Abstract
The absolute concentrations of nitrogen (N) and phosphorus (P) and their relative availabilities (N:P stoichiometry) can influence numerous ecological processes. In streams, N:P stoichiometry is influenced by different hydrologic and biogeochemical processes that also affect the downstream transport of these nutrients to receiving waters. Calcium carbonate (CaCO3) deposition, a widespread geochemical process in alkaline streams and other aquatic ecosystems, can lower phosphate concentrations and, potentially, decrease P availability relative to N availability. We evaluated the effects of CaCO3 deposition on stream water stoichiometry using a 3-year dataset of stream physicochemistry and several metrics of CaCO3 deposition across three streams in the Huachuca Mountains of southern Arizona, USA. CaCO3 deposition rates varied across and within streams, with benthic coverage of travertine as high as 70 % and deposition rates up to 8.3 μg Ca2+ L−1 m−1. Redundancy analysis revealed a strong, negative correlation between stream water phosphate concentrations and CaCO3 deposition rates, a relationship that also extended to total P concentrations, and a strong, positive correlation between inorganic N concentrations and CaCO3 deposition rates. Furthermore, we found a significant positive relationship between CaCO3 deposition rates and N:P ratios. These results support the role of coprecipitation of phosphate with CaCO3 deposition in reducing P supply. They also suggest that reduced concentrations of P in the water column may reduce biological N uptake, amplifying the stoichiometric signal of CaCO3 deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) and phosphorus (P) are two of the most important constituents in the elemental composition of life. The biological availability of these nutrients, as well as the availability of these nutrients relative to each other (N:P stoichiometry), has important implications for a variety of ecological processes (Sterner and Elser 2002; Sardans et al. 2012) including water quality regulation (Smith 1983; Dodds and Oakes 2008). In streams, numerous hydrologic and biogeochemical processes can influence N and P cycling (Alexander et al. 2007) and nutrient flows to receiving rivers, lakes, and estuaries via longitudinal transport (Peterson et al. 2001). In this study, we examine how multiple processes affect N and P concentrations, with a particular emphasis on the interactions of P with calcium carbonate (CaCO3) deposition. We also consider how these processes might uncouple or couple N and P cycles, affecting N:P stoichiometry.
Surface (lateral) flow can regulate material transport, and therefore nutrient transport, from watersheds into streams. However, while lateral transport may couple N and P inflows to streams, it may not necessarily result in a distinct N:P stoichiometric signature of the influx. The form and stoichiometry of nutrients in the material being transported will be a reflection of the watershed characteristics. Therefore, the relationship between discharge and dissolved or particulate matter, while often positive, is variable (Austnes et al. 2010; Mattsson et al. 2015; Meyer et al. 1988). For example, in forested watersheds, spates can transport greater amounts of dissolved organic matter (DOM) to the stream (Mulholland and Hill 1997), while in a desert stream, spates can increase inorganic nutrient concentrations (Grimm 1992). Conversely, drought can lead to decreased stream water concentrations of DOM, and therefore organic N and P (Dahm et al. 2003).
Numerous instream processes influence nutrient dynamics as well. Some biological processes, such as microbially mediated nutrient assimilation or mineralization, tend to couple N and P flows because of the relatively restricted range of N:P ratios present in living biomass (Mooshammer et al. 2012; Schade et al. 2011). Other biological processes, like the microbially mediated conversion of \({\text{NO}}_{3}^{ - }\) to N2 via denitrification, lead to the selective removal of one nutrient over another (Grimm and Fisher 1986). Physical sorption processes can also reduce stream nutrient concentrations, although the nutrient-specific effect depends on the characteristics of the particulate matter (Meyer and Likens 1979; Triska et al. 1994). Indeed, P, as phosphate, tends to interact more readily with suspended particles or sediment than N and, as a result, physical adsorption is thought to be more important in removing P from the water column than N (Avilés et al. 2006; Dorioz et al. 1989).
