Abstract
Carbon cycling associated with biological soil crusts, which occupy interspaces between vascular plants in drylands globally, may be an important part of the coupled climate-carbon cycle of the Earth system. A major challenge to understanding CO2 fluxes in these systems is that much of the biotic and biogeochemical activity occurs in the upper few mm of the soil surface layer (i.e., the ‘mantle of fertility’), which exhibits highly dynamic and difficult to measure temperature and moisture fluctuations. Here, we report a multi-sensor approach to simultaneously measuring temperature and moisture of this biocrust surface layer (0–2 mm), and the deeper soil profile, concurrent with automated measurement of surface soil CO2 effluxes. Our results illuminate robust relationships between biocrust water content and field CO2 pulses that have previously been difficult to detect and explain. All observed CO2 pulses over the measurement period corresponded to surface wetting events, including when the wetting events did not penetrate into the soil below the biocrust layer (0–2 mm). The variability of temperature and moisture of the biocrust surface layer was much greater than even in the 0–5 cm layer of the soil beneath the biocrust, or deeper in the soil profile. We therefore suggest that coupling surface measurements of biocrust moisture and temperature to automated CO2 flux measurements may greatly improve our understanding of the climatic sensitivity of carbon cycling in biocrusted interspaces in our study region, and that this method may be globally relevant and applicable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The sensitivity of carbon (C) cycling to climate change in global drylands influences the interannual variability and decadal trajectory of the global terrestrial carbon sink (Ahlström et al. 2015; Poulter et al. 2014). In contrast to many other terrestrial ecosystems, drylands exhibit low cover of vascular plants, where patches of shrubs and perennial grasses form “islands of fertility” (Schlesinger and Pilmanis 1998), and high cover of interspaces dominated by biological soil crusts (biocrusts) form “mantles of fertility” (Garcia-Pichel et al. 2003). Biocrusts are photosynthetic soil surface communities composed of some mix of cyanobacteria, lichens, algae, bryophytes, and heterotrophs concentrated in the top few millimeters of soil (Belnap et al. 2016). Biocrusts create a thin surface layer of high biotic activity, with high C and nutrient pools and fluxes relative to deeper soil layers where the soil tends to be biotically and biogeochemically less active (Pointing and Belnap 2012). Accordingly, understanding C and nutrient cycling within drylands requires explicit consideration of the dynamics of this narrow band of activity.
A challenging aspect of such consideration is the extremely dynamic and difficult to measure temperature and moisture fluctuations of the biocrust surface layer. Concomitantly, measuring pulse-driven fluxes of CO2 in concert with surface temperature and moisture measurements has proven challenging, yet is also likely critical to our understanding of the climatic sensitivity of dryland C cycling (Austin et al. 2004; Huxman et al. 2004). Wetting events that do not penetrate below the biocrust surface layer can have enormous consequences for community composition and biogeochemistry of dryland ecosystems (Austin et al. 2004; Cable and Huxman 2004; Coe et al. 2012; Darrouzet-Nardi et al. 2015; Reed et al. 2012). For example, in a Spanish site similar to our Colorado Plateau ecosystem, biocrusted microsites accounted for a larger percentage of soil CO2 efflux than microsites with vascular plants (Castillo-Monroy et al. 2011), such that climate controls on biocrust CO2 effluxes may determine much of the ecosystem C balance.
Here, we report a multi-sensor approach to measuring temperature and moisture of the upper few millimeters of biocrusted soils (Weber et al. 2016), with simultaneous measurements of temperature and moisture of the deeper soil profile, and with automated measurement of surface CO2 effluxes. The purpose of this study was to evaluate the use of a coupled vertical profile soil microclimate sensor × surface CO2 efflux chamber approach during a time when pulse precipitation events were likely. We hypothesized that soil surface microclimate would be decoupled from that of deeper soils during certain pulse events, and that a vertically-resolved view of soil microclimate would improve our understanding of CO2 fluxes from biocrusted soils.
