Abstract
Plant root associations with microbes such as mycorrhizal fungi or N-fixing bacteria enable ecosystems to tap pools of nitrogen (N) that might otherwise be inaccessible, including atmospheric N or N in large soil organic molecules. Such microbially assisted N-foraging strategies may be particularly important in late-successional retrogressive ecosystems where productivity is low and soil nutrients are scarce. Here, we use natural N-stable isotopic composition to constrain pathways of N supplies to different plant functional groups across a well-studied natural soil fertility gradient that includes a highly retrogressive stage. We demonstrate that ectomycorrhizal fungi, ericoid mycorrhizal fungi, and N-fixing bacteria support forest N supplies at all stages of ecosystem succession, from relatively young, N-rich/phosphorus (P)-rich sites, to ancient sites (ca. 500 ky) where both N supplies and P supplies are exceedingly low. Microbially mediated N sources are most important in older ecosystems with very low soil nutrient availability, accounting for 75–96% of foliar N at the oldest, least fertile sites. These isotopically ground findings point to the key role of plant–microbe associations in shaping ecosystem processes and functioning, particularly in retrogressive-phase forest ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant–microbe interactions are critical drivers of ecosystem functioning and nutrient cycling (Phillips et al. 2013; Averill et al. 2014; van der Putten 2017). These interactions can shape ecosystem processes and properties throughout primary succession, as nutrient capital builds during early pedogenic phases and then declines over the course of millennia (Wardle et al. 2004). In ecosystems that have developed beyond peak biomass and into a retrogressive stage, characterized by declining standing biomass, net primary productivity, and soil nutrient availability (Peltzer et al. 2010), plant–microbe relationships may be key in maintaining nutrient cycling (Dickie et al. 2013). However, changes in plant–microbe symbioses and nutrient dynamics throughout long-term ecosystem development are not well understood, with a number of possible outcomes which likely differ between plant functional types.
In retrogressive-stage ecosystems, soil nitrogen (N) supplies are typically depleted relative to earlier, more fertile stages, and what little N remains is often found in complex, organic forms such as protein–tannin complexes (Qualls et al. 1991; Kraus et al. 2003; Wardle et al. 2004). Under these circumstances, root-associated microbes such as N-fixing bacteria or mycorrhizal fungi can assist in plant N acquisition (van der Heijden et al. 2008). These ecological partnerships incur a carbon (C) cost to the plant hosts but provide access to N pools that would otherwise be unavailable (Chapman et al. 2005; Fisher et al. 2010).
Ectomycorrhizae, a symbiotic relationship between fungi from a phylogenetically diverse group of approximately 25,000 fungal species (Tedersoo et al. 2010) and a broad group of plants spanning both gymnosperms and angiosperms (Brundrett 2009), are one such partnership. Ectomycorrhizal fungi can acquire soil N in both mineral and organic forms (Rains and Bledsoe 2007), as well as degrade complex organic molecules to liberate N (Read and Perez-Moreno 2003), making them valuable for plant nutrition. Ericoid mycorrhizae are another important symbiosis, occurring between fungi spanning many phyla (Leopold 2016) and plants mainly in the Ericaceae family. Similar to ectomycorrhizal fungi, ericoid mycorrhizal fungi can produce extracellular enzymes and organic acids to mineralize dissolved organic N (DON) compounds and facilitate plant N uptake (Bending and Read 1997a, b; Read and Perez-Moreno 2003; Schimel and Bennett 2004). However, both lab and field experiments suggest that ericoid mycorrhizal fungi have a particular advantage in depolymerizing and accessing N found in protein–tannin complexes due to greater enzymatic potential as well as greater specific root length compared to ectomycorrhizal fungi (Read 1991; Bending and Read 1997a; Read and Perez-Moreno 2003; Wurzburger and Hendrick 2009).
Both ecto- and ericoid mycorrhizal fungi can be an important source of plant N nutrition, and have been found to supply up to 80% of plant N in N-limited ecosystems (Read and Perez-Moreno 2003; Hobbie and Hobbie 2006). Indirect evidence suggests that plant reliance on ecto- and ericoid mycorrhizal fungi partners for N foraging may increase as plant-available soil N supplies decline during ecosystem retrogression. For example, ectomycorrhizal fungi have been found to contribute more to plant nutrition under conditions of environmental stress (Pena and Polle 2014), and declining nutrient availability has been linked with greater abundance of ectomycorrhizal fungi (Courty et al. 2018) as well as more intensive fungal foraging traits (Moeller et al. 2014). Root colonization by ericoid mycorrhizal fungi has also been observed to increase in response to declines in soil nutrient availability (Wurzburger and Bledsoe 2001). However, there remains little direct characterization of changes in plant–mycorrhizal nutrient dynamics throughout ecosystem development, especially with respect to ericoid mycorrhizal fungi (Dickie et al. 2013).
Symbiotic N fixation is another strategy through which plants can meet their N demands. High rates of symbiotic N fixation are most often observed in early successional environments in the temperate zone, where soil N is still accumulating (Vitousek et al. 2002; Menge et al. 2012); in late-successional temperate forests, symbiotic N-fixing plants are often absent (Vitousek and Howarth 1991). This may be due to the high C cost of N fixation compared to soil N acquisition (Rastetter et al. 2001), climate constraints on nitrogenase activity (Houlton et al. 2008), evolutionary factors (Crews 1999; Menge et al. 2009; Sheffer et al. 2015), limitation of symbiotic N fixers by nutrients other than N (Uliassi and Ruess 2002), or all of these. In contrast, work in the boreal suggests that at least in some cases, low soil N availability in late succession can drive higher rates of N fixation (Zackrisson et al. 2004). Further work is, therefore, needed to resolve the role of symbiotic N fixation in plant N nutrition throughout succession, particularly in retrogressive ecosystems where N is depleted.
The natural abundance of stable N isotopes (15N/14N) in plant foliage can be a useful tool for examining plant N-foraging strategies and their response to changes in nutrient availability (Robinson 2001; Hobbie and Hobbie 2006; Houlton et al. 2007). Foliar 15N/14N can be used to estimate the proportion of foliar N derived from mycorrhizal transfer, as mycorrhizal fungi preferentially retain 15N in their biomass and transfer low 15N/14N to their plant hosts, resulting in lower foliar 15N/14N in mycorrhizal vs. non-mycorrhizal plants (Hobbie et al. 2005). Foliar 15N/14N can also be used to calculate the proportion of N derived from symbiotic N fixation, because N derived from atmospheric N2 is often distinguishable from soil N sources. In addition, foliar 15N/14N can be compared to that of specific soil N pools (DON, NH4+, and NO3−) to further constrain plant N sources using probabilistic mixing models (Houlton et al. 2007).
Here, we investigate plant reliance on microbial symbioses to acquire N throughout ecosystem retrogression across a well-studied chronosequence, the Ecological Staircase in Mendocino County, California (Jenny 1973), using natural stable N isotopic techniques. This chronosequence is a model for understanding plant response to nutrient deficiency throughout succession due to an extreme range in ecosystem development and soil fertility across the sequence. At the youngest terraces (100,000–200,000 years old), soils are fertile and have relatively greater concentrations of extractable mineral N forms, and support productive coniferous forests. At the older terraces (300,000–500,000 years old), soils are highly weathered and infertile, and the soil N pool is dominated by organic compounds such as protein–tannin complexes (Northup et al. 1995). Three distinct plant–microbe symbioses that can contribute to plant N acquisition are present at all sites: ectomycorrhizae, ericoid mycorrhizae, and an actinorhizal N-fixation symbiosis between the Frankia actinobacteria and shrub Myrica californica. Although M. californica is also taxonomically capable of forming ectomycorrhizae, it has not been observed to do so at these sites (Wurzburger and Bledsoe 2001).
We test the specific hypothesis that plants increasingly rely on root-associated microbes (ectomycorrhizal fungi, ericoid mycorrhizal fungi, or N-fixing bacteria) to obtain N as soil fertility declines throughout succession and into retrogression. If this is the case, we expect to observe a systematic decline in foliar 15N/14N in ectomycorrhizal and ericoid mycorrhizal plant species across the chronosequence, reflecting greater reliance on N derived from mycorrhizal associates as soil N becomes increasingly held in complex organic forms. We also expect a positive correlation between foliar 15N/14N and foliar N concentration, with increased divergence of plant and soil 15N/14N reflecting isotopic discrimination during N transfer from mycorrhizal fungi to plants. In the N-fixing shrub M. californica, we expect that foliar 15N/14N to approach that of atmospheric N2 when N fixation is an important source of plant N, using the standard mean δ15N of 0‰, where \(\delta^{15} N = \left( {\frac{{\frac{{{}^{15}{\text{N}}}}{{{}^{14}{\text{N}}}}{\text{sample}}}}{{\frac{{{}^{15}{\text{N}}}}{{{}^{14}{\text{N}}}}{\text{atmospheric}} \,{\text{N}}}} - 1} \right) \times 1000\), consistent with N-fixing plant shoot sampling (Unkovich 2013). Further, we hypothesize that ericoid mycorrhizal plants will demonstrate a greater ability to take up soil N via mycorrhizal transfer than ectomycorrhizal plants, especially at the oldest terraces, reflecting the enhanced ability of ericoid mycorrhizal fungi to access N within protein–tannin complexes via enzyme production (Bending and Read 1997a). If this is the case, we expect to observe lower foliar 15N/14N in ericoid mycorrhizal plant species than in ectomycorrhizal plant species, reflecting greater reliance on N transferred from mycorrhizal fungi.
Materials and methods
Study sites
Study sites were located along a well-characterized soil chronosequence known as the Ecological Staircase. The Ecological Staircase is located in the Jug Handle State Reserve near Fort Bragg, CA, which experiences a Mediterranean climate with warm, dry summers and cool, wet winters. The chronosequence consists of five marine-cut terraces formed through a combination of ocean erosion and tectonic uplift, ranging from approximately 100,000–500,000 years in age (Jenny 1973). Soils are formed from beach and eolian deposits overlying greywacke sandstone and are classified as Typic Hapludults at the youngest terraces (T1 and T2) and Aquic Hapludults and Typic Duraquods at the older terraces (T3–T5) (Table S1). One 20 m × 20 m sampling plot was established at each of the five terraces.
Terraces 1 and 2 support productive Bishop pine (Pinus muricata) forests, while terraces 3–5 are characterized by pygmy forests of stunted (< 4 m at 100 years) Bishop pine and Bolander pine (Pinus contorta var. bolanderi), Mendocino cypress (Cupressus pigmea) and a thick shrub understory (dominantly Vaccinium ovatum, Rhododendron macrophyllum, Arctostaphylos nummularia, and Gaultheria shallon). Individual M. californica shrubs are scattered infrequently across all terraces.
Previous research at the Ecological Staircase has shown that the speciation of N in soil changes dramatically when examining subsequently older terraces. The size of the mineral N pool decreases sharply with soil age, from ~ 300 mg mineral N m−2 at the T1 to close to 0 mg mineral N m−2 at T3–T5. At all stages of development, the mineral N pool is dominated by NH4+, with < 10% of the mineral N pool present as NO3−, (Yu et al. 2003). On the other hand, soil-extractable DON concentrations increase from ~ 100 mg N m−2 at the T1 to ~ 3000 mg N m−2 at T3–T5 (Yu et al. 2003). Protein–tannin complexes are a particularly important part of the organic N pool at the pygmy forest terraces and contain 60% of the dissolved soil organic N (Northup et al. 1995), while free amino acids account for only 1.5–10.6% of DON (Yu et al 2002).
Foliage sampling
Current-year, fully expanded foliage was collected using a pole-saw from replicate individual plants of common species that composed the majority of aboveground biomass within the established sampling plots in August 2008. Foliage was collected from ectomycorrhizal plant species: P. muricata, P. contorta ssp. bolanderi; arbutoid mycorrhizal plant species: A. columbiana, A. nummularia; ericoid mycorrhizal plant species: R. macrophyllum, V. ovatum, G. shallon; and the N-fixing shrub M. californica. Symbiotic type was assigned based on plant taxonomy and known mycorrhizal capabilities based on the literature. Because the fungi that form arbutoid mycorrhizae are frequently the same fungi that form ectomycorrhizae in other plants, arbutoid mycorrhizae can be considered a subset of ectomycorrhizae (Brundrett 2004); thus, we assigned A. columbiana and A. nummularia to the ectomycorrhizal plant species category, though they are not technically ectomycorrhizal. Although M. californica is capable of hosting arbuscular mycorrhizal fungi, this association has not been observed at this set of sites (Wurzburger and Bledsoe 2001). Thus, M. californica was not considered to be mycorrhizal in this study.
Only cosmopolitan species found in both fertile (i.e., T1–T2) and retrogressive (i.e., T3–T5) terraces were sampled; hence, Cupressus pigmaea was excluded because it is only found at retrogressive terraces. When possible, foliage samples were collected from at least five individual plants of each species at each plot. When five individuals of a species were not present within a plot, foliage was sampled from as many individuals as were present. The number of individual plants sampled of each species at each terrace is shown in Table S2. While all species sampled in this study were present in fertile and retrogressive terraces, only P. muricata and M. californica were observed in all five sites across the chronosequence.
All foliage samples were placed in paper bags and air-dried upon collection. Upon returning to the lab, undamaged leaves were selected by hand, rinsed three times with Nanopure water (Thermo Scientific, Waltham, MA, USA) to remove dust and leaf debris, and dried for three days at 50 °C. Replicate leaves collected from individual plants were then ground to a fine powder and homogenized using a Wiley mill followed by ball mill pulverization. Powdered samples were analyzed for C concentration, 13C/12C, N concentration, and 15N/14N using an PDZ Europa ANCA-GSL elemental analyzer (Sercon Ltd., Cheshire, UK) interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) at the UC Davis Stable Isotope Facility. Sample isotope ratios were corrected for instrument variation between runs using laboratory standards that were previously calibrated against NIST Standard Reference Materials. Significant differences in foliar chemistry between plant species and across sites were determined via a two-way ANOVA with species and site as factors, followed by Tukey’s honestly significant difference post hoc test. Our choice of ANOVA as a statistical test is based on a wealth of biogeochemical studies that have also used ANOVA to compare single sites spanning a wide biogeochemical gradient arrayed along a time axis (e.g., Vitousek 2004), including numerous such studies at this same chronosequence (e.g., Yu et al. 2003; Izquierdo et al. 2013; Winbourne and Houlton 2018). Linear regression was used to analyze the relationship between foliar ẟ15N and N concentration.
Soil collection and analysis
In April 2015, ten soil samples were collected from each sampling plot from the top 0–10 cm mineral horizon (the depth of greatest soil N based on previous sampling of soil pits at these sites) using a hand coring device and placed in a plastic bag. At each sampling plot, five of the soil samples were collected from underneath known mycorrhizal plant species, and five of the soil samples were collected from underneath M. californica individuals. At each terrace, concentrations and isotopic composition of N in bulk soil and individual pools did not significantly differ between the soil samples collected underneath mycorrhizal plant species and the soil samples collected underneath M. californica individuals, so soil data at each terrace were pooled prior to statistical analyses. Soil samples were collected from no closer than 3 m and sampling locations were strategically chosen to be representative of the sampling plot. All soil samples were immediately sieved to 2 mm and 10 g was subsampled for soil solution extraction. Soil solution was extracted with 50 mL of 2 M potassium chloride (KCl) and frozen immediately.
Samples were returned to the lab at UC Davis and a subsample of each field-moist soil sample was oven-dried and field moisture content was determined by mass difference. The remaining soil samples were air-dried and ground to a fine powder using a ball mill. Ground soil samples were weighed into tin capsules on a microbalance before being submitted to the UC Davis Stable Isotope Facility. C concentration, 13C/12C, N concentration, and 15N/14N were measured using an Elementar Vario EL Cube (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). Significant differences in soil chemistry between sites were determined via one-way ANOVA followed by Tukey’s honestly significant difference post hoc test.
Comparing ẟ15N between foliar samples and soil samples that were collected 7 years apart required the assumption that each site was near steady state on this timeframe, with little foliar ẟ15N variation from year to year. To validate this assumption, we collected additional foliage from selected species at each terrace in 2016 (data not shown). Within individual plant species, foliar ẟ15N did not vary significantly between 2008 and 2016, supporting this assumption.
Analysis of soil solution extracts
NH4+ concentrations of soil solution extracts were measured using the indophenol formation colorimetric method described in Verdouw et al. (1978). NO3− concentrations of soil solution extracts were quantified using the vanadium reduction colorimetric method described in Doane and Horwath (2003). Total dissolved N (TDN) in soil solution extracts was determined by a modified persulfate oxidation protocol, which oxidizes all dissolved N species to NO3− (Knapp et al. 2005), followed by vanadium reduction colorimetry. All concentrations were adjusted for soil moisture and converted to µg N g−1 dry soil.
Because no quantifiable NO3− could be detected in the extracts, N isotopes were only determined for the NH4+ and TDN pools. To determine the δ15N of TDN, the persulfate-digested TDN samples were submitted to the UC Davis Stable Isotope Facility for 15N analysis of NO3− in water using the denitrifier method.
To determine the δ15N of NH4+, the NH4+ in each extract was converted to NO3− using the acid trap diffusion method followed by persulfate oxidation (Sigman et al. 1997; Houlton et al. 2007). The NO3− concentration in each sample was measured colorimetrically using the vanadium reduction method. δ15N of NO3− was determined using the denitrifier method (Sigman et al. 2001) at the UC Davis Stable Isotope Facility. Significant differences in concentration and δ15N between extractable soil N pools and across sites were determined via two-way ANOVA with pool and site as factors, followed by Tukey’s honestly significant difference post hoc test.
Isotopic blanks and standards
Nitrogen isotope composition of dissolved N forms (TDN, NH4+) was converted to NO3− via persulfate oxidation (see above). Hence, method blanks were included for every step of the extraction, oxidation, and/or acid trap diffusion followed by oxidation processes and analyzed for NO3− concentration and isotopic ratio as with the rest of the samples: 2.0 M KCl blanks, persulfate blanks, and diffusion blanks. Sample TDN concentrations were adjusted by subtracting the average measured NO3− concentration of KCl blanks that were oxidized along with the rest of the samples. Sample NH4+ concentrations were adjusted by subtracting the average measured NO3− concentration of KCl blanks that were subjected to acid trap diffusion and then oxidized along with the rest of the samples. In all cases, the background concentration of TDN and NH4+ in the KCl blanks constituted < 20% of the actual sample concentration. The δ15N of TDN and NH4+ samples were corrected for the δ15N of KCl blanks using the following equation:
To check the accuracy of isotopic results, USGS-26 ammonium sulfate standard (δ15N = 53.75‰\()\) was treated as a sample for both the persulfate oxidation and the acid trap diffusion followed by persulfate oxidation procedures. The measured δ15N of the standard was always < 4% different from the expected δ15N.
Soil solution extract δ15N calculations
The concentration of DON was calculated using the following equation:
The δ15N of DON was then calculated using the following equation:
Isotopic mixing model
Foliar δ15N was corrected for internal N transfer. Assuming an isotope effect of internal fractionation of 4‰, plant steady state of N uptake and loss, and an equal proportion of belowground and aboveground losses, foliar δ15N should be 2‰ higher than source N (Houlton et al. 2007):
We considered four possible sources of foliar N:soil NH4+, soil DON, NH4+-N transferred from mycorrhizae and DON-N transferred from mycorrhizal fungi. To calculate the δ15N of mycorrhizal transferred N, the isotope effect of mycorrhizal transfer (\(\Delta\)) was assumed to be 9‰ (Hobbie and Hobbie 2006; Hobbie et al. 2008), and the δ15N of N from mycorrhizal transfer was estimated using the following equation:
Fractional contributions of direct soil uptake and mycorrhizal transfer to foliar N were calculated using the Stable Isotope Analysis in R package (SIAR). SIAR uses a Markov Chain Monte Carlo framework to calculate the fractional contribution (f, expressed as a decimal fraction) of each isotopic source (direct soil DON uptake, direct soil NH4+ uptake, mycorrhizal transfer of DON-N, mycorrhizal transfer of NH4+-N) to the mixture (total N of plant foliage). This model accounts for uncertainty in the isotopic values of mixtures and sources. The model input was the recycling-corrected foliar δ15N of each sample, the mean and standard deviation δ15N of each N source at each terrace (Tables S3–S4). Foliar samples were grouped by terrace and mycorrhizal type (ectomycorrhizal and ericoid mycorrhizal), and the model was run for 500,000 iterations, with the initial 50,000 iterations discarded. The model output was an estimated mean f for each N source at each terrace for both mycorrhizal types, as well as an estimated 95% confidence interval for each f, which takes into account uncertainty of isotopic sources as well as variability between plants in each group (Table S5). Non-overlapping confidence intervals were interpreted to indicate significantly different f values.
The full mixing model included four potential N sources (i.e., unknowns) and one isotopic system (known), resulting in a mathematically undetermined system of equations (Houlton et al. 2007). This resulted in model runs with significant uncertainty, in which the different potential sources were not readily distinguished (Table S5, Fig. S1). To facilitate analysis, a simplified model was run using the same parameters and input mixtures, but with the N sources simplified to soil TDN (without differentiating between DON and NH4+) and mycorrhizal transfer TDN (Tables S6–7). This reduced the uncertainty of estimated f values, and is likely more representative of biological processes, as fungal mixing and processing of soil N occurs prior to transfer to plant hosts (Hobbie and Hobbie 2006). Our analysis thus focused on this simplified model.
The same modeling techniques were used to calculate fractional contributions of direct soil uptake and symbiotic N fixation in M. californica, except the N sources were either soil TDN or atmospheric N (Tables S8–10).
All mixing models were also run without applying the 2‰ correction factor to foliar δ15N values to test the validity of applying the correction factor (Tables S11–S13, Figs. S2–S4). Because similar results were obtained using the corrected vs. the uncorrected data, the uncorrected foliar δ15N values were outside the range of the source δ15N values causing model errors, and the 2‰ correction factor has been validated in previous studies (Houlton et al. 2007), we chose to use corrected foliar δ15N values in our analysis.
Results
Foliar δ 15 N across terraces
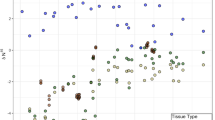
Ericoid mycorrhizal plant foliar δ15N significantly declined between T2 and T3–T5 (Fig. 1, F3,93 = 23.897, p < 0.001), ectomycorrhizal plant foliar δ15N significantly declined between T1 and T2–T5 (Fig. 1, F4,71 = 22.769, p < 0.001), and N-fixing foliar δ15N significantly declined between T1–T2 and T3–T5 (Fig. 1, F4,25 = 13.016, p < 0.001). There was a significant correlation between δ15N and N concentration in ectomycorrhizal plant foliage (Fig. 2), (R2 = 0.18, F1,77 = 16.65, p < 0.001) and N-fixing plant foliage (Fig. 2, R2 = 0.18, F1,28 = 6.35, p = 0.018), but not in ericoid mycorrhizal plant foliage (Fig. 2, R2 = 0.001, F1,92 = 0.10, p = 0.75). Additional foliar chemistry data are shown in Table S14.
Mean ± SD δ15N (‰) in soil total dissolved nitrogen (black diamonds), ectomycorrhizal plant foliage (open circles), ericoid mycorrhizal plant foliage (black circles), N-fixing plant foliage (gray triangles) across the Ecological Staircase chronosequence. The terraces shown on the x-axis increase in age from ca. 100 ky at terrace 1 to ca. 500 ky at terrace 5. The vertical dashed line divides the younger, fertile terraces from the retrogressive-phase pygmy forest terraces. Ericoid mycorrhizal plant foliar δ15N significantly declined between terrace 2 and terraces 3–5 (F3,93 = 23.897, p < 0.001), ectomycorrhizal plant foliar δ15N significantly declined between terrace 1 and terrace 2–5 (F4,71 = 22.769, p < 0.001), and N-fixing plant foliar δ15N significantly declined between terraces 1–2 and terraces 3–5 (F4,25 = 13.016, p < 0.001). No ericoid mycorrhizal plants were present at terrace 1
Foliar δ15N (‰) plotted against foliar N concentration (%) in ectomycorrhizal plant foliage (open circles and solid regression line), ericoid mycorrhizal plant foliage (black circles and short-dash regression line), and N-fixing plant foliage (gray triangles and long-dash regression line). Linear regression was used to analyze the relationship between foliar δ15N and foliar N concentration within each symbiotic type individually. There is a significant correlation between δ15N and N concentration in ectomycorrhizal plant foliage (R2 = 0.18, F1,77 = 16.65, p < 0.001) and N-fixing plant foliage (R2 = 0.18, F1,28 = 6.35, p = 0.018), but not in ericoid mycorrhizal plant foliage (R2 = 0.001, F1,92 = 0.10, p = 0.75)
Soil chemistry across terraces
DON was the most abundant form of soil-extractable N, with NH4+ concentrations consistently low, and no detectable NO3− at any of the terraces (Table 1). There was significantly more DON than NH4+ at all terraces (F1,90 = 124.827, p < 0.001), although there was no significant change in the size of either N pool across the chronosequence (F4,90 = 1.898, p = 0.118). The δ15N of DON was significantly higher than the δ15N of NH4+ by ~ 2–5 per mil across sites (F1,90 = 65.169, p < 0.001); neither δ15N of DON or NH4+ changed significantly across the chronosequence (Table 1, F4,90 = 1.848, p = 0.127). Additional soil chemistry data are shown in Table S15.
Foliar δ15N isotopic mixing models
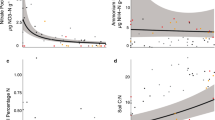
Foliar δ15N was consistently lower than bulk soil δ15N and the δ15N of all soil-extractable N pools across sites (Fig. 1). In ectomycorrhizal plant species, the estimated percentage of foliar N derived from mycorrhizal transfer (fmycorrhizal) ranged from 54% ± 0.10% at the T1 to 78% ± 15% at T5, and exhibited significantly higher fmycorrhizal at T3–5 vs. at T1 (Fig. 3). In ericoid mycorrhizal plant species, fmycorrhizal ranged from 68% ± 11% at the T1 to 97% ± 5% at T5, and increased significantly between T2 (68% ± 11%) and T3 (97% ± 5%) (Fig. 3). At T2, both ericoid mycorrhizal plant foliage and ectomycorrhizal plant foliage displayed similar fmycorrhizal values (68% ± 11% in ericoid mycorrhizal species and 66% ± 22% in ectomycorrhizal species). At T3–T5, fmycorrhizal in ericoid mycorrhizal plant foliage was higher than fmycorrhizal in ectomycorrhizal plant foliage (~ 96% vs. ~ 75%), though this difference was only statistically significant at T3.
Proportion of foliar N derived from mycorrhizal transfer (fmycorrhizal) in ectomycorrhizal (open circles) and ericoid mycorrhizal (filled circles) plant foliage. Values are shown as the mean calculated fractional contribution of each N source calculated using a Markov Chain Monte Carlo framework in SIAR. Error bars represent the 95% confidence interval for f estimates, which take into account uncertainty of isotopic sources as well as variability between plants in each group. Non-overlapping confidence intervals indicate significantly different f estimates. The vertical dashed line divides the younger, fertile terraces from the retrogressive-phase pygmy forest terraces
The fraction of M. californica foliar N derived from N fixation (ffixation) ranged from 76% ± 17% at T1 to 98% ± 4% at T5 (Fig. 4). At T3, ffixation was significantly higher than at T1–T2. At the retrogressive terraces (T3-T5), ffixation was uniformly high (> 85%) and not significantly different between terraces.
Proportion of foliar N derived from atmospheric N fixation (ffixation) in N-fixing plant foliage (gray triangles). Values are shown as the mean calculated fractional contribution of each N source calculated using a Markov Chain Monte Carlo framework in SIAR. Error bars represent the 95% confidence interval for f estimates, which take into account uncertainty of isotopic sources as well as variability between plants in each group. Non-overlapping confidence intervals indicate significantly different f estimates. The vertical dashed line divides the younger, fertile terraces from the retrogressive-phase pygmy forest terraces
Discussion
Plant reliance on microbial partnerships across the chronosequence
Our findings support the hypothesis that plants of various symbiotic types rely heavily on microbial partners to access diverse N forms throughout ecosystem succession and retrogression. Across all sites, plants with all types of microbial associates (ectomycorrhizal, ericoid mycorrhizal, and N-fixing) obtain a significant fraction of their foliar N via symbiotic pathways, with increasing reliance on microbial partners in the retrogressive pygmy forest ecosystems (i.e., T3–T5). In ectomycorrhizal plant species, foliar N concentrations are significantly correlated with foliar δ15N, indicating that when N is more limiting, plant foliage expresses a greater mycorrhizal transfer isotopic signature (Fig. 2). However, this relationship is not observed in ericoid mycorrhizal plant species, suggesting that N obtained from mycorrhizal fungi may prevent their plant hosts from experiencing N limitation.
These results support past work hypothesizing that declines in nutrient availability may drive plants at retrogressive terraces to rely more heavily on mycorrhizae for N acquisition. Past studies have utilized fungal DNA sequencing to examine changes in ectomycorrhizal fungi traits across the Ecological Staircase chronosequence, and found that ectomycorrhizal fungi are more abundant at the retrogressive pygmy forest terraces and exhibit more C-intensive nutrient foraging traits than ectomycorrhizal fungi at the younger terraces (Moeller et al. 2014; Courty et al. 2018). This may be reflective of greater tree nutrient limitation, and thus greater investment in ectomycorrhizae, at the pygmy forest sites. Our findings provide a non-disruptive test of such previous hypotheses and show that mycorrhizae indeed supply a significantly greater fraction of plant N at the highly retrograded and stunted forest pygmy forest sites.
Ericoid mycorrhizal fungi are predicted to dominate over ectomycorrhizal fungi in late-successional and retrogressive ecosystems (Read 1991; Lambers et al. 2008). Several factors contribute to this expectation, including the capacity for such root-associates to degrade protein–tannin complexes (Bending and Read 1997a), thereby outcompeting ectomycorrhizal fungi for soil N when protein–tannin complexes are prevalent (Yu et al. 1999). This could result in a plant–soil feedback wherein ericaceous plants produce more foliar tannins and polyphenols when growing in acidic and infertile environments (Kraus et al. 2004); upon litterfall, these compounds can then be mineralized and taken back up by ericoid mycorrhizal fungi, contributing to a conservative soil N cycle (Chapman et al. 2005; Wurzburger and Hendrick 2009; Phillips et al. 2013). This alteration of the N cycle has been hypothesized as allowing ericaceous plants to monopolize the soil N economy in acidic and infertile soils (Northup et al. 1998; Yu et al. 2002). Indeed, past research at this chronosequence has demonstrated greater ericoid mycorrhizal colonization and decreased ectomycorrhizal colonization at the oldest terraces (Wurzburger and Bledsoe 2001). In accordance, our results reveal that ericoid mycorrhizal plants at the oldest terraces obtain significantly more of their N from mycorrhizal transfer than do ectomycorrhizal plants. Although our approach cannot address if this shift translates to a significantly greater gross amount of N, the fact that ericoid mycorrhizal plants derived nearly all of their foliar N from fungal transfer at the retrogressive-stage sites is suggestive of a closed, conservative N economy.
Symbiotic N fixation
A body of research maintains that symbiotic N fixation is not likely to be favorable in late-successional temperate and boreal forest environments (Rastetter et al. 2001). Although the N-fixing shrub M. californica is not a dominant species at the Ecological Staircase, we found that those individuals present are heavily reliant on symbiotic N fixation, particularly in late succession. As hypothesized, M. californica foliar ẟ15N approaches 0‰ as site age increased, indicating an increased contribution of atmospheric N to foliar N. The isotopic mixing model reveals similar results, estimating that 98% of foliar N is derived from N fixation at the oldest site. Symbiotic N fixers are known to have a high demand for soil resources other than N, especially P, mostly to support the higher rates of photosynthesis needed to supply N-fixing bacteria with C (Augusto et al. 2013). The soils of the Ecological Staircase chronosequence are extremely low in available forms of P (Izquierdo et al. 2013), which may limit photosynthesis by M. californica. P limitation has been shown to limit free-living N fixation at the Ecological Staircase (Winbourne and Houlton 2018) and symbiotic N fixation during primary succession in Alaska (Uliassi and Ruess 2002); this could explain why M. californica is not a dominant species at the oldest terraces, despite its ability to access atmospheric N. However, our study clearly demonstrates that those M. californica individuals that do succeed in this nutrient-poor environment rely heavily on atmospheric N access made possible by their root symbionts. This supports past research in boreal forests showing increased N fixation in later successional stages (Zackrisson et al. 2004), and calls into question the assumption that all symbiotic N fixation ceases in older ecosystems, despite low overall abundance of symbiotic N-fixing trees at high latitudes (Vitousek and Howarth 1991).
Assumptions, limitations, and future research
A number of assumptions were applied to our isotopic modeling approach. We assumed that our study sites are at steady state, and that foliar and soil δ15N values, therefore, reflect quasi-equilibrium conditions across each site. We assumed the isotope effect of mycorrhizal transfer was 9‰, based on an 8–10‰ range estimated by Hobbie and Hobbie (2006). However, as we were not able to directly measure the isotope effect of mycorrhizal transfer, the actual isotope effect may differ from our estimate. Implicit in our simplified mixing model for mycorrhizal plant functional types are the assumptions that there is a uniform δ15N value for the entire DON pool at each site, and that all forms of DON (e.g., amino acids, peptides, and protein–tannin complexes) are equally available for direct plant root and mycorrhizal uptake. Previous research has suggested that the δ15N accessible to ectomycorrhizal fungi does not differ from that of bulk DON (Hobbie et al. 2000). However, given the large differences in DON between T2 and T3 (from ~ 25 µg DON-N g−1 dry soil at T2 to ~ 15 µg DON-N g−1 dry soil at T3, Table 1), it is likely that not all DON is available for plant uptake, resulting in hydrologic losses throughout soil development. Although the methods used here cannot distinguish between available and unavailable DON, isotopic depletion of foliar δ15N values to that of soil N pools points to the likelihood of mycorrhizal transfer as a foliar N source, as observed in numerous studies of tundra ecosystems (Hobbie and Hobbie 2006; Hobbie and Högberg 2012). Given the importance of protein–tannin complexes in N cycling at the ecological staircase, future research into the availability and isotopic signature of different organic N compounds, particularly protein–tannin complexes, is worth pursuing.
Our calculations assume that changes in foliar δ15N not explained by variation in soil available δ15N were due to isotopic discrimination against 15N via mycorrhizal transfer of N to plant hosts (∆). However, when significant plant N is derived from mycorrhizal transfer, foliar δ15N can be affected by discrimination during transfer as well as by the amount of N that mycorrhizae transfer to their plant hosts (Hobbie et al. 2005). Nutrient limitation can prompt mycorrhizal fungi to sequester a greater proportion of their N in their biomass, rather than transferring it to their plant hosts (Hobbie and Agerer 2010), thus causing foliar δ15N to be lower. Previous studies of mycorrhizal nutrient transfer have similarly found that mycorrhizal fungi can regulate how much N they transfer to their plant hosts (Albarracín et al. 2013). At the old, retrogressive-stage sites, mycorrhizal fungi may, therefore, decrease the amount of N that they allocate to plants, which could also be responsible for the observed declines in foliar δ15N at those sites.
Conclusions
Our study clearly demonstrates the importance of plant–microbe interactions in maintaining aboveground productivity throughout ecosystem succession, particularly in retrogressive ecosystems. At retrogressive-phase sites, plant–microbe associations involving mycorrhizal fungi and N-fixing bacteria provided the majority of plant N. This work supports illustrates the critical role that plant–microbe symbioses play in maintaining aboveground ecosystem function and productivity in response to environmental stressors (Phillips et al. 2013; Averill et al. 2014; Pena and Polle 2014), and serves as a model for exploring the biogeochemical limits to which plant–soil–microbe interactions can maintain ecosystem functioning.
References
Albarracín MV, Six J, Houlton BZ, Bledsoe CS (2013) A nitrogen fertilization field study of carbon-13 and nitrogen-15 transfers in ectomycorrhizae of Pinus sabiniana. Oecologia 173:1439–1450
Augusto L, Delerue F, Gallet-Budynek A, Achat DL (2013) Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Global Biogeochem Cycles 27:1–12
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545
Bending GD, Read DJ (1997a) Nitrogen mobilization from protein-polyphenol complex by ericoid and ectomycorrhizal fungi. Soil Biol Biochem 28:1603–1612
Bending GD, Read DJ (1997b) Lignin and soluble phenolic degradation by ectomycorrhizal and ericoid mycorrhizal fungi. Mycol Res 101:1348–1354
Brundrett M (2004) Diversity and classification of mycorrhizal associations. Biol Rev 79(3):473–495. https://doi.org/10.1017/S1464793103006316
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Chapman SK, Langley JA, Hart SC, Koch GW (2005) Plants actively control nitrogen cycling: uncorking the microbial bottleneck. New Phytol 169:27–34
Courty PE, Buée M, Tech JJT, Brulé D, Colin Y, Leveau JHJ, Uroz S (2018) Impact of soil pedogenesis on the diversity and composition of fungal communities across the California soil chronosequence of Mendocino. Mycorrhiza 28:343–356
Crews TE (1999) The presence of nitrogen fixing legumes in terrestrial communities: evolutionary vs ecological considerations. Biogeochemistry 46:233–246
Dickie IA, Martínez-García LB, Koele N, Grelet GA, Tylianakis JM, Peltzer DA, Richardson SJ (2013) Mycorrhizas and mycorrhizal fungal communities throughout ecosystem development. Plant Soil 367:11–39
Doane TA, Horwath WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722
Fisher JB, Sitch S, Malhi Y, Fisher RA, Huntingford C, Tan S-Y (2010) Carbon cost of plant nitrogen acquisition: a mechanistic, globally applicable model of plant nitrogen uptake, retranslocation, and fixation. Global Biogeochem Cycles 24:1–17
Hobbie EA, Agerer R (2010) Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327:71–83
Hobbie JE, Hobbie EA (2006) 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in arctic tundra. Ecology 87:816–822
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196:367–382
Hobbie EA, Macko SA, Williams M (2000) Correlations between foliar delta N-15 and nitrogen concentrations may indicate plant-mycorrhizal interactions. Oecologia 122:273–283
Hobbie EA, Jumpponen A, Trappe J (2005) Foliar and fungal 15 N:14 N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: testing analytical models. Oecologia 146:258–268
Hobbie EA, Colpaert JV, White MW, Ouimette AP, Macko SA (2008) Nitrogen form, availability, and mycorrhizal colonization affect biomass and nitrogen isotope patterns in Pinus sylvestris. Plant Soil 310:121–136
Houlton BZ, Sigman DM, Schuur EA, Hedin LO (2007) A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc Natl Acad Sci USA 104:8902–8906
Houlton BZ, Wang Y-P, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330
Izquierdo JE, Houlton BZ, van Huysen TL (2013) Evidence for progressive phosphorus limitation over long-term ecosystem development: Examination of a biogeochemical paradigm. Plant Soil 367:135–147
Jenny H (1973) The pygmy forest ecological staircase: a description and interpretation. Monograph University of California, Berkeley, Ca
Knapp AN, Sigman DM, Lipschultz F (2005) N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic Time-series study site. Global Biogeochem Cycles 19:1–15
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems—a review. Plant Soil 256:41–66
Kraus TEC, Zasoski RJ, Dahlgren RA (2004) Fertility and pH effects on polyphenol and condensed tannin concentrations in foliage and roots. Plant Soil 262:95–109
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Leopold DR (2016) Ericoid fungal diversity: challenges and opportunities for mycorrhizal research. Fungal Ecology 24:114–123
Menge DN, Levin SA, Hedin LO (2009) Facultative versus obligate nitrogen fixation strategies and their ecosystem consequences. Am Nat 174:465–477
Menge DNL, Hedin LO, Pacala SW (2012) Nitrogen and phosphorus limitation over long-term ecosystem development in terrestrial ecosystems. PLoS One 7:1–17
Moeller HV, Peay KG, Fukami T (2014) Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol Ecol 87:797–806
Northup R, Dahlgren R, Yu Z (1995) Intraspecific variation of conifer phenolic concentration on a marine terrace soil acidity gradient; a new interpretation. Plant Soil 171:255–262
Northup RR, Dahlgren RA, McColl JG (1998) Polyphenols as regulators of plant-litter-soil interactions in northern California’s pygmy forest: a positive feedback? Biogeochemistry 42:189–220
Peltzer DA, Wardle DA, Allison VJ, Baisden WT, Bardgett RD, Chadwick OA, Condron LM, Parfitt RL, Porder S, Richardson SJ, Turner BL, Vitousek PM, Walker J, Walker LR (2010) Understanding ecosystem retrogression. Ecol Monogr 80:509–529
Pena R, Polle A (2014) Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J 8:321–330
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy : a new framework for predicting carbon—nutrient couplings in temperate forests. New Phytol 199:41–51
Qualls RG, Haines BL, Swank WT (1991) Fluxes of dissolved organic nutrients and humic substances in a deciduous forest. Ecology 72:254–266
Rains KC, Bledsoe CS (2007) Rapid uptake of 15N-ammonium and glycine-13C, 15N by arbuscular and ericoid mycorrhizal plants native to a Northern California coastal pygmy forest. Soil Biol Biochem 39:1078–1086
Rastetter E, Vitousek P, Field C (2001) Resource optimization and symbiotic nitrogen fixation. Ecosystems 4:369–388
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 8:591–602
Sheffer E, Batterman SA, Levin SA, Hedin LO (2015) Biome-scale nitrogen fixation strategies selected by climatic constraints on nitrogen cycle. Nat Plants 1:15182
Sigman DM, Altabet MA, Michener R, McCorkle DC, Fry B, Holmes RM (1997) Natural abundance-level measurement of the nitrogen isotopic composition of oceanic nitrate: an adaptation of the ammonia diffusion method. Mar Chem 57:227–242
Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Böhlke JK (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73:4145–4153
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Uliassi DD, Ruess RW (2002) Limitations to symbiotic nitrogen fixation in primary succession on the Tanana river floodplain. Ecology 83:88–103
Unkovich M (2013) Isotope discrimination provides new insight into biological nitrogen fixation. New Phytol 198:643–646
van der Putten WH (2017) Belowground drivers of plant diversity. Science 355:134–135
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Verdouw H, Van Echteld CJA, Dekkers EMJ (1978) Ammonia determination based on indophenol formation with sodium salicylate. Water Res 12:399–402
Vitousek P (2004) Nutrient Cycling and Limitation: Hawai’i as a Model System. Page In: Molina MJ, Morel FMM, Morgan JJ, Tilman D (eds) Princeton University Press
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Vitousek P, Cassman K, Cleveland CC, Crews TE, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli LA, Rastetter EB, Sprent JI (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57(58):1–45
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–512
Winbourne JB, Houlton BZ (2018) Plant-soil feedbacks on free-living nitrogen fixation over geological time. Ecology 99:2496–2505
Wurzburger N, Bledsoe CS (2001) Comparison of ericoid and ectomycorrhizal colonization and ectomycorrhizal morphotypes in mixed conifer and pygmy forests on the northern California coast. Can J Bot 79:1202–1210
Wurzburger N, Hendrick RL (2009) Plant litter chemistry and mycorrhizal roots promote a nitrogen feedback in a temperate forest. J Ecol 97:528–536
Yu Z, Dahlgren RA, Northup RR (1999) Evolution of soil properties and plant communities along an extreme edaphic gradient. European Journal of Soil Biology 35:31–38
Yu Z, Zhang Q, Kraus T, Dahlgren R, Anastasio C, Zasoski RJ (2002) Contribution of amino compounds to dissolved organic nitrogen in forest soils. Biogeochemistry 61:173–198
Yu Z, Kraus T, Dahlgren R, Horwath W, Zasoski R (2003) Mineral and dissolved organic nitrogen dynamics along a soil acidity-fertility gradient. Soil Sci Soc Am J 67:878–888
Zackrisson O, Deluca TH, Nilsson M, Sellstedt A, Berglund LM, Zackrisson O (2004) Nitrogen fixation increases with successional age in boreal forests. Ecology 85:3327–3334
Acknowledgements
We thank Joyce Wong, Laura Losmozos, Christina Day, Andy Parks, Elisa Fernandes-McDade, Emma Hansen-Smith, Sara Hutton, Avery Kruger, and Kelly Norris for their assistance in sample collection. This study was funded by National Science Foundation Grant DGE-1148897 awarded to KAD and DEB-1150246 awarded to BZH.
Author information
Authors and Affiliations
Contributions
KAD and BZH designed the research and the methodology. KAD conducted the fieldwork and laboratory work, analyzed the data, and wrote the original manuscript draft. KAD and BZH revised the manuscript.
Corresponding author
Additional information
Communicated by Hakan Wallander.
Here, we use stable N isotopes to quantify the contribution of plant-microbe partnerships to forest N supplies throughout succession. We demonstrate that symbioses are critical to plant nutrition in the retrogressive ecosystems of late-stage succession, where soil nutrient availability is especially low.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dynarski, K.A., Houlton, B.Z. Isotopic constraints on plant nitrogen acquisition strategies during ecosystem retrogression. Oecologia 192, 603–614 (2020). https://doi.org/10.1007/s00442-020-04606-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04606-y