Phosphate can interact with a variety of sediment components: it can bind with aluminum and iron, adsorb to organic matter, and/or coprecipitate with CaCO3 (Chave 1965; House and Donaldson 1986; Meyer and Likens 1979). P coprecipitation with CaCO3 occurs in a variety of aquatic ecosystems and is found across the world: benthic photosynthetic mats (Noe et al. 2001 and references therein, Borovec et al. 2010), wetland soils (Boyer and Wheeler 1989), lakes (Lawrence Lake, Michigan, USA, Otsuki and Wetzel 1972; Lake Wallersee, Austria, Jäger and Röhrs 1990; Lake Constance, Europe, Kleiner 1988; Gull Lake, Michigan, USA, Hamilton et al. 2009), sea ice (Hu et al. 2014), and rivers (House and Donaldson 1986; Jarvie et al. 2006; Cohen et al. 2013). As coprecipitation reactions depend on the chemical equilibrium between bottom substrata and overlying stream water, the ability of CaCO3 deposition to remove P from the water column can be saturated; therefore, at high levels of P, coprecipitation loses effectiveness in reducing water column PO4 concentrations, as phosphate may inhibit CaCO3 formation (House 1990; House and Denison 1997; Neal 1999). Despite the attenuation of CaCO3 formation at high phosphate concentrations, in many rivers phosphate coprecipitation with CaCO3 is considered the most important endogenous process removing P from the water column (Avilés et al. 2006). It is also likely important in reducing P concentrations in headwater streams (Withers and Jarvie 2008) and, particularly, in streams with low nutrient inputs. However, microbes may interact with CaCO3 deposition and influence patterns of phosphate coprecipitation in unexpected ways (Jarvie et al. 2006). Therefore, to understand the extent to which CaCO3 deposition effects phosphate concentrations in aquatic ecosystems, it is necessary to study these processes in oligotrophic streams as well.
In this study, we focus on the relative importance of watershed (e.g., hydrologic) and in-stream (e.g., biologic and geochemical processes, particularly CaCO3 deposition) processes in influencing absolute and relative supplies of N and P in headwater streams draining the Huachuca Mountains in southeastern Arizona. Despite geomorphic similarities (Jaeger and Olden 2012), preliminary observations of these streams suggest that they exhibit different rates of travertine deposition (Corman unpublished data). As travertine is a form of CaCO3, this natural gradient provides a unique opportunity to assess the impacts of a geochemical process on the stoichiometry of potentially limiting nutrients in headwater streams (Fisher et al. 2004). We used estimates of hydrologic processes (stream discharge), biological processes (stream temperature and season, both which can regulate microbial activities), and geochemical processes (estimates of CaCO3 deposition) to assess the relative influence of each on stream water nutrient concentrations over 3 years. To determine if CaCO3 deposition could account for any observed differences in P concentrations, we also analyzed P content within travertine samples. Based on the hypothesis that CaCO3 coprecipitation of phosphate differentially affects P relative to N, we predicted that we would observe lower absolute P concentrations and higher N:P ratios in the water of streams with higher rates of carbonate deposition. This study complements growing knowledge of how the interactions between hydrologic and biogeochemical processes influence nutrient concentrations and flows in aquatic ecosystems.
Methods
Field site description
This study was carried out in the Huachuca Mountain Range within the Upper San Pedro River Basin, southeastern Arizona. The peaks of the Huachuca Mountains rise to nearly 2800 m above sea level. The streams in the eastern portion of the Huachuca Mountains are part of the watershed of the San Pedro River, the last major free-flowing, undammed river in the southwestern US. The climate in this region is semi-arid with about half of the mean annual precipitation of ~80 cm associated with the North American monsoon season and the other half with Pacific fronts in the winter (WRRC 2014).
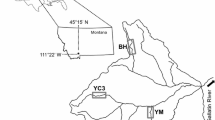
The three streams used in this study, Garden Canyon, Ramsey Canyon, and Huachuca Canyon, drain the northeastern side of the Huachuca Mountains (Fig. 1). The streams are spring-fed and perennial above ~1500 m elevation (Jaeger and Olden 2012). Stream channel morphology is characterized by cascade and bedrock reaches in the upper canyons and step-pool, plane bed, and pool-riffle reaches downstream. Substrata are travertine or cobbles and boulders. In some of the reaches, CaCO3 deposition has cemented the channel bed and contributed to travertine step-pool morphology. Stream flow is monitored in each stream by the United States Geological Survey (USGS Gaging Stations: Garden Canyon: 09470800, Huachuca Canyon: 09471310, and Ramsey Canyon: 09470750). Broadleaf deciduous trees, including Arizona sycamore (Platanus wrightii) and bigtooth maple (Acer grandidentatum), dominate the riparian zones along the streams (Brown 1994).