Materials and methods
Site description
Our study was conducted in a cool desert ecosystem on the Upper Colorado Plateau (36.675N, −109.416W; near Castle Valley, UT, USA) at an elevation of 1310 m above sea level. Mean annual temperature for the surrounding area is 13 °C and mean annual precipitation is 269 mm, of which 65% comes in the winter and spring (based on 1981–2010 data; WRCC 2014). The location is the site of an ongoing long-term climate manipulation experiment, which has explored the role of temperature and altered precipitation in regulating dryland communities and their function (e.g., Ferrenberg et al. 2015; Reed et al. 2012; Wertin et al. 2015). The climate manipulation plots also maintained multiple years of automated CO2 measurements (Darrouzet-Nardi et al. 2015), although very shallow (i.e., 0–2 cm) soil climate was not assessed. The vascular plant community at the site is dominated by the native C3 perennial grass Achnatherum hymenoides, the native C4 perennial grass Pleuraphis jamesii, the native C4 perennial shrub Atriplex confertifolia, and the exotic invasive C3 grass Bromus tectorum (Wertin et al. 2015). Soils at the site are relatively shallow (with exposed bedrock in places) and are classified as sandy loam, calcareous, Rizno series Aridisols. The biocrust community at the site is dominated by lightly-pigmented cyanobacterium (Microcoleus vaginatus), darkly-pigmented cyanobacteria (Scytonema spp.), the cyanolichens Collema tenax and C. coccophorum, and the moss Syntrichia caninervis. We chose four biocrusted interspaces to install CO2 flux chambers (Fig. 1), as well as a vertically-refined soil profile system for soil temperature and moisture measurements (Fig. 2). Chambers 1, 2, and 4 were dominated by Syntrichia caninervis with lower abundance of other mosses, lichens, and cyanobacteria, while chamber 3 had a greater relative abundance of darkly-pigmented cyanobacteria and an otherwise similar biocrust community composition.
The research site along with three LI-8100-104 opaque automated soil CO2 flux chambers. Each chamber was installed in biocrusted interspaces >5 cm from the nearest perennial grass or shrub canopy. During installation, rock walkways were installed to allow access to each chamber while minimizing repeated soil surface disturbance near the chamber because of concerns about minimizing dust production
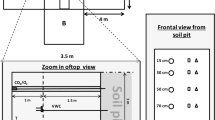
Diagram of the soil CO2 efflux collars, and the temperature and soil moisture measurement system. The surface biocrust layer is green, the subsurface soil layer is brown. The interface between biocrust and deeper soil is considered the 0 cm soil depth. The shown distances between the collar and different probes are for convenience, in reality the CS 650 probes were installed approximately 1 m from each collar, and the CS109 and E-C5 probes were ~5–10 cm from the collar wall. (Color figure online)
Automated CO2 measurements
Soil surface CO2 efflux is the result of biocrust respiration, soil heterotrophic respiration throughout the profile, and likely a relatively small contribution from inorganic C processes and vascular plant roots. Automated measurements of soil surface CO2 efflux rates were conducted hourly from March 10, 2016 to June 20, 2016 using an infrared gas analyzer (IRGA: LI-8100A; Li-Cor Inc., Lincoln, NE, USA) connected to a multiplexer (LI-8150; Li-Cor Inc.) operating four 20 cm-diameter opaque chambers (8100-104; Li-Cor Inc.) (Fig. 1). Chambers were oriented to face south when closed to minimize shading effects in collars. Thick-walled (~6.5 mm) polyvinyl chloride (PVC) 20.3 cm-diameter collars (LI 6581-157: Li-Cor Inc.) were permanently installed on February 20, 2016. Collars were installed to 9 cm depth in the soil profile, in interspaces among vascular plants, outside of the canopy of any perennial shrubs or grasses. Fluxes were measured in each chamber for 2.5 min every hour. Prior to each CO2 flux measurement, the chamber, gas line, and IRGA were purged for 1 min and purged again for 45 s following each measurement. After each chamber closed, there was a 30 s deadband, where no flux was recorded in order to allow biocrusts to acclimate to the dark. The order of measurements between chambers remained consistent over the length of this study. A small number of measurements (less than 0.3% of the total number over the approximately 3 month measurement period) indicated net CO2 uptake by the biocrust; however, CO2 accumulation curves indicated that these few uptake estimates were not reliable and thus they were excluded. Within the <0.3% of data excluded, in some cases, it appeared that high photosynthetic rates prior to chamber closure resulted in continued CO2 assimilation for as much as ~30 s to 1 min into the measurement period, after which the CO2 accumulation curve (viewed as raw data using the Li-COR File Viewer software) switched from decreasing to increasing, indicating a cessation of CO2 assimilation and a switch to respiration entirely driving the flux. In other cases, it was unclear whether we were measuring actual CO2 uptake or some other source of CO2 that entered the chamber prior to measurement and made values inexplicably high when measurements began.
Biocrust and soil temperature and moisture profile measurement system
Soil temperature and moisture were measured (1) inside the collars and (2) in soil profiles adjacent to the collars; due to the disturbance caused by probe installation, deeper probes could not be installed beneath the automated soil CO2 efflux chambers (Fig. 2). Within each collar, we installed three biocrust surface moisture probes and one biocrust surface temperature probe (Weber et al. 2016). Within 10 cm from each collar, two sets of Decagon Devices, Inc. EC-5 moisture probes and Campbell Scientific, Inc. CS 109 temperature probes were inserted between 0 and 5 cm depth in the sub-crust soil at opposite sides of each collar. Additionally, two sets of four Campbell Scientific, Inc. CS650 soil temperature and moisture probes were inserted at 5, 10, 15 and 20 cm depth in the subcrust soil. More details are given below.
Biocrust surface probes
Within each of the four collars, one surface temperature sensor and three surface moisture sensors were installed in the biocrust surface, to a depth of ~2 mm (Fig. 3). More detail about these probes can be found in Weber et al. (2016), where they are referred to as ‘biocrust wetness probes’. In brief, the water content of the biocrust or soil surface layer is calculated based on the conductance of two 3 mm long copper electrodes. These probes are minimally destructive: the sensor covers less than 1 cm2 surface area and the probes and stabilization pins require four small punctures in the biocrust/soil surface, each with a diameter of less than 0.5 mm. Sensor calibration was critical for this approach, and is described below. Because these probes are small, minimally destructive and easy to install, several probes could be installed in near proximity allowing us to better measure the potentially highly variable surface water content. Probes were distributed evenly through the chambers (with ~10 cm between them) to minimize interference between them. Each probe made measurements every 5 min. We used the average reading from the three moisture probes within each chamber as the chamber values. The surface temperature sensor consisted of a fine wire thermocouple inserted to 2 mm depth in the biocrust. The surface temperature sensor was weighted in place because the thin filament and shallow insertion depth lead to repeated wire displacement in the initial phase of the study (none of the data from this phase are presented here).
Soil temperature and moisture profile
In the field, soil moisture and temperature 0–5 cm below the bottom of the biocrust layer were measured on two sides of each collar, within 10 cm from the edge of the collar, using Decagon EC-5 probes for moisture measurements and Campbell Scientific CS109 probes for temperature (Fig. 2). Data from these probes were averaged for each collar. Measurements were made every 1 min and averaged into 10 min intervals. To install probes, we first examined nearby biocrust to determine the depth of the biocrust-soil interface (i.e., where the bottom of the biocrust layer met the top of the underlying mineral soil), and then gently pushed probes through the biocrust so that they were oriented vertically with the top even with the soil surface (Fig. 2). The probes were not inserted inside the collars because the height of the connection where the cable joined the probe would have interfered with the autochamber operation.
Deeper soil moisture and temperature values were measured using Campbell Scientific CS650 water content reflectometers, installed at 5, 10, 15 and 20 cm depth below the bottom of the biocrust layer (Fig. 2). Factory calibration curves were used for processing the raw data per the manufacturer’s instruction. To install the probes, we first dug two soil pits to approximately 25 cm depth, being careful not to disturb the wall on the side where the probes were to be inserted. The probes were then inserted horizontally 30 cm into the soil, until the sensor head was flush with the wall. As probes were installed from deepest to shallowest, we backfilled the soil pit with the soil that had been removed during digging. The process of digging the soil pit inevitably affected soil physical properties relevant to temperature and moisture, but we minimized this effect by putting the probes into untouched soil.
Surface probe calibration
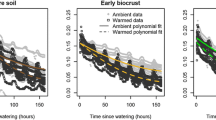
To calibrate the surface moisture probes in the laboratory, we placed intact biocrust specimens, with the subcrust soil removed by careful brushing, in Styrofoam basins with perforations in the bottom. We then saturated the biocrust and allowed it to drain freely until no water was visibly pooled on the surface or draining from the bottom. At this point, the surface probe was inserted and the first reading was taken. Then, the probe was removed and the mass of the biocrust + Styrofoam basin was measured, after which we reinstalled the surface probe. The original approach [detailed in Weber et al. (2016)] involved fixing the sensors in place and measuring the mass over a dry down period (from saturated to air dry), but with our infrastructure the settling of the sensor and sensor wire caused a difficult-to-correct increase in the total mass of the biocrust + sensor. Therefore, during each mass measurement, we carefully removed the sensor, measured the biocrust + container mass, and then carefully reinserted the sensor in the exact same place. This approach admittedly may have led to some differences in biocrust water content near the sensors, but the effect appeared to be small relative to the effect of the settling sensor wire. Mass measurements were taken each half hour for 9 h, and then 12 and 17 h following wetting, at which time the biocrust was air dry. Surface probe readings (documented as conductance in mV) were made every 5 min. Each mass measurement was paired with the surface probe measurement made immediately prior for creating calibration curves.
We calibrated the probes using three replicates each of S. caninervis moss biocrust, Psora decipiens squamulose lichen biocrust, C. tenax gelatinous lichen biocrust, and darkly- and lightly-pigmented cyanobacterial biocrusts. For the purposes of this paper we present calibrations for S. caninervis and the darkly pigmented cyanobacteria, because chambers 1, 2, and 4 were dominated by S. caninervis, while chamber 3 primarily comprised dark pigmented cyanbacteria. We fit the calibration curve using non-linear regression based on a Michaelis–Menten function in R (Fig. 4).
Calibration curve for surface moisture probes. The y-axis is the gravimetric water content determined by repeatedly weighing the biocrust through a dry-down phase, and correcting for the total mass of the biocrust and container, and normalizing by oven dry weight. The x-axis is the surface moisture probe reading in mV
All data analysis was conducted using R software version 3.3.2 (“Sincere Pumpkin Patch”). For fitting calibration data, we implemented a Michaelis–Menten function within the R package ‘drc’. CO2 flux and microclimate data were merged by time; in general, readings were less than 5 min apart. To compare the exponential temperature sensitivity of CO2 efflux, using temperatures from either the biocrust surface or the 0–5 cm soil layer, we first excluded data where water content of the respective layer was less that 0.08 cm3 H2O cm−3 soil or biocrust, based on previous analysis that demonstrate a soil moisture threshold for respiration at this value (Tucker and Reed 2016). Then, we fitted a simple exponential model, using a linear fit of log transformed CO2 efflux via R function ‘lm’, between temperature of the respective layer and CO2 efflux and estimated the Q10 = e(10 * B) where B is the temperature coefficient of the log-transformed linear regression.
Results
Between March 10 and June 20, 2016, temperatures throughout the biocrust and soil profile generally increased, while moisture generally decreased (Fig. 5). Diurnal and seasonal temperature changes were largest at the biocrust surface (min. = −5.82 °C, max. = 74.0 °C, max. diurnal range = 61.4 °C) and decreased throughout the soil profile (at 20 cm depth: min. = 4.95 °C, max. = 33.1 °C, max. diurnal range = 4.2 °C), such that the biocrust surface was the most dynamic region of soil in terms of temperature and moisture variability. Soil moisture at the biocrust surface was mostly pulse-driven, while deeper layers showed initially high water content and a gradual decline over the study period (Fig. 5). Of 31 biocrust surface wetting events (across all chambers) registered with biocrust surface moisture probes, 20 resulted in detectable moisture in the 0–5 cm layer in the soil, while only 15 were detectable at 10 cm in the soil, and only 4 at 20 cm depth. The magnitude of pulse wetting events was always increasingly dampened deeper in the soil profile. Biocrust surface moisture and temperature were, with the exception of the pulse wetting events that did not penetrate the soil beneath the biocrust, positively correlated with the temperature and moisture of the 0–5 cm soil layer (shallow soil immediately beneath the biocrust) of the soil profile (temperature: r = 0.887, p < 0.001; moisture: r = 0.656, p < 0.001). This correlation decreased throughout the soil profile such that at 20 cm depth the positive correlation of temperature with the biocrust surface was smaller (r = 0.445, p < 0.001) and moisture at the biocrust surface was only very weakly correlated with moisture at 20 cm depth (r = 0.089, p < 0.001).
Surface CO2 effluxes and soil microclimate for the four measured chambers. The top row shows surface CO2 efflux rates for each of the four automated soil CO2 efflux chambers. Each of the lower rows shows soil water content (black lines) and temperatures (grey lines) for deeper soils along the profile for each of four replicate chambers, beginning with surface biocrust soils and moving into deeper soil layers. Arrows in the upper row of panels indicate CO2 efflux pulses that correspond to biocrust surface moisture pulses, but that do not correspond to moisture at any of the deeper levels. Surface CO2 efflux measurements have some gaps due to instrumentation power source failure. ‘Biocrust surface’ soil moisture and temperature are measured in the upper 0–2 mm of the biocrust layer, the soil depth of measurement below the surface layer is shown in the first column and refers to sensor depth below the biocrust-soil interface. Thus, ‘Soil (0–5 cm)’ moisture and temperature refer to measurements starting just below the biocrust, 5 cm measurements are 5 cm below the biocrust, and so on. The 5, 10, 15, and 20 cm measurements come from two sensor profiles, one that was placed near chambers 1 and 2, and the other that was placed near chambers 3 and 4. Data from those two soil profiles are replicated for chambers 1 and 2 and for 3 and 4, respectively to show a full profile for each
Across the 102 day study period, CO2 effluxes showed a pulse-dynamic pattern, with each chamber showing 6–8 pulse events of CO2 efflux (total of 31 events), with a low-level background flux (0–1.2 μmol CO2 m−2 s−1) between pulses (Fig. 5). Efflux rates increased as seasonal soil temperatures warmed, but then decreased in mid-June as surface soil temperatures exceeded 70 °C (Fig. 5). Each CO2 pulse was associated with a biocrust surface wetting event, and each biocrust surface wetting event was associated with a CO2 pulse (Fig. 5). “Standard” surface soil temperature and moisture measurements (i.e., 0–5 cm in the soil profile) were associated with many but not all CO2 pulses. In fact, 11 of 31 CO2 pulse events (across all chambers) were correlated with biocrust wetting events only (black arrows in Fig. 5), and not with deeper soil moisture. Deeper wetting events resulted in more sustained CO2 effluxes, suggesting that more total CO2 was released from the biocrust and soil, but the magnitude of the instantaneous flux was not substantially larger. CO2 efflux increased with increasing soil moisture and with increasing temperature within a given moisture level for both the biocrust surface layer and the shallow soil layer (Fig. 6). Due to the differences in magnitude of temperature variation in these layers, the estimated temperature sensitivity of CO2 effluxes was significantly lower using temperature of the biocrust surface versus temperature of the shallow soil layer (Q10,biocrust = 2.17 vs. Q10,shallow = 2.49 at VWC >0.08). CO2 fluxes were very low when the soil was dry (Figs. 5, 7). Nonetheless, there was a strong diurnal cycle of CO2 efflux even during a very dry period, corresponding to the diurnal temperature cycle (Fig. 7).
CO2 efflux during periods with soil moisture above (blue) or below (tan) 0.08 cm3 H2O cm−3 soil, compared to temperature. The left figure shows the CO2 efflux compared to the temperature of the biocrust surface layer, and the moisture threshold is based on biocrust moisture. The right figure shows the same, but using the temperature and moisture of the 0–5 cm layer in the soil below the biocrust. The x-axis scale is based on the minimum and maximum temperature at the biocrust surface layer. The vertical line at 40 °C is to help orient the reader to the different apparent temperature thresholds. (Color figure online)
Discussion
Our results demonstrate that a depth resolved approach to soil microclimate assessment, including surface (0–2 mm depth) soil measurements, illuminate robust relationships between biocrust water content and CO2 pulses that have previously been difficult to detect and explain. By exploring soil microclimate along the depth profile in conjunction with soil CO2 efflux measurements, we were able to explore new questions about the relationships between microclimate and a core aspect of soil C cycling: soil respiration. Surface CO2 efflux was strongly coupled to temperature and moisture of the surface biocrust layer. The temperature and moisture of this surface layer showed extreme diurnal and seasonal variation. Microclimate of deeper soil layers was less variable and somewhat less closely related to soil CO2 efflux. While previous work in drylands has demonstrated the occurrence of CO2 pulses in response to small precipitation events (e.g. Cable and Huxman 2004) and dew events (e.g. Lange et al. 1994; McHugh et al. 2015; Wilske et al. 2008), as well as evaluated moisture sensitivity of biocrust respiration in the lab at very low moisture (Coe et al. 2012; Grote et al. 2010), the data presented here are, to our knowledge, the first to directly measure the water content of the biocrust surface layer coupled to CO2 flux measurements in the field. Because of the highly dynamic nature of water and temperature in the surface layer, and the concentration of biotic and biogeochemical activity in the biocrust, we believe that this approach has the potential to add new insight into dryland biogeochemistry beyond the study presented here.
It is worth noting that the temperature regime experienced by biocrust organisms is much more severe than would be indicated by air temperatures or deeper soil temperatures. Over the 100 day period, the lowest surface temperature was ~−6 °C while the highest was ~74 °C, and on several days, the diurnal range was >60 °C. These extreme environmental conditions were significantly ameliorated deeper in the soil profile. Yet, biocrust organisms persist on the surface where they form a mantle of fertility (Garcia-Pichel et al. 2003). Due to the severity of the biocrust surface microclimate in desert ecosystems, carbon and nutrient cycling within biocrusts is highly coupled to temperature and moisture. Indeed, the ability of biocrusts to withstand the harsh environment—and form the dominant landcover in most drylands—is due to the fact that the component organisms exhibit poikilohydric response to water availability. That is, they are dormant during hot, dry periods, with a very low level of “survival respiration”, and respond quickly to even small available water pulses. For context, in the Sonoran Desert, biocrusts contributed ~80% of soil CO2 efflux during small precipitation events, but root and soil heterotrophic respiration contributed nearly all of the flux during large precipitation pulses (Cable and Huxman 2004).
The occurrence of diurnal cycles of CO2 efflux during an extended hot and dry period in late June (Fig. 7), when presumably most organisms were inactive, merits some explanation. These cycles correspond to diurnal temperature cycles and, while it is possible that the fluxes represent biotic enzyme kinetics as is traditionally inferred from soil temperature responses of soil respiration (Davidson and Janssens 2006), it also seems plausible that abiotic effects on soil C cycling explain these fluxes. One possibility is that as the air in the soil pore space warms it expands, causing pressure-induced efflux of air with a higher CO2 concentration into the atmosphere (Rey 2015). This possible mechanism may not change the ecosystem C balance significantly over time (i.e., it is C that has been lost from the system into the atmosphere of the soil pore space), but better capturing it would help constrain our estimates of the climatic sensitivity of soil C cycling. A second possible mechanism is UV-induced photodegradation. During hot dry periods at this site, solar radiation is intense (Rutherford et al. 2017) and correlated with diurnal temperature, and previous work in other drylands indicates photodegradation may be a principle pathway of loss of litter C (Austin and Vivanco 2006). While there is not significant standing litter atop our measured soil columns, UV effects on living biocrust tissue and biocrust litter and its decomposition remain highly uncertain. A final possibility is that we are seeing the efflux of soil respiration (either from plant roots or microbes) from deeper soils where some moisture persists, and that these fluxes respond to diurnal temperature cycles in those layers. However, due to the time lags of heat and gas diffusion, the resulting CO2 efflux cycles should in that case show a significant time lag compared to the surface temperature(Phillips et al. 2011), which we do not see.
A few caveats of this study are worth consideration and provide room for future improvement. Here, we evaluate the response of CO2 effluxes to temperature and moisture at different levels in the biocrust and soil profile. At the biocrust surface, the shallow soil layer (0–5 cm depth), and deeper in the profile from 5 to 20 cm, we used different soil moisture and temperature sensors. In each case, we used the appropriate sensor for that measurement, but direct comparison of data from different sensor types should be tempered by a recognition that different sensors have different inherent variability, sensitivity to other aspects of the environment and so on. Another important caveat is, that we evaluate CO2 efflux in this study, yet many biocrust organisms are photosynthetic. We measured CO2 flux in dark chambers, but a small number of measurements ambiguously indicated net CO2 uptake (less than 0.3% of the total) and were excluded from this study, because the shape of the CO2 accumulation curve showed a switch from net uptake to net efflux during the measurement period. It is reasonable to speculate that CO2 uptake rates were high enough prior to the chamber closing to reduce the apparent respiration rate in the dark with the chamber closed on some fraction of the data we did include in the study.
The use of surface moisture and temperature probes, coupled to microclimate measurements of deeper soils, and automated measurements of CO2 effluxes, allowed us to detect a number of subtle patterns of climatic sensitivity of CO2 cycling in biocrusted interspaces. These patterns are not entirely novel, but the data do allow greater clarity about processes underlying soil C cycling. We therefore suggest that combining surface measurements of biocrust moisture and temperature with automated CO2 flux measurements may greatly improve our understanding of the climatic sensitivity of C cycling in biocrusted interspaces in our study region. Because biocrusts are a dominant cover type in many dryland regions, which cover >40% of the terrestrial Earth surface, this method may be globally relevant and applicable. Moreover, while here we restrict our analysis to CO2 effluxes, biological soil crust also contribute substantially to dryland nitrogen (N) cycles [e.g., via N2 fixation and gaseous N loss; (Barger et al. 2016; Weber et al. 2015)] and this same proposed approach used with N cycling assays could provide valuable insights into the N cycle in drylands. Overall, these results highlight the power of a multi-sensor approach to elucidate the patterns and controls over biogeochemical cycles in biocrust soils.
References
Ahlström A, Raupach MR, Schurgers G, Smith B, Arneth A, Jung M, Reichstein M, Canadell JG, Friedlingstein P, Jain AK, Kato E, Poulter B, Sitch S, Stocker BD, Viovy N, Wang YP, Wiltshire A, Zaehle S, Zeng N (2015) The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 348(6237):895–899
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442(7102):555–558
Austin A, Yahdjian L, Stark J, Belnap J, Porporato A, Norton U, Ravetta D, Schaeffer S (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141(2):221–235
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J (2016) Patterns and controls on nitrogen cycling of biological soil crusts. Biological soil crusts: an organizing principle in drylands. Springer, New York, pp 257–285
Belnap J, Weber B, Büdel B (2016) Biological Soil Crusts as an Organizing Principle in Drylands. In: Biological Soil Crusts: An Organizing Principle in Drylands. Springer International Publishing. p 3-13
Cable JM, Huxman TE (2004) Precipitation pulse size effects on Sonoran Desert soil microbial crusts. Oecologia 141(2):317–324
Castillo-Monroy AP, Maestre FT, Rey A, Soliveres S, Garcia-Palacios P (2011) Biological soil crust microsites are the main contributor to soil respiration in a semiarid ecosystem. Ecosystems 14(5):835–847
Coe KK, Belnap J, Sparks JP (2012) Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology 93(7):1626–1636
Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J (2015) Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 126(3):363–378
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440(7081):165–173
Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc Natl Acad Sci 112(39):12116–12121
Garcia-Pichel F, Johnson SL, Youngkin D, Belnap J (2003) Small-scale vertical distribution of bacterial biomass and diversity in biological soil crusts from arid lands in the Colorado Plateau. Microb Ecol 46(3):312–321
Grote EE, Belnap J, Housman DC, Sparks JP (2010) Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Glob Change Biol 16(10):2763–2774
Huxman T, Snyder K, Tissue D, Leffler AJ, Ogle K, Pockman W, Sandquist D, Potts D, Schwinning S (2004) Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141(2):254–268
Lange OL, Meyer A, Budel B (1994) Net photosynthesis activation of a desiccated cyanobacterium without liquid water in high air humidity alone—experiments with microcoleus-sociatus isolated from a desert soil crust. Funct Ecol 8(1):52–57
McHugh TA, Morrissey EM, Reed SC, Hungate BA, Schwartz E (2015) Water from air: an overlooked source of moisture in arid and semiarid regions. Sci Rep 5:13767
Phillips CL, Nickerson N, Risk D, Bond BJ (2011) Interpreting diel hysteresis between soil respiration and temperature. Glob Change Biol 17(1):515–527
Pointing SB, Belnap J (2012) Microbial colonization and controls in dryland systems. Nat Rev Microbiol 10(8):551
Poulter B, Frank D, Ciais P, Myneni RB, Andela N, Bi J, Broquet G, Canadell JG, Chevallier F, Liu YY, Running SW, Sitch S, van der Werf GR (2014) Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 509(7502):600–603
Reed SC, Coe KK, Sparks JP, Housman DC, Zelikova TJ, Belnap J (2012) Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat Clim Change 2(10):752–755
Rey A (2015) Mind the gap: non-biological processes contributing to soil CO2 efflux. Glob Change Biol. doi:10.1111/gcb.12821
Rutherford WA, Painter TH, Ferrenberg S, Belnap J, Okin GS, Flagg C, Reed SC (2017) Albedo feedbacks to future climate via climate change impacts on dryland biocrusts. Sci Rep 7:44188
Schlesinger W, Pilmanis A (1998) Plant-soil interactions in deserts. Biogeochemistry 42(1–2):169–187
Tucker CL, Reed SC (2016) Low soil moisture during hot periods drives apparent negative temperature sensitivity of soil respiration in a dryland ecosystem: a multi-model comparison. Biogeochemistry 128(1–2):155–169
Weber B, Wu D, Tamm A, Ruckteschler N, Rodríguez-Caballero E, Steinkamp J, Meusel H, Elbert W, Behrendt T, Sörgel M, Cheng Y, Crutzen PJ, Su H, Pöschl U (2015) Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. Proc Natl Acad Sci 112(50):15384–15389
Weber B, Berkemeier T, Ruckteschler N, Caesar J, Heintz H, Ritter H, Braß H (2016) Development and calibration of a novel sensor to quantify the water content of surface soils and biological soil crusts. Methods Ecol Evol 7(1):14–22
Wertin T, Reed S, Belnap J (2015) C3 and C4 plant responses to increased temperatures and altered monsoonal precipitation in a cool desert on the Colorado Plateau, USA. Oecologia 177(4):997–1013
Wilske B, Burgheimer J, Karnieli A, Zaady E, Andreae M, Yakir D, Kesselmeier J (2008) The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert, Israel. Biogeosci Discuss 5(3):1969–2001
Acknowledgements
This material is based upon work supported by the U.S. Department of Energy Office of Science, Office of Biological and Environmental Research Terrestrial Ecosystem Sciences Program, under Award Number DE-SC-0008168 and by the U.S. Geological Survey Ecosystem Mission Area. TAM was supported by a National Science Foundation Postdoctoral Research Fellowship in Biology under Grant No. 1402451 and BW by a Paul Crutzen Nobel Laureate Fellowship. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stephen Porder.
Rights and permissions
About this article
Cite this article
Tucker, C.L., McHugh, T.A., Howell, A. et al. The concurrent use of novel soil surface microclimate measurements to evaluate CO2 pulses in biocrusted interspaces in a cool desert ecosystem. Biogeochemistry 135, 239–249 (2017). https://doi.org/10.1007/s10533-017-0372-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-017-0372-3