al. 2019), and deep root networks can be important promoters of long-term soil development (Jobbagy and Jackson 2004; Brantley et al. 2012). For example, even after ~ 80 years of forest regeneration rooting depth distributions lag those of older reference forests, with consequences for biogeochemical fluxes far below the zone of maximum rooting density (Billings et al. 2018; Cherkinsky et al. 2018). Although some of these changes may be a consequence of species changes across successional stages, the shift to an earlier successional stage is itself a consequence of land use change. Thus, a relative dearth of deep roots, whether due to species or ecosystem age, can serve as an important signal of the Anthropocene in many subsoils.
Second, regenerating rooting systems grow where anthropogenically accelerated erosion (Haff 2010; Brecheisen et al. 2019) and historic mineral fertilizer use (Richter and Markewitz 2001; Richter et al. 2006; Yoo et al. 2015) have altered the geomorphology of Earth’s surface and the distribution of mineral nutrients across it. Compared to less disturbed soil profiles, contemporary soils can exhibit proportionately more mineral-bound nutrients near the surface due to historically accelerated erosion of topsoil typically enriched in organically-bound nutrients (Richter and Markewitz 2001; Yoo et al. 2015), as well as past application of mineral fertilizers (Richter et al. 2006). Redistribution of the depths and relative abundances of mineral vs. organic nutrients in disturbed ecosystems compared to what is observed in relatively undisturbed ecosystems represents another way in which anthropogenic signals emerge in Earth’s subsurface where the aboveground system may otherwise appear natural.
Combined, these widespread phenomena—shallower roots where ecosystems have been disturbed frequently and a greater relative abundance of mineral-bound nutrients in shallower horizons—suggest that trees’ means of obtaining nutrients may be different where historic, agricultural land use has been extensive. Rooting system nutritional strategies are complex, encompassing both root proliferation and distinct mechanisms to access organic vs. mineral nutrient forms. Here, we use “rooting system” to acknowledge that roots and the microbes that reside in and around them jointly exercise these nutrient acquisition strategies. One such strategy is organic anion production, which enhances mineral-bound nutrient mobility via desorption, ligand exchange, and pH-based reactions (Landeweert et al. 2001; Marschner and Rengel 2007). Rooting systems also can exude extracellular enzymes that hydrolyze organic molecules, transforming nutrients bound in relatively large organic compounds into bioavailable forms (Marschner and Rengel 2007). These two mechanisms incur distinct costs of fixed C. Extracellular enzymes exhibit nearly double the concentration of C as organic acids (Smith 1976; Hauser and Billings unpublished data), suggesting that release of bioavailable nutrients via extracellular enzymes may require relatively greater C investment. However, the relatively shallow rooting systems of young, regenerating forests may be restricted to more shallow nutrient pools, which tend to accumulate organic stocks as forests regrow (Cross and Schlesinger 1995; Vitousek et al. 1997; Mobley et al. 2013). In contrast, more deeply rooted, older forests may have greater access to deeper nutrient sources that are dominantly mineral-bound (Jin et al. 2010; Brantley et al. 2017; Hasenmueller et al. 2017) and may be subject to redistribution via nutrient uplift (Jobaggy and Jackson 2001).
One nutrient element for which these acquisition strategies are especially evident is phosphorus (P), which we use as an element of interest in this study due to its presence in both organic- and mineral-derived sources, and its status as a macronutrient in high demand across Earth’s ecosystems (Vitousek et al. 1997). Rooting systems display the mechanisms described above as a means of obtaining P. For example, phosphatase enzyme exudation of rooting systems occurs in the presence of organic P (Bunemann et al. 2011). Additionally, production of organic acids such as oxalic acid, though not specific to P acquisition, can manipulate soil pH and increase mineral-bound P mobilization (Marschner and Rengel 2007; Thorley et al. 2015). Numerous studies also suggest that root and mycorrhizal morphology reflects P bioavailability. Proliferation of high surface area fine roots, root hairs, and mycorrhizal mycelia represent key plant mechanisms for tapping into heterogeneous soil P supplies (Hodge 2004; Liu et al. 2015; Lugli et al. 2019) that preferentially occur near concentrated P sources (Drew 1975; Jackson et al. 1990). Plant P acquisition thus represents a considerable investment of C because it requires multiple mechanisms including both root and mycorrhizal proliferation and exudate production.
Because of these varying C costs for obtaining different P forms and the unpredictable belowground conditions regenerating ecosystems can experience, we might expect ecosystems regenerating on lands that experienced intense land use in the past to exhibit different P acquisition mechanisms relative to less disturbed systems. These strategies should reflect efficient tradeoffs between C costs and the potential nutrient benefits offered by varying depth distributions of organic vs. mineral nutrient stocks. We thus hypothesize that plant C allocation to mechanisms of nutrient acquisition is strategically depth dependent as a function of (1) degree of rooting system development and (2) the relative abundance of mineral vs. organic P forms in the rooting zone. Further, we posit that, because land use history influences rooting system depth development as well as stocks and depths of nutrient sources, forest C allocation to specific nutrient acquisition mechanisms reveals anthropogenic signatures lasting for many decades after initiation of forest regrowth. If validated, these hypotheses suggest that the functional signatures of land use legacies dictate contemporary nutrient economies that serve as underappreciated drivers of soil organic C (SOC) distributions. Thus, forest disturbance and subsequent regrowth may prompt shifts in deep critical zone phenomena that drive ecosystem C allocation, mineral weathering and soil development on timescales relevant to human lifetimes.
Methods
Site and soil sampling description
We collected soil samples from the Calhoun Critical Zone Observatory, SC, USA, which consists of several research sites in the area of 34° 36′ 25″ N, 81° 43′ 13″ W. The region has a mean annual precipitation of 1250 mm y−1 and mean average temperature of 16 °C (NADP et al. 2017). These sites comprise paired, spatially replicated reference hardwood and regenerating pine forest plots that represent distinct land use histories. Reference hardwood forests are comprised of trees that are approximately 130 to 140 years old, dominated by Quercus alba, Q. rubra, Q. montana, and multiple Carya spp. Estimates of aboveground biomass in these forests averaged 21.1 ± 6.7 kg m−2 in 2014 (W. Cook and D. Richter, unpublished data). To the best of our knowledge, the soils in these forests have never been plowed or otherwise subjected to agricultural practices (Brecheisen et al. 2019). We compare these relatively old hardwood stands with regenerating pine stands, growing on land that was cleared for agriculture and cultivated for up to 150 years until its abandonment and regrowth both by natural reestablishment and by planting mainly of Pinus taeda and P. echinata from the 1930s through 1950s. The trees in these forests are approximately 60 to 80 years old, according to historical records of site abandonment. Estimates of aboveground biomass in these pine-dominated forests are statistically similar to those of the reference hardwood forests, averaging 14.6 ± 2.24 kg m−2 in 2014 (W. Cook and D. Richter, unpublished data), and modeling analyses indicate that biomass accumulation in regenerating forests has declined in recent decades (Mobley et al. 2015).
On May 1, 2018, we hand-augured five sampling cores in each of the two forest types at six fixed intervals (0–15 cm, 15–35 cm, 35–60 cm, 60–100 cm, 100–150 cm, and 150–200 cm) using a 10 cm diameter bucket auger. We homogenized soil collected in each of these depth intervals and collected a representative subsample. We froze samples as soon as possible and shipped them to the University of Kansas where they were maintained at − 20 °C except when briefly thawed for chemical analyses. These subsamples were then subjected to analyses for rooting system C exudates and soil P forms, detailed below.
Fine root abundances
To estimate differences across forest types in rooting depth distributions most relevant for nutrient acquisition, we quantified fine root abundances to two meters in soil profiles using the method described in Billings et al. (2018). For this analysis, we relied on soil data collected during the Big Dig 2016 sampling campaign at the Calhoun, timed late in the growing season when root biomass was maximized. During the campaign, we dug ten > 2 m deep soil pits and captured high-resolution photos along the length of each pit face that had been cleaned with a knife to expose natural planes of weakness. Six of these pits (i.e. three regenerating forests, three reference hardwood forests) are located in the same forest sites as hand-augured locations from May 2018. We then overlaid a 1 × 1 cm grid on each soil profile image using ImageJ software. In each 1 × 1 cm grid cell, we noted the presence or absence of roots, as well as its classification as fine or coarse, generating a measure of rooting density (units of roots cm−2). We classified fine roots as roots < 1 mm in diameter, consistent with the idea that designating multiple size classes of roots < 2 mm in diameter is important for understanding root system functioning, and in acknowledgement of the importance of very fine roots for nutrient uptake (Pregitzer 2002; McCormack et al. 2015; McCormack and Iversen 2019).
Soil P stocks
Organic, mineral-bound, and total P
We parsed organic P (Po) and mineral-bound P (Pi) using the first two extractions of the Hedley soil P sequential extraction protocol (Tiessen and Moir 1993), which extracts nearly all Po present in these soils (Richter et al. 2006), permitting us to divide total P estimates into an estimate of Po vs. Pi in these soil samples. Briefly, we extracted 0.5 g of each soil sequentially with 30 mL 0.5 M NaHCO3 followed by 30 mL 0.1 M NaOH. Each solution was shaken at 120 rpm for ~ 20 h, centrifuged for 10 min at 3400 rpm, and filtered through a 0.45 μm polyvinylidene fluoride syringe filter prior to P analyses. We measured the Pi in these extracts according to the malachite green colorimetric method (D’Angelo et al. 2001) on a Bio Tek SynergyHT plate reader (VT, USA). We sent the remaining solution to Kansas State University to quantify the total P contained in these extracts via inductively coupled plasma—optical emission spectroscopy (ICP-OES, Varian 720-ES, Palo Alto, USA). The difference between values provided by the Kansas State testing lab and Pi in each extract provides an estimate of Po in each solution, with the sum of Po from NaOH and NaHCO3 extracts approximating the total Po in a given soil sample.
We also dried and pulverized five to ten gram soil subsamples for total soil P. We passed these subsamples through a 2 mm soil sieve and sent them to the Kansas State University Soil Testing Lab for total P analysis via salicylic-sulfuric acid digestion and ICP-OES analysis. The difference between previously summed Po estimates and total P measured on dried, salicylic-sulfuric digested soils provides an estimate of overall Pi and Po in each sample.
Bio-extractable P
To investigate the potential of rooting system exudates to generate bioavailable P from organic- vs. mineral-bound P sources, we extracted soils with acid phosphatase and oxalic acid solutions (DeLuca et al. 2015). We chose concentrations of each exudate well in excess of that found in a natural soil system based on trial extractions demonstrating greater P release with increasing extraction solution concentration. As such, P concentrations observed from these exudate-based extracts represent the maximum potential nutrient benefit to a plant with unlimited C allocation toward the given exudate.
For acid phosphatase (APase), we used purified APase (P1146, Sigma-Aldrich, USA) mixed with deionized water to make a solution with an enzyme activity approximately 1000 times that previously measured in Calhoun soils (Billings et al. 2018). We mixed this solution with soil samples in a 5:1 solution:soil ratio, agitated the soil solution for 30 min, centrifuged the solutions and filtered the resulting supernatant with 45 μm syringe filters. We then analyzed extracted liquid for orthophosphate P (PO4−3-P) on the microplate reader using the malachite green colorimetric method (D’Angelo et al. 2001).
To quantify oxalic acid extractable P, we made a 10 mM solution of powdered oxalate (129601000, Acros Organics, USA) and deionized water. We mixed the solution with each soil sample in a 5:1 solution:soil ratio and agitated samples for 30 min. After shaking, we centrifuged the samples and filtered the extracts with 0.45 μm syringe filters. Extracts were analyzed for PO4−3-P as above.
Evidence of rooting system exudates
As a means of estimating rooting system C investment in mineral vs. organic nutrient acquisition strategies, we analyzed rooting system exudate activities and concentrations in sampled soils. First, we examined extracellular APase activity—a representation of C investment in organic P acquisition—via protocols detailed in previous publications (Deforest 2009; Lehmeier et al. 2013; Min et al. 2014). We performed this analysis within two months of freezing soils given previously observed propensity for extracellular enzyme activity to decay in old samples (DeForest 2009). Briefly, we subsampled 1 g of each soil sample and mixed it with a sodium acetate buffer solution of pH ~ 5.5 for 30 s using a hand-held blender. We quickly transferred the solutions to black 96 well plates, to which we added either a fluorescently tagged phosphatase substrate (methylumbelliferyl phosphate, M8883, Sigma-Aldrich, USA), the fluorescent methylumbelliferyl label itself (MUB, M7008, Sigma-Aldrich, USA), or pH adjusted buffer to provide appropriate controls. The substrate, when cleaved by its relevant enzyme, releases a fluorescent signal that can be quantified as a metric of potential enzymatic activity. After plating, we incubated the plates for ~ 18 h before analyzing the fluorescence at excitation wavelength 360, emission wavelength 460 on the plate reader (Lehmeier et al. 2013). All activities are reported in nmol g−soil h−1.
We further transformed these potential activities into estimates of soil APase C concentrations to assess rooting system C investment in organic P acquisition. We divided the APase activity quantified in environmental samples (nmol g−soil h−1) by the P we extracted from the same soils using a known amount of APase C (nmol g−1 h−1 gC−1), as described in bioextractable P forms above. This calculation reveals the maximum potential C in APase enzymes in these soils, given the greatest P benefit that these soils can contribute with no limitation of enzyme C addition. We further normalized these APase C concentration estimates by unit root abundance and assessed how these estimates varied with the fraction of total soil P present as organic P.
To assess rooting systems’ propensity to use oxalic acid as a nutrient acquisition strategy, we extracted each soil sample by shaking soils with water (5:1 ratio) for 30 min. We centrifuged and filtered extracts with 0.45 μm syringe filters and froze them for subsequent analyses. We analyzed organic acid concentrations in these extracts using ion chromatography. We conducted the analysis using an Ion Chromatography System (ICS) 2000 (Dionex, USA) fitted with Dionex IonPac AS11-HC 4 mm analytical and guard columns, following a modified version of the organic acid analytical protocol outlined by Thermo Fisher (2012). Briefly, we ran samples on a KOH eluent gradient of 1 mM, 15 mM, 30 mM, and 60 mM KOH, increasing concentrations at 15, 25, and 35 min respectively. Sample chromatogram peak areas were then analyzed using Chromeleon 6.8 Software build 2212. To determine retention time of oxalate anions, we compared chromatogram peaks produced by deionized water both with and without the addition of 5 μM oxalic acid. After we discerned the retention time, we produced analytical standards containing 0, 0.05, 0.1, 0.5, 1, and 5 μm oxalic acid using the Organic Acid Kit from Sigma Aldrich (47264) to determine oxalic acid concentrations in unknowns. We normalized oxalic acid C concentrations by rooting abundance metrics as for APase, and assessed how these normalized values vary with the inorganic fraction of total soil P.
Statistical analyses
To assess the relative importance of forest history, soil depth and their interaction on root distributions, enzyme activities, organic acid concentrations, and all P fractions, we used linear mixed effects models. Two data sets, inorganic and organic soil P concentrations, required natural log transformation prior to linear mixed effect analyses to comply with model assumptions. For all response variables, we generated four statistical models: one with forest type as a fixed effect (1); one with depth as a fixed effect (2); one with both variables included as independent fixed effects but without their interaction (3), and one including all effects and their interaction (4) (Winter 2013). Models including depth as an effect were structured to account for the lack of independence across depths. In all models, we included sampling site as a random effect. We then ran ANOVAs between models 3 and 4 to test for a significant interaction. We interpreted a significant interaction between forest type and depth as a difference between forest types in the depth-associated pattern of the response variable; this approach embraces the lack of independence across depths and does not require post-hoc tests of the effect of forest type in each soil depth interval. When the interaction effect of forest type and depth on the response variable was insignificant, we tested models 1 and 2 against model 3 to assess the significance of forest type and depth individually as influences on the response variables.
As a means of assessing mechanisms of rooting system nutrient acquisition, we tested correlations between exudate C (i.e. APase and oxalic acid) per unit root and potential soil organic and inorganic P stocks both as concentrations and as proportion of total soil P, across all soil depths assayed in each of the ten plots. We log transformed metrics of exudate C concentrations per unit root to meet model assumptions. We used Cook’s distance to check for outliers and influential points, and ran all analyses both with and without extreme points to examine their effects on tested relationships. We then tested for differences between the slopes of lines fitted to the five reference forest plots vs. the five regenerating forest plots via a paired t-test. The positive or negative nature of correlations between root exudate C metrics and potentially bioavailable soil P forms serves three roles: an index of rooting system C investment toward mineral vs. organic P acquisition; the extent to which those rooting system mechanisms differ with land use history; and the robustness of those observations across sampling space given the presence or absence of influential points.
Because fine root data exist at a much finer depth resolution (i.e. every 1 cm layer across 2 m) than the P fraction and exudate datasets (six fixed depth points), we used linear interpolation to generate values of P fractions. We considered this approach to be reasonable given monotonic trends with depth in P data. In one instance interpolations generated distinct clusters of points where the examined soil profile characteristic varied greatly between sites within a given forest type. We considered these interpolated profiles as replicates and the calculated variability as part of a standard error. We then performed correlation analyses between root density and measured P fractions across soil depths and forest types as a means of inferring root abilities to acquire P from the measured P pools. Where correlations exhibited non-linear relationships, we fitted logarithmic, power law, and exponential equations to the data and selected the model with the lowest AIC value as the best representation of the relationship. In those cases, P-values representing significance reflect rejection of the null hypothesis that there is no relationship between X and Y variables.
Errors presented are one standard error of the mean. We report all P values suggesting meaningful trends in the data (Amrhein et al. 2019). All analyses were run using R via R Studio, Version 1.0.153 (RStudio Team 2017) with packages lme4 Version 1.1–21 and ggplot2 Version 3.1.1 (Bates et al. 2015; Wickham 2016).
Results
Fine roots
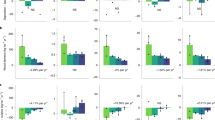
Forest type and depth both exhibited a significant influence on fine root abundances in these forests. Reference hardwood forests had higher fine root densities across soil profiles than regenerating forests (P = 0.047, Fig. 1). The fraction of fine roots in each 1 cm soil depth interval declined exponentially with increasing soil depth in both forest types (P = 0.0001). Reference hardwood fine root abundances declined from 49 to 4% and regenerating pine root abundances declined from 43 to 1.5% in the top 1 cm to 200 cm, respectively. The legacy of land use history in metrics of fine root density is particularly evident one meter deep in the soil profile, a point below which we observed far smaller abundances of fine roots in both forest types but consistently greater abundance of fine roots in reference forests, approximately double that in regenerating forest profiles.
Fraction of each 1-cm depth interval containing fine roots (< 1 mm diameter) to 200 cm depth in older reference and regenerating forest profiles at the Calhoun CZO, SC, USA. Each data point represents the average of five sites. Error bars represent one standard error of the mean. See Statistical Analyses in “Methods” section for explanation of the model used to generate P-values
Soil phosphorus pools
There was a significant interaction between forest type and depth driving inorganic P concentration patterns in Calhoun soils (P = 0.03, Fig. 2a). In reference forests, inorganic P showed little variation in concentration across depths and sites (Fig. 2a), with an average of 82 mg kg−1 across soil samples. In regenerating forest soils, we encountered more inorganic P on average (100 mg kg−1) than in reference forests, but there were less consistent inorganic P concentration patterns when compared with reference forest P depth distributions. Each regenerating site has a distinct land use history, likely producing high variability in P concentrations across regenerating forest sites. The large standard errors (Fig. 2a, b) and range of inorganic P values from 1.33 to 496 mg kg−1 across all regenerating forest soil samples highlight this subsurface variability in P metrics. Organic P concentration declined significantly with depth in both forest types (P = 0.0003 Fig. 2b), having high concentrations in the soil surface declining to near-zero values in deep soils.
Soil P stocks to 200 cm in regenerating and older reference forest soils at the Calhoun CZO, SC, USA. Each data point represents the mean of five sites. Error bars represent one standard error of the mean. Pools include a mineral-bound NaOH- and NaHCO3-extractable Hedley P fractions; b organic P stocks estimated via the difference between salicylic acid-digested total P and inorganic P in a. We also examined proportion of total P comprised of c inorganic P and d organic P across depths and sites. Note that statistical results for c and d are identical given that organic P pools are derived using inorganic P pool sizes, and that soil depths for regenerating forests have been offset by 1.5 cm for error bar visualization. See Statistical Analyses in “Methods” section for explanation of the model used to generate P-values
In general, inorganic P comprised an increasingly larger proportion of the total P with soil depth (P = 1.17e−6, Fig. 2c), and made up a larger fraction of the total P than organic P in all except the shallowest soil horizons across forest sites (Fig. 2c, d). Correspondingly, organic P comprised an increasingly smaller proportion of total P with increased soil depth (P = 1.17e−6, Fig. 2d) in both forest types.
Potential linkages between P stocks and rooting system activities
Root distribution and bioavailable P
Below 100 cm in Calhoun regenerating forests, fine roots occupied at most 1.5% of each 1 cm soil depth interval. Although few in number, fine roots in reference forests were nearly double that of regenerating forests, hinting at potential disparities in trees’ resource-acquiring activities in these deep soils. Indeed, within the depth interval just above this depth (60 to 100 cm), we observed differences in the relationships between fine root abundances and potentially bioavailable P stocks. Across forests, this depth interval represented part of the clay-rich Bt horizon although was nearer the C horizon in regenerating forests than reference forests (W. Cook and D. Richter, unpublished data). Thus, this interval in both forests is a potential zone of material accumulation, but regenerating fine roots may not have to extend as deeply before reaching less mineralogically-altered material. In these forests, oxalate-extractable P, a metric of potential bioavailable mineral-bound P, declined where roots were more abundant (Fig. 3a, P = 0.0002). Acid phosphatase-extractable P, a measure of potentially bioavailable organically-bound P, also exhibited a negative trend with fine root abundance (Fig. 3b, P = 0.0002).
Fraction of fine roots in 1-cm depth intervals vs. soil P extracted with synthetic root exudate solutions comprised of a 10 mM oxalic acid (relationship for reference forests is y = 0.3e−1.5x; regenerating forests, y = 0.18e−2.6x) and b sufficient APase to generate an activity of 100 nmol g−1 h−1 in samples collected from 60 to 100 cm deep soils in CCZO forests (reference forests, y = 33.2x − 0.66; regenerating, y = −1.81x + 0.18) . Inset plots have the same x-axis labels and units as their main plots and show P concentrations obtained from a oxalate and b phosphatase extraction across all soil depths in these forests, with black boxes denoting the sampling region represented by interpolated data points in main plots. Data clusters are an artifact of interpolation. For further explanation, see “Methods”
In this same depth interval in reference forests, where fine roots were relatively more abundant, oxalate-extractable P was present only in soils where we observed few to no roots (Fig. 3a, P = 0.001). Unlike regenerating forest soils, reference forest fine roots displayed a positive relationship with APase-extractable P (Fig. 3b, P < 0.0001) suggesting that organically-bound P remained relatively abundant in the presence of roots in these same soils.
Potential rooting system exudate production
In both forest types, oxalic acid-C concentrations per unit root abundance tended to be greater where inorganic, mineral-bound P comprised a greater proportion of the total soil P content (P = 0.058 and 0.07 for reference and regenerating forests respectively, Fig. 4a, c). In contrast, estimates of phosphatase-C per unit root abundance were greater where organic P comprised a relatively small proportion of soil P stocks, in both forest types (P = 6.8e−8; P = 0.0003, Fig. 4b, d). In both cases, we observed no significant difference in the slope of the lines representing the exudate vs. soil P relationship in regenerating forests when compared to reference forests, although the absolute concentration of both exudates produced per root was consistently greater in regenerating forests compared to reference forests. In both exudate datasets, we encountered up to three outliers as defined by Cook’s distance test for influential points. The APase dataset contained one outlier in reference forests and three in regenerating forests. For oxalic acid, we observed two outliers in each forest type. These points generally represented soil samples in which APase or oxalic acid concentrations were below detection limits and thus generated an unrealistic fit to otherwise linear, log-transformed data. We tested the relationship between X and Y with and without these influential points; in most cases, these analyses revealed significant relationships between root responses and P forms when outliers were omitted, but not when outliers were included. One dataset, oxalic acid per unit root vs. proportion inorganic P (Fig. 4c), did not exhibit a change in significance regardless of outlier inclusion. We report results both with and without outliers in Fig. 4.
Rooting system exudate concentrations as they relate to inorganic and organic P proportions of total P across all depths in CCZO forest soils. In reference forests, a oxalic acid per unit root abundance in each depth increment vs. inorganic proportion of soil P, and b acid phosphatase per unit root abundance in the soil depth increment vs. organic proportion of soil P. c, d Represent analogous data sets for regenerating forests, respectively. All plots depict untransformed data with outliers removed. Symbols of the same shape represent paired reference and regenerating sites. Black lines depict the average fitted trend across all data points. P values are reported on log-transformed data both with three outliers (values in parentheses) and without outliers, where outlier inclusion produced statistical differences. Reference and regenerating forests displayed no significant differences in the slope of fitted curves for the same relationship (i.e. a vs. c, or b vs. d), but note greater absolute exudate production in regenerating forests highlighted by y-axis scales, which are different across all panels to highlight trends
Discussion
This study illuminates persistent anthropogenic legacies deep within Earth’s CZ even after six to eight decades of forest regeneration on post-agricultural land. These legacies are manifest most obviously as truncated fine root depth distributions (Fig. 1) and altered depth distributions of P nutrient stocks (Fig. 2). These two attributes appear to drive subtle shifts in forest functioning that nevertheless represent persistent legacies of anthropogenic land use that influence these forests’ C economies. Because fine roots are plants’ primary methods of acquiring resources (Jackson et al. 1997; Liu et al. 2015; Kong et al. 2016), any redistribution of fine roots resulting from anthropogenic disturbances both represents and necessitates shifts in forest mechanisms of obtaining nutrients. Land use history may further promote changes in forest nutrient acquisition strategies by altering the nutrient stocks those fine roots can encounter as trees reestablish. Here, we provide one, place-based example that supports our hypothesis that changes in rooting system development and nutrient stocks incurred as a result of historic land use alter the depths at which, and mechanisms with which, forest ecosystems acquire nutrition. We propose that shallower rooting system depths in regenerating forests compared to older, reference forests may promote distinct C for P tradeoffs reflective of divergent nutrient economies (Fig. 5).
Conceptual representation of rooting system nutrient acquisition economies in regenerating forests compared to their older, reference forest counterparts. We depict three distinct belowground C for P exchange regions: a shallow exchange in both forest types governed by C-rich enzyme exudate-driven organic matter decomposition; a region from 60 to 100 cm where rooting systems in regenerating forests diverge from reference stands, accessing both mineral- and organic matter-bound P; and a deep exchange region governed by low-C organic acid-induced mineral-bound P release, occurring only in older reference forests. Arrow length and thickness represents the degree to which each exchange plays a role in a rooting system’s nutrient economy, and opposite directions of arrows indicate loss of exudate and gain of P. The CO2 feature associated with enzyme C exchange for Po refers to microbially-mediated CO2 losses typically linked to organic matter decay, a phenomenon that mineral P release does not necessitate
Carbon investments: rooting systems display depth-dependent C allocation strategies to acquire nutrition
Consistent with our first hypothesis, rooting system C distribution in these forests’ soils reflects time since disturbance and the nutrient forms present in the rooting zone. Foremost, more rooting system C is present in the form of fine roots more deeply in the reference forest soil profiles than in the regenerating forests (Fig. 1), consistent with differences in total root abundances in these same forests (Billings et al. 2018). This phenomenon likely results from several factors. First, regenerating rooting systems have had only ~ 80 years to establish compared to well over 100 years in reference forests and may not have had the time needed to develop such extensive networks (Knops and Bradley 2009; Yuan and Chen 2012; Pierret et al. 2016). In addition, succession-associated tree species differences—early successional pines in regenerating forests and late successional oaks in reference forests—typically exhibit different rooting structures (Shukla and Ramakrishnan 1984; Finér et al. 1997; Lambers et al. 2008), a feature that emphasizes another way in which time since disturbance can contribute to belowground root abundances and functioning. Though regenerating rooting systems could eventually extend to comparable depths as reference forests, there may be limited incentive for roots in regenerating forests to grow into deeper soils because surface soils accumulate organic matter in the reestablishing O horizon in the early years of forest regrowth (Mobley et al. 2013) that re-growing roots can access as a source of relatively abundant nutrients (Fig. 2; Crews et al. 1995; Cross and Schlesinger 1995; Vitousek et al. 1997). This may be particularly relevant for species such as the CCZO’s loblolly pines, which invest significant C stores in ectomycorrhizal fungi that produce exudates—including phosphatase—at relatively high rates (Lambers et al. 2008; Yin et al. 2014). Though potentially expensive, a shallow C allocation strategy for nutrient acquisition might be resource-effective for these early successional, shade intolerant pines that can invest C in nutrient acquisition, but have not yet allocated C to deep root construction (Phillips et al. 2013).
Rooting systems in these forests also provide clues of depth-dependent C allocation strategies other than fine root distributions: their rooting system exudation patterns appear to reflect organic vs. mineral nutrient stocks in the rooting zone. Across soil depth, rooting systems appear to allocate more C per unit root to oxalic acid generation where inorganic P stocks comprised a greater proportion of total soil P, regardless of forest type (Fig. 4a, c). Numerous authors demonstrate that oxalic acid liberates P from mineral-bound forms while requiring relatively little plant C to produce (Aoki et al. 2012; Keiluweit et al. 2015; Lugli et al. 2019). Thus, our results suggest that roots may leverage relatively low C-cost nutrient stocks when possible to optimize nutrient returns on their resource expenditures.
In contrast, APase concentrations per root were high only when relatively little organically bound P was present in the rooting zone, regardless of forest type (Fig. 4b, d). Numerous studies have observed rooting system phosphatase production in soils primarily where P is relatively scarce (Bunemann et al. 2011), which may be a function of the high C-cost of enzyme exudates and mycorrhizal partners, both of which can mobilize organic nutrients. Andrino et al. (2019), for example, demonstrate that rooting systems without mycorrhizae are less able to acquire organically-bound P as well as inorganically-bound P. However, they demonstrate that mycorrhizae, which enable plant access to organic P stocks they might not otherwise obtain, confer a smaller net P benefit for plants’ C investments than inorganic P (Andrino et al. 2019). Thus, C allocated to organic nutrient acquisition likely is beneficial in soil conditions where organic nutrient stocks are the prevailing nutrient source, as in shallow horizons or highly chemically denuded soils. Our study suggests that rooting systems appear to invest in these C-expensive mechanisms only when necessary, such that nutritional benefits should exceed high C costs.
Because depth distributions of SOC influence forest soils’ capacity to retain C over decades to centuries (Rumpel and Kogel-Knaber 2011), distinctions among forests’ strategies of belowground C allocation for nutrient acquisition may contribute to longer-term belowground C budgets. Roots themselves represent one of the largest contributors to soil C stocks, with increasing relevance to soil C accumulation in deeper soils (Rasse et al. 2005; Crow et al. 2009). Further, their exudates are implicated in soil C retention (Rasse et al. 2005; Keiluweit et al. 2015). Indeed, some studies hint that soil retention of C-rich exudates may exceed C lost as CO2 due to mineralization of SOC induced by those exudates (Qiao et al. 2013). Exudate fate depends on its identity, however. Exo-enzymes, for example, likely enhance CO2 release due to their specific role as catalysts of organic matter decay and thus as provisioners of microbially available nutrient and C resources (Schlesinger 1984; Schneckenberger et al. 2008). Organic acids, on the other hand, have a high propensity for adsorption onto Fe- and Al-minerals (Rasse et al 2005; Ganor et al. 2009) and the ensuing ligand exchange process can result in soil retention of this exudate while simultaneously displacing nutrient-containing ions into soil solution (Ganor et al. 2009; Keiluweit et al. 2015; Hasegawa et al. 2015). Rooting systems leveraging this exchange for nutrients may mineralize relatively less C to CO2 than an exo-enzyme induced transaction, given that nutrients are made available via C-binding ligand exchange and not by microbial activities that incur a respiratory cost (Schlesinger 1984; Rasse et al. 2005). Indeed, Richter et al. (1999) observed net C loss in regenerating forest soils during 40 years of rooting system development. We cannot know the degree to which these phenomena drive SOC depth distributions in these forests, but it seems reasonable to infer that distinct patterns of vegetation C exchange for different P forms may influence SOC generation and retention.
Depth-dependent P acquisition strategies reveal persistent legacies of land use
These data are also consistent with our second hypothesis. Potential rooting system P acquisition from mineral vs. organic stocks appears to vary with both depth and forest type because historic agricultural practices have redistributed plants’ primary mechanism to obtain nutrients—fine root proliferation—relative to distributions of nutrient forms (Figs. 2, 3). Fine root proliferation in these regenerating soils is approximately half that of reference forests below ~ 100 cm (Fig. 1). Just above this depth (60–100 cm), reference and regenerating rooting systems appear to employ different nutritional strategies, exhibiting apparent uptake of the distinct P forms present in the rooting zone of each forest type. In reference forests, roots between 60 and 100 cm appear more successful at acquiring inorganic P compared to organic P stocks (Fig. 3). Roots in regenerating forests in this same depth interval, though relatively few, appear to deplete both organic and inorganic forms (Fig. 3).
This distinction in P resource use between forest types likely reflects the interaction of altered fine root distributions and distinct abundances of P stocks in the rooting zone of each forest type. Reference forests display relatively less variable inorganic P concentrations across soil depths compared to regenerating forests (Fig. 2a). This implies that, in these older forests, investment in low C-cost oxalic acid would be a reliable mechanism for rooting system P acquisition (Fig. 3a), given that these mechanisms promote P release from mineral-bound P forms (Fig. 5; Crews et al. 1995; Keiluweit et al. 2015; Hasegawa et al. 2015; Ellsworth et al. 2017). Like reference forests, regenerating forest roots encounter both organic and inorganic P, but these stocks are more variable across depths and sites (Fig. 2). Reduced predictability of soil P stocks might explain the appearance of an “omnivorous” or opportunistic nutrient strategy in regenerating forests, reflecting the benefits of synergistic nutrient acquisition strategies where P stocks are variable (Fig. 5; Marschner and Rengel 2007; Darch et al. 2016; Brantley 2017; Lugli et al. 2019). Given that these regenerating forests appear thus far to have constructed fewer fine roots between 1 and 2 m than in the reference forests, they may have more C resources to spend on C-costly extracellular enzymes than reference forest trees, either directly or through mycorrhizal fungi, promoting shallow soil P for C exchanges in place of deep mineral-P uptake.
Conclusions
Our results suggest that rooting systems develop depth-dependent C distribution patterns in response to organic vs. mineral bound nutrient stocks, perhaps optimizing the nutrients plants obtain for the C resources they invest. Further, the relative availability of organic vs. mineral nutrient stocks reflects historic land use, prompting anthropogenic legacies to produce distinct ecosystem nutritional economies. These distinct economies have implications for soil C fate. Deep rooting systems in relatively old forests represent nutritional economies that can sequester root C and root exudate C meters deep into Earth’s surface. Forests regenerating post-disturbance, in contrast, exhibit nutritional strategies focused on relatively surficial horizons’ nutrient stores for decades post-disturbance. In these forests, the relative dearth of deep roots and the C-rich, exo-enzyme dominated mechanisms of liberating surficial, organically-bound nutrient stores emphasize surface-focused strategies for nutrient acquisition. The demonstration of the multiple ways in which reference forests contain and possibly retain C more deeply in the subsurface compared to regenerating forests adds support to propositions that older forests may continue to serve as a net C sink well after aboveground biomass appears to reach steady-state (Magnani et al. 2007; Baldocchi 2008; Luyssaert et al. 2008). Similarities in regenerating and reference forests’ aboveground biomass in this study suggest that aboveground growth can mask lags in rooting system development in regenerating forests that can inhibit their capacity to establish the C sink and SOC retention mechanisms present in older forests. Distinctions across rooting system economies likely have meaningful implications for soil development via both mineral weathering and C cycling dynamics on time scales relevant to human lifetimes, representing subtle but consequential signals of the Anthropocene.
References
Aber JD, Melillo JM (2001) Terrestrial ecosystems. Academic, Burlington
Adams MA, Pate JS (1992) Availability of organic and inorganic forms of phosphorus to lupins (Lupinus spp.). Plant Soil 145:107–113
Amrhein V, Greenland S, McShane B (2019) Scientists rise up against statistical significance. Nature 567:305–307
Andrino A, Boy J, Mikutta R, Sauheitl L, Guggengerger G (2019) Carbon investment required for mobilization of inorganic and organic phosphorus bound to goethite by an arbuscular mycorrhiza (Solanum lycopersicum x Rhizophagus irregularis). Front Environ Sci 7:1–15
Aoki M, Fujii K, Kitayama K (2012) Environmental control of root exudation of low-molecular weight organic acids in tropical rainforests. Ecosystems. https://doi.org/10.1007/s10021-012-9575-6
Austin JC, Perry A, Richter DD, Schroeder PA (2018) Modifications of 2:1 clay minerals in a kaolinite dominated ultisol under changing land use regimes. Clay Clay Miner 66:61–73
Baldocchi D (2008) ‘Breathing’ of the terrestrial biosphere: Lessons learned from a global network of carbon dioxide flux measurement systems. Aust J Bot 56:1–26
Band LE, Mcdonnell JJ, Duncan JM, Barros A, Bejan A, Burt T, Dietrich WE, Emanuel R, Hwang T, Katul G, Kim Y, McGlynn B, Miles B, Porporato A, Scaife C, Troch PA (2014) Ecohydrological flow networks in the subsurface. Ecohydrology. https://doi.org/10.1002/eco.1525
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Berner RA (1992) Weathering, plants and the long-term carbon cycle. Geochim Cosmochim Acta 56:3225–3231
Berner RA (2003) The long-term carbon cycle, fossil fuels and atmospheric composition. Nature 426:323–326
Billings SA, Hirmas D, Sullivan PL, Lehmeier CA, Bagchi S, Min K, Brecheisen Z, Hauser E, Stair R, Flournoy R, Richter DD (2018) Loss of deep roots limits biogenic agents of soil development that are only partially restored by decades of forest regeneration. Elem Sci Anth 6:34
Bond BJ, Meinzer FC, Brooks JR (2008) How trees influence the hydrological cycle in forest ecosystems. In: Wood PJ, Hannah DM, SadlerJP (eds) Hydroecology and ecohydrology: past, present and future. Wiley, Sussex, pp 7–28.
Brantley SL, Lebedeva M, Hausrath EM (2012) A geobiological view of weathering and erosion. In: Knoll AH, Canfield DE, Konhauser KO (eds) Fundamentals of geobiology. Blackwell, Oxford
Brantley SL, Eissenstat DM, Marshall JA, Godsey SE, Balough-Brunstad A, Karwan DL, Papuga SA, Roering J, Dawson TE, Dvaristo J, Chadwick O, McDonnell JJ, Weathers KC (2017) Reviews and synthesis: on the roles trees play in building and plumbing the critical zone. Biogeosciences. https://doi.org/10.5194/bg-2017-61
Brecheisen ZS, Cook CW, Heine PR, Richter D (2019) Micro-topographic roughness analysis (MTRA) highlights minimally eroded terrain in a landscape severely impacted by historic agriculture. Rem Sens Environ 222:78–89
Bunemann EK, Oberson A, Frossard E (eds) (2011) Phosphorus in action: biological processes in soil phosphorus cycling. Springer, Berlin
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595
Cherkinsky A, Brecheisen ZS, Richter D (2018) Carbon and oxygen isotope composition in soil carbon dioxide within deep ultisols at the Calhoun CZO, South Carolina, USA. Radiocarbon 60:1357–1366
Crews TE, Kitayam K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombolis D, Vitousek PM (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214
Crow SE, Lajtha K, Filley TR, Swanston CW, Bowden RD, Caldwell BA (2009) Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob Change Biol 15:2003–2019
D'Angelo E, Crutchfield J, Vandiviere M (2001) Rapid, sensitive, microscale determination of phosphate in water and soil. J Environ Qual 30:2206–2209
Darch T, Blackwell MSA, Chadwick D, Haygarth PM, Hawkins JMB, Turner BL (2016) Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma 284:93–102
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol Biochem 41:1180–1186
Deluca TH, Glanville HC, Harris M, Emmet BA, Pingree MRA, de Sosa LL, Jone DL (2015) A novel biologically-based approach to evaluating soil phosphorus availability across complex landscapes. Soil Biol Biochem 88:110–119
Devine S, Markewitz D, Hendrix P, Coleman D (2011) Soil carbon change through 2 m during forest succession alongside a 30-year agroecosystem experiment. For Sci 57:36–50
Drew MC (1975) Comparison of the effect of a localized supply of phosphate, nitrate, ammonium, and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75:479–490
Dupouey JL, Dambrine E, Laffite JD, Moares C (2002) Irreversible impacts of past land use on forest soils and biodiversity. Ecology 83:2978–2984
Eaton JM, Lawrence D (2006) Loss of carbon sequestration potential after several decades of shifting cultivation in the Southern Yucatan. For Ecol Manag 258:949–958
Ellis EC (2011) Anthropogenic transformation of the terrestrial biosphere. Philos Trans R Soc 369:1010–1135
Ellsworth DS, Anderson IC, Crous KY, Cooke J, Drake JE, Gherlenda AN, GimenoTE MCA, Medlyn BE, Powell JR, Tjoelker MG, Reich PB (2017) Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat Clim Change 7:279–282
Fan J, McConkey B, Wang H, Janzen H (2016) Root distribution by depth for temperate agricultural crops. Field Crop Res 189:68–74
Fan Y, Miguez-Macho G, Jobbagy EG, Jackson RB, Otero-Casal C (2017) Hydrologic regulation of plant rooting depth. Proc Natl Acad Sci USA 114:10572–10577
Finér L, Messier C, Grandpré LD (1997) Fine-root dynamics in mixed boreal confer-broad-leaved forest stands at different successional stages after fire. Can J For Res 27:304–314
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Change Biol 21:2082–2094
Ganor J, Reznik IJ, Rosenberg YO (2009) Organics in water-rock interactions. Rev Mineral Geochem 70:259–369
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Haff PK (2010) Hillslopes, rivers, plows, and trucks: mass transport on Earth’s surface by natural and technological processes. Earth Surf Process Landf 35:1157–1166
Hasegawa S, MacDonald CA, Power SA (2015) Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited Eucalyptus woodland. Glob Change Biol 22:1628–1643
Hasenmueller EA, Gu X, Weitzman JN, Adams TS, Stinchcomb GE, Eissenstat DM, Drohan PJ, Brantley SL, Kaye JP (2017) Weathering of rock to regolith: the activity of deep roots in bedrock fractures. Geoderma 300:11–31
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Jackson RB, Manwaring JH, Caldwell MM (1990) Rapid physiological adjustment of roots to localized soil enrichment. Nature 344:58–60
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA 94:7362–7366
Janzen HH (2006) The soil carbon dilemma: Shall we hoard or use it? Soil Biol Biochem 38:419–424
Jin L, Ramesh R, Ketchum PR, Heany P, White T, Brantley SL (2010) Mineral weathering and elemental transport during hillslope evolution at the Susquehanna/Shale Hills Critical Zone Observatory. Geochim Cosmochim Acta 74:3669–3691
Jipp PH, Nepstad DC, Cassel DK, de Carvalho CR (1998) Deep soil moisture storage and transpiration in forests and pastures of seasonally-dry Amazonia. In: Markham A (eds) Potential impacts of climate change on tropical forest ecosystems. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-2730-3_11
Jobbagy EG, Jackson RB (2001) The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53:51–77
Jobbagy EG, Jackson RB (2004) The uplift of soil nutrients by plants: biogeochemical consequences across scales. Ecology 85:2380–2389
Keiluweit M, Bourgoure JJ, Nico PS, Pett-Ridge J, Weber PK, Kleber M (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Change 5:588–595
Kelly EF, Chadwick OA, Hilinski ET (1998) The effect of plants on mineral weathering. Biogeochemistry 42:21–53
Knops JMH, Bradley KL (2009) Soil carbon and nitrogen accumulation and vertical distribution across a 74-year chronosequence. Soil Sci Soc Am J 73:2096–2104
Kong DL, Wang JJ, Kardol P, Wu HF, Zheng H, Deng XB, Deng Y (2016) Economic strategies of plant absorptive roots vary with root diameter. Biogeosciences 13:415–424
Lambers H, Atkin OK, Millenaar FF (2000) Respiratory patterns in roots in relation to their function. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots. The hidden half, 1st edn. Marcel Dekker, New York, pp 521–552.
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breeman T (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Lehmeier CA, Min K, Niehues ND, Ballantyne F, Billings SA (2013) Temperature-mediated changes of exoenzyme-substrate reaction rates and their consequences for the carbon to nitrogen flow ratio of liberated resources. Soil Biol Biochem 57:374–382
Liu B, Hongbo L, Zhu B, Koide RT, Eissenstat DM, Guo D (2015) Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol 208:125–136
Lucas Y (2001) The role of plants in controlling rates and products of weather: importance of biological pumping. Annu Rev Earth Planet Sci 29:135–163
Lugli L, Andersen KM, Aragao LEOC, Cordiero AL, Cunha HFV, Fuchslueger L, Meir P, Mercado LM, Oblitas E, Quesada CA, Rosa JS, Schaap KJ, Valverde-Barrantes O, Hartley IP (2019) Multiple phosphorus acquisition strategies adopted by fine roots in low-fertility soils in Central Amazonia. Plant Soil. https://doi.org/10.1007/s11104-019-03963-9
Luyssaert S, Schulze ED, Borner A, Knohl A, Hessenmoller D, Law BE, Ciais P, Grace J (2008) Old-growth forests as global carbon sinks. Nature 455:213–215
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Maeght JL, Rewald B, Pierret A (2013) How to study deep roots and why it matters. Front Plant Sci 4:299
Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzon S, Grelle A, Hari P, Jarvis PG, Kolari P, Kowalski AS, Lankreijer H, Law BE, Lindroth A, Loustau D, Manca G, Moncrieff JB, Rayment M, Tedeschi V, Valentiti R, Grace J (2007) The human footprint in the carbon cycle of temperate and boreal forests. Nature. https://doi.org/10.1038/nature05847
Marschner P, Rengel Z (2007) Nutrient cycling in terrestrial ecosystems. Springer, New York
Martin PA, Newton AC, Bullock JM (2013) Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc Roy Soc 280:20132236
Mayer A, Hausfather Z, Jones AD, Silver WL (2018) The potential of agricultural land management to contribute to lower global surface temperatures. Sci Adv 4:eaaq0932
McCormack LM, Iversen CM (2019) Physical and functional constraints on viable belowground acquisition strategies. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01215
McCormak LM, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppalammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of belowground contributions to terrestrial biosphere processes. New Phytol 207:505–518
Min K, Lehmeier CA, Ballantyne F, Tatarko A, Billings SA (2014) Differential effects of pH on temperature sensitivity of organic carbon and nitrogen decay. Soil Biol Biochem 76:193–200
Mobley ML, Richter DD, Heine PR (2013) Accumulation and decay of woody detritus in a humid subtropical secondary pine forest. Can J For Res 43:109–118
Mobley ML, Lajtha K, Kramer MG, Bacon AR, Heine PR, Richter DD (2015) Surficial gains and subsoil loses of soil carbon and nitrogen during secondary forest development. Glob Change Biol 21:986–996
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73:1139–1147
NADP, NOAA, others (2017) CZO dataset: national—air temperature, flux tower, meteorology (2017)—NADP and NOAA or other weather stations. http://criticalzone.org/calhoun/data/dataset/6112/. Accessed 5 Dec 2019
Newmann G (2007) Root exudates and nutrient cycling. In: Marschner P, Rengel Z (eds) Nutrient cycling in terrestrial ecosystems. Springer, New York, pp 123–157
Pawlik L (2013) The role of trees in the geomorphic system of forested hillslopes—a review. Earth Sci Rev 126:250–265
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51
Pierret A, Maeght JL, Clement C, Montoroi JP, Hartman C, Gonkhamdee S (2016) Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann Bot 118:621–635
Pregitzer KS (2002) Fine roots of trees—a new perspective. New Phytol 154:267–270
Qiao NA, Schaefer D, Blagodatskaya E, Zou X, Xu X, Kuzyakov Y (2013) Labile carbon retention compensates for CO2 released by priming in forest soils. Glob Change Biol. https://doi.org/10.1111/gcb.12458
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 269:341–356
Richter DD, Billings SA (2015) ‘One physical system’: Tansley’s ecosystem as Earth’s critical zone. New Phytol 206:900–912
Richter DD, Markewitz D (2001) Understanding soil change: soil sustainability over millennia, centuries, and decades. Cambridge, New York
Richter DD, Markewitz D, Trumbore SE, Wells CG (1999) Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 400:56–58
Richter DD, Markewitz D, Heine PR, Jin V, Raikes J, Tian K, Wells CG (2000) Legacies of agriculture and forest regrowth in the nitrogen of old-field soils. For Ecol Manag 138:233–248
Richter DD, Allen HL, Li J, Markewitz D, Raikes J (2006) Bioavailability of slowly cycling soil phosphorus: major restructuring of soil P fractions over four decades in an aggrading forest. Oecologia 150:259–271
Richter DD, Oh NH, Fimmen R, Jackson J (2007) The rhizosphere and soil formation. In: Cardon ZG, Whitbeck JL (eds) The rhizosphere: An ecological perspective. Elsevier, London, pp 179–200
Rodina ABL, Tonon BC, Marques LEA, Hungria LM, Nogueria AM, Zangaro W (2019) Plants of distinct successional stages have different strategies for nutrient acquisition in an Atlantic rain forest ecosystem. Int J Plant Sci 180:186–199
Roering JJ, Marshall J, Booth AM, Mort M, Jin Q (2010) Evidence for biotic controls on topography and soil production. Earth Planet Sci Lett 298:183–190
RStudio Team (2017) RStudio: integrated development for R. RStudio Inc, Boston. https://www.rstudio.com/. Accessed 8 May 2019.
Rumpel C, Kogel-Knaber I (2011) Deep soil organic matter—a key but poorly understood component of the terrestrial C cycle. Plant Soil 338:143–158
Ryan MG, Law BE (2005) Interpreting, measuring and modeling soil respiration. Biogeochemistry 73:3–27
Schlesinger WH (1984) Soil organic matter: a source of atmospheric CO2. In: Woodwell GM (ed) The role of terrestrial vegetation in the global carbon cycle: measurement by remote sensing. Wiley, New York, pp 111–127
Schneckenberger K, Demin D, Stahr K, Kuzyakov Y (2008) Microbial utilization and mineralization of [14C]glucose added in 6 orders of concentration to soil. Soil Biol Biochem 40:1981–1988
Scientific T (2012) IonPac AS11-HC product manual. Thermo Fisher Scientific, Waltham
Shukla RP, Ramakrishnan PS (1984) Biomass allocation strategies and productivity of tropical trees related to successional status. For Ecol Manag 9:315–324
Smith WH (1976) Character and significance of forest tree root exudates. Ecology 57:324–331
Soper FM, Chamberlain SD, Crumsey JM, Gregor S, Derry LA, Sparks JP (2018) Biological cycling of mineral nutrients in a temperate forested shale catchment. J Geophys Res Biogeosciences 1:1. https://doi.org/10.1029/2018jg004639
Thorley RMS, Taylor LL, Banwart SA, Leake JR, Beerling DJ (2015) The role of forest trees and their mycorrhizal fungi in carbonate rock weathering and its significance for global carbon cycling. Plant Cell Environ 38:1947–1961
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil Sampling and methods of analysis. Louis, Boca Raton, Florida, USA, pp 75–86
van Vuuren MMI, Robinson D, Griffiths BS (1996) Nutrient inflow and root proliferation during the exploitation of a temporally and spatially discrete source of nitrogen in the soil. Plant Soil 178:185–192
Vitousek P, Chadwick O, Crews T, Fownes J, Hendricks D, Herbert D (1997) Soil and ecosystem development across the Hawaiian islands. GSA Today 7:3–7
Waters CN, Zalasiewicz J, Summerhayes C, Barnosky AD, Poirier C, Galuszka A, Cearreta A, Edgeworth M, Ellis EC, Ellis M, Jeandel C, Leinfelder R, McNeill JR, Richter DD, Steffen W, Syvitski J, Vidas D, Wagreich M, Williams M, Zhisheng A, Grinevald J, Odada E, Oreskes N, Wolfe AP (2016) The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351:aad2622
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Winter B (2013) Linear models and linear mixed effects models in R with linguistic applications. arXiv:1308.5499. https://arxiv.org/pdf/1308.5499.pdf. Accessed 9 May 2019.
World Bank Group (2015) Forest area (% of land area). Food and Agricultural Organization, Rome. https://data.worldbank.org/indicator/ag.lnd.frst.zs. Accessed 9 May 2019.
Yin H, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221
Yoo K, Fisher B, Ji J, Aufdenkampe A, Klaminder J (2015) The geochemical transformation of soils by agriculture and its dependence on soil erosion: an application of the geochemical mass balance approach. Sci Total Environ 251:326–335
Yuan ZY, Chen HYH (2012) Fine root dynamics with stand development in the boreal forest. Funct Ecol 26:991–998
Acknowledgements
We thank Dr. Dan Reuman for enriching our understanding of some of the intricacies of post hoc statistical testing, Rena Stair for her work developing the fine root dataset, the assistance of the Kansas State University Soil Testing Lab, and National Science Foundation grant EAR-1331846.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Sasha C. Reed.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hauser, E., Richter, D.D., Markewitz, D. et al. Persistent anthropogenic legacies structure depth dependence of regenerating rooting systems and their functions. Biogeochemistry 147, 259–275 (2020). https://doi.org/10.1007/s10533-020-00641-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00641-2