Study site locations in Huachuca, Garden, and Ramsey Canyon streams in the Huachuca Mountains, south eastern Arizona, USA. Crosses denote sampling locations and dashed lines indicate topographic contour lines. Background colors correspond to geologic units. Jg/Yg granite/granite–diorite, Jv/KJs sedimentary rocks and volcanics, MC limestone, Pz quartzite, Q surficial alluvium deposits, Tsm/Tsy sedimentary rocks. Inset shows the State of Arizona, USA, and the location of the Town of Sierra Vista
Stream sampling
Stream water was sampled at least every 2–3 months from February 2011 to May 2014 (Fig. 2). Four reaches were sampled per stream; reaches spanned 4.6, 2.4, and 0.8 km in Garden, Huachuca, and Ramsey Canyons, respectively (Fig. 1). On dates when parts of the stream were dry, only wet reaches were sampled. At each reach, temperature (°C), specific conductivity (μS cm−1), and dissolved oxygen (mg L−1) were determined using a YSI 85 meter (Yellow Springs, OH, USA) and pH using a Beckman-Coulter 255 pH/mV (Beckman Coulter, Inc., Brea, CA, USA) hand-held meter. Alkalinity was quantified by Gran titration (APHA 2005). Stream water samples were collected in acid-cleaned HDPE bottles and, when necessary, filtered (Supor® membrane filter, 0.45 µm) and/or preserved with acid. Cation concentrations (Ca2+, Mg2+, Na+, K+) were analyzed on filtered samples preserved with 2 % HNO3 using a Thermo iCAP6300 inductively coupled plasma optical emission spectrometer (ICP-OES; Waltham, MA, USA). Anion concentrations (\({\text{SO}}_{4}^{ 2- } ,\) Fl−, Cl−, Br−) were assessed on filtered samples using ion chromatography (Dionex ICS-2000, Sunnyvale, CA, USA). Soluble reactive P (SRP) was quantified spectrophotometrically using filtered samples with the ammonium molybdate colorimetric method (APHA 2005). Total dissolved P (TDP) and total P (TP) were determined on filtered and unfiltered samples, respectively, by persulfate digestion (Solorzano and Sharp 1980) after which P concentrations were analyzed as described for SRP. Nitrate and ammonium (\({\text{NO}}_{3}^{ - }\) and \({\text{NH}}_{4}^{ + }\)) were determined on filtered samples using a Lachat QuikChem 8000 flow injection automated ion analyzer (Hach, Loveland, CO, USA); dissolved inorganic N (DIN) concentrations were calculated by summing \({\text{NO}}_{3}^{ - }\) and \({\text{NH}}_{4}^{ + }\) concentrations. Total dissolved N (TDN) was analyzed concomitantly with dissolved organic carbon (DOC) on filtered samples preserved with 2 % HCl using a Shimadzu TOC-VN/TN analyzer. Total N (TN) was determined on unfiltered samples by the in-line persulfate/UV oxidation method using a Lachat QC8000. Dissolved organic P (DOP) and dissolved organic N (DON) concentrations were calculated as the difference between total dissolved and dissolved inorganic P or N, respectively.
A subset of acidified stream water samples (six events between 2012 and 2014) were analyzed for iron (Fe) concentrations using ICP mass spectrometry (Thermo Fisher Scientific X Series 2, Waltham, MA, USA). However, as mean concentrations were low (<1.75 µg L−1) and did not vary between streams, potential mineral interactions between phosphate and Fe or Fe oxides were considered of negligible importance and not considered further in the analysis.
In the streams with active CaCO3 deposition, Garden and Huachuca Canyon, we also collected travertine samples to assess P content. Travertine samples were collected in 2011 and 2012 from the upper three reaches using a rock hammer to remove rock to a depth of <3 cm. Samples were frozen until return to the lab where they were dried for at least 48 h at 60 °C. P content was estimated following the methods of Aspila et al. (1976). Briefly, we extracted ashed travertine material (4 h, 550 °C) in 1 M HCl for 16 h with constant agitation on a shaker table, diluted the extractant to 4 % in Nanopure water, and analyzed the solution for SRP using spectrophotometry, as described above.
CaCO3 deposition rate determinations
We quantified the natural gradient of CaCO3 deposition across the streams using three metrics of CaCO3 deposition rates: travertine cover, deposition on a natural substrate, and Ca2+ longitudinal mass transfer reactions. To determine travertine cover, an indication of past CaCO3 depositional patterns, benthic surveys were conducted at each site on 31 August 2011, 30 July 2012, 11 October 2013, and 5 May 2014. A 0.25 m2 quadrat was thrown haphazardly in the stream at each site in three different locations. The percent cover of travertine was estimated visually at each location. Travertine deposits on leaves were included in the estimations of travertine cover (Supplementary Material, Fig. S1).
We determined net CaCO3 deposition rates over several months in the three upstream reaches in each stream using leaves as natural substrata (Supplementary Material, Fig. S1). Dried, senescent leaves of Arizona sycamore (P. wrightii) were collected in December 2010 from trees in the riparian zone of Ramsey Canyon. Leaves were transported immediately to the laboratory at Arizona State University where they were dried at 50 °C and then stored in a climate-controlled storage facility until use. In November 2011, we re-dried the leaves and placed them into mesh pecan bags (Gulf Coast Bag and Bagging Company, Houston, TX, USA) containing about 5 g of dried leaves each. We deployed litter bags at each site on 20 November 2011, and retrieved one bag per site at four intervals over the following 3 months. Three control bags were brought to the field and processed without deployment in the stream to measure the amount of material lost in transport, which we subtracted from all final dry mass totals. We processed litter bags by rinsing leaves to remove all macroinvertebrates that had colonized them, drying the remaining leaf material at 50 °C, combusting a homogenized subsample at 500 °C to measure ash-free dry mass, and, finally, dissolving the remaining material in 2 % HNO3 to analyze Ca2+ concentrations as described above. The Ca2+ content in leaves from the control bags was used to correct final Ca2+ values of incubated leaves to reflect only Ca2+ accumulated on the substrate. The linear change in the amount of accumulated calcium per dry leaf mass over time was used to determine the CaCO3 accumulation rate (mg CaCO3 g−1 dry leaf day−1). As the accumulation rate was based on the change in Ca2+ per initial dry mass of the leaf, and leaf mass was likely lost during the incubation due to decomposition or physical breakdown, these rates serve as conservative estimates of CaCO3 deposition.
CaCO3 deposition or dissolution rates in each stream were determined from Ca2+ transfer reactions estimated on each sampling day (Lorah and Herman 1988). We used the aqueous geochemical modeling software PHREEQC and the phreeqc.dat database to determine mineral (e.g., calcite) formation tendencies in the streams on each sampling date with the full suite of stream water physicochemical parameters (e.g., temperature, pH, alkalinity, ion concentrations, nutrient concentrations) (Parkhurst and Appelo 1999). For each mineral, PHREEQC calculates the saturation index, based on the ion activity product and the equilibrium solubility product, where SI >0 indicates supersaturation, SI = 0 indicates equilibrium, and SI <0 indicates undersaturation. CaCO3 longitudinal mass transfer from solution to channel bed (mg L−1) was determined using the change in Ca2+ between the upper and lower most reach sampled within each stream. Mass transfers were normalized across streams by dividing the change in Ca2+ concentration by the distance between reaches (reported in units of μg Ca2+ L−1 m−1). The reaction rate of CaCO3 was determined by dividing the mass transfer by estimates of the reaction time, determined from discharge and stream area, following Lorah and Herman (1988). The reaction rates are reported in units of μg CaCO3 L−1 s−1. Positive mass transfers or reaction rates indicate CaCO3 precipitation, while negative indicate CaCO3 dissolution. A preliminary analysis of changes to concentrations of conservative ions across reaches to detect groundwater intrusion showed negligible changes in conservative ion concentration (Supplementary Material, Fig. S2). Therefore, groundwater flux to the stream is assumed to be negligible and all changes to Ca2+ ions are assumed to be due to CaCO3 deposition or dissolution.
Statistical analyses
Descriptive statistics were calculated for the physicochemical variables, nutrient chemical parameters, and the proxies of hydrologic and biogeochemical processes. All values are reported as means ±1 standard error unless otherwise noted. To determine univariate differences in basic physicochemical parameters (temperature, pH, specific conductivity, dissolved oxygen), nutrient chemical parameters, or travertine cover across streams over time, we used one-way repeated measures ANOVA fit with maximum likelihood estimation and using stream as a repeated factor. To determine seasonality of parameters, season was added as a fixed factor. Due to the usually biseasonal rainfall pattern in the region, with monsoon storms occurring in late summer and Pacific fronts in winter, as well as the decrease in canopy cover in the riparian areas in the winter, seasons are defined as monsoon (July–September), fall (October–November), winter (December–March), or summer (April–June). To determine rates of CaCO3 accrual on leaves, we used linear regression within each stream and reach.
Next, we investigated the relationships between stream nutrient chemical parameters (the mean concentration of DOC, SRP, DOP, TDP, TP, DIN, DON, TDN, and TN across reaches within a site) and various potential processes (stream descriptor variables) across each sampling date. The stream descriptor variables included stream temperature, flow, CaCO3 deposition rate, and SIcalcite. Stream temperature and SIcalcite were estimated for each sampling day based on the mean value across reaches within each stream. To determine stream discharge at each site, we used the average stream discharge at the gaging station on the day of sampling. The stream gages were located no more than 2.6 km upstream or downstream of the sampling reaches in Garden, Huachuca, and Ramsey Canyons. Although the discharge data from the gages may not indicate the precise discharge at each site, it is unlikely that using gages would bias estimates of discharge at the different sites within a stream. We chose CaCO3 mass transfer and reaction rate as proxies of CaCO3 deposition instead of travertine cover or CaCO3 deposition on leaves, because these proxies were determined at the same temporal scale as the nutrient data (i.e., we were able to calculate mass transfer and reaction rates for every date on which we sampled stream water).
We analyzed the relationship between stream chemistry and biologic, hydrologic, or edaphic processes using redundancy analysis (RDA) to simultaneously examine the relationship across the two datasets with all variables. RDA is an ordination analysis that describes the variation between two multivariate datasets (ter Braak et al. 1988). The technique summarizes all the variance of the response variable dataset (e.g., stream nutrient chemistry data) that is related to the explanatory variable dataset (e.g., stream descriptor variables). Prior to performing the RDA, we tested for linearity between the variables using detrended correspondence analysis. A complete data matrix is needed for multivariate analysis (Tabachnick and Fidell 2001). Therefore, missing values were imputed based on the median value of the parameter. Next, distributions of univariate variables were checked for normality; if variables were not normally distributed, either a log-transformation or square root transformation was performed to achieve normality (McGarigal et al. 2000). Then, univariate variables were normalized to a mean of zero and a variance of one to reduce the effect of different measurement scales and the RDA was performed. The significance of each RDA ordination axis and explanatory variable was tested statistically (p < 0.05) using a Monte Carlo permutation test with forward selection. The final result is presented in an RDA triplot in which the main patterns of variation in stream nutrient chemistry and descriptor variables are presented as lines and arrows, respectively. Vectors pointing in roughly the same direction or in opposite directions indicate a strongly positive or negative correlation, respectively; vectors at right angles indicate a near zero correlation (ter Braak et al. 1988). The interset correlations between the stream descriptor variables with the RDA axes indicate which variables have the strongest influence in the ordination (ter Braak et al. 1988).
Finally, we use a regression model to assess the relationship between CaCO3 deposition and N:P stoichiometry in terms of inorganic nutrients (as a reflection of what is readily available to the biota) and in terms of total nutrient concentrations (as the relative availabilities of TN and TP can be an indicator of nutrient limitation; Downing and McCauley 1992).
All statistical tests were performed in R (v. 3.0.2) (R Core Team 2014). ANOVA was performed using the ‘nlme’ package (Pinheiro et al. 2014) and post hoc comparisons were made using Tukey’s honestly significant difference test with the ‘multcomp’ package (Hothorn et al. 2008). We assessed homoscedasticity and normality of residuals visually for each model with a plot of model residuals versus fitted values and a normal probability plot, respectively. For the repeated-measures ANOVA, we tested the assumption of sphericity and, if needed, corrected with the Greenhouse–Geisser estimate of epsilon (1958). RDA was performed with the ‘vegan’ package (Oksanen et al. 2013).
Results
Water physicochemistry
Discharge ranged from 0 to 6.26 m3 s−1 (Fig. 2). Median discharge was similar among streams, ranging from 0.003 m3 s−1 in Ramsey to 0.006 m3 s−1 in Huachuca and Garden Canyons. The highest flow events occurred in July during the monsoon season, the highest mean daily discharge on a date that was sampled was 0.069 m3 s−1 in Ramsey Canyon on 30 July 2012 (Fig. 2). Temperature, specific conductivity, and pH differed significantly among streams (temperature: F2,22 = 16.77, p < 0.001, specific conductivity: F2,22 = 56.60, p < 0.001, pH: F2,21 = 7.91, p < 0.01); dissolved oxygen concentrations did not (Fig. 3). Temperature was highest in Huachuca Canyon, 15.2 ± 1.0 °C, compared to Garden Canyon (13.0 ± 1.0 °C) or Ramsey Canyon (11.3 ± 1.2 °C). Specific conductivity and pH were highest in Garden Canyon. Temperature and specific conductivity showed significant seasonality (temperature: F3,32 = 42.71, p < 0.001, specific conductivity: F3,32 = 6.97, p = 0.001) with values usually highest during the monsoon season (Fig. 3). Across all streams, cation concentrations followed the order: Ca2+ ≫ Mg2+ > Na+ > K+, and anion concentrations followed the order: \({\text{SO}}_{4}^{ 2- }\) > alkalinity > Cl− > Fl− > Br− (Supplementary Material, Tables S1, S2).
CaCO3 deposition dynamics
Proxies of CaCO3 deposition (travertine cover, deposition on leaves, and Ca2+ transfer reactions) indicated that rates during the study period were generally higher in Garden Canyon than Huachuca Canyon and low or undetectable in Ramsey Canyon. The spatially-explicit method, CaCO3 deposition on leaves, showed that within a stream, CaCO3 deposition rates may vary across reaches while tests for significant seasonality of CaCO3 deposition rates were unsupported.
Travertine cover varied significantly among the three streams (F2,40 = 16.79, p < 0.001; Fig. 4). Travertine cover was lowest in Ramsey Canyon stream (9 ± 2 %) and higher in Huachuca Canyon stream (23 ± 7 %) and Garden Canyon stream (70 ± 18 %). Indeed, travertine nearly coated the entire benthic substrate in some reaches (Supplementary Material, Fig. S1).
CaCO3 accumulation rates on dry leaves varied among and within streams. CaCO3 accumulation rates were nearly undetectable in Ramsey Canyon. Only one site exhibited significant rates of CaCO3 deposition, Reach 2. However, the rate was about 75 % less than the average at Garden or Huachuca Canyon: 0.70 mg CaCO3 g dry leaf−1 day−1. Garden Canyon and Huachuca Canyon had similar average accumulation rates (2.50 ± 1.06 mg CaCO3 g dry leaf−1 day−1 and 2.78 ± 1.81 mg CaCO3 g dry leaf−1 day−1, respectively), though accumulation rates were only described significantly by a linear relationship in two of the three reaches in each site (Fig. 5). Huachuca Canyon had the highest rate at a single location: 6.17 mg CaCO3 g dry leaf−1 day−1 in the middle reach, Reach 2.
Calcite saturation indices and CaCO3 deposition rates, in terms of mass transfer and reaction rates, also varied significantly among streams (SIcalcite: F2,19 = 5.773, p < 0.05, mass transfer: F2,28 = 9.308, p < 0.001, reaction rate: F2,26 = 8.583, p < 0.01; Fig. 6). SIcalcite varied seasonally (F3,19 = 9.471, p < 0.01) with lowest values across all streams in the winter (0.40 ± 0.11). While SIcalcite indicated CaCO3 could be depositing in all streams (SIcalcite >0), CaCO3 mass transfer and reaction rates were generally only indicative of CaCO3 deposition in Huachuca and Garden Canyon. In Ramsey Canyon, Ca2+ concentrations generally increased downstream along the study reach, 3.1 ± 2.6 μg Ca2+ L−1 m−1, suggesting some dissolution of CaCO3 along the reach (Fig. 6). In Huachuca Canyon, changes in Ca2+ concentrations along the study reach were variable throughout the study period, 1.55 ± 3.96 μg Ca2+ L−1 m−1. In Garden Canyon, downstream reductions in the concentration of Ca2+ were observed consistently (−10.5 ± 4.8 μg Ca2+ L−1 m−1), indicating regular deposition of CaCO3 throughout the study period. Similarly, reaction rates were near zero in Ramsey Canyon (0.07 ± 0.22 μg CaCO3 L−1 s−1) and Huachuca Canyon (−0.08 ± 0.20 μg CaCO3 L−1 s−1), but always positive in Garden Canyon (0.43 ± 0.40 μg CaCO3 L−1 s−1; Fig. 6). Variation in mass transfer or reaction rates was not significantly related to seasonality.
Calcium carbonate (CaCO3) saturation index (as calcite) and dissolution or deposition rates across Ramsey (RA), Huachuca (HU), and Garden (GA) Canyon streams, AZ, during 2011–2014. Values of Ca2+ mass transfer per stream length or CaCO3 reaction rates below zero indicate dissolution, values above zero indicate deposition. Different letters indicate significant differences (Tukey’s post hoc test, p < 0.05). Error bars represent ±1 standard error
Results of the travertine chemical analysis are reported together as Huachuca Canyon was only sampled in 2012. Across both streams, P content in the travertine was 123 ± 16 mg P kg−1 CaCO3 within the range of P content observed in travertines sampled from across the globe (Pentecost 2005). This concentration equates to a molar Ca:P in the travertine of 2515, dramatically lower than the molar ratio of Ca2+:SRP in the stream water of either Garden or Huachuca Canyons (>20,000).
Nutrients
In general, we found stream water P concentrations were highest in Ramsey Canyon and lowest in Garden Canyon (SRP: F2,22 = 16.031, p < 0.001, TDP: F2,22 = 8.833, p < 0.01, TP: F2,32 = 5.327, p < 0.05; Fig. 7). This pattern was reversed for DOC and N concentrations, although the pattern was not significant for any constituent (DOC: F2,22 = 2.802, p = 0.08, DIN: F2,22 = 2.766, p = 0.08, DON: F2,32 = 0.723, p = 0.49, TDN: F2,32 = 1.691, p = 0.20, TN: F2,22 = 1.486, p = 0.25; Fig. 8). Dissolved organic compounds represented a substantial proportion of total dissolved nutrients. For P, DOP represented over half of TDP, but with a greater proportion in Garden (79 %) compared to Huachuca (67 %) or Ramsey (56 %) Canyon. For N, DON also represented over half of TDN concentrations, with the greatest proportions in Huachuca (91 %) and Ramsey (75 %) compared to Garden (56 %). DIN was comprised mostly of \({\text{NO}}_{3}^{ - }\) across all streams, with \({\text{NH}}_{4}^{ + }\) representing 7, 18, and 4 % of DIN in Ramsey, Huachuca, and Garden Canyon, respectively. The molar ratio of TN to TP (TN:TP) was generally below or similar to the Redfield ratio, 16:1 (Redfield 1958), in Ramsey Canyon and Huachuca Canyon but exceeded the Redfield ratio in Garden Canyon (Fig. 9).
Stream water phosphorus concentrations in Ramsey (RA), Huachuca (HU), and Garden (GA) Canyon streams including soluble reactive phosphorus (SRP), dissolved organic phosphorus (DOP), total dissolved phosphorus (TDP), and total phosphorus (TP). Outliers defined as values greater than 1.5 times the inner quartile range, are removed
Stream water dissolved organic carbon (DOC) and N concentrations in Ramsey (RA), Huachuca (HU), and Garden (GA) Canyon streams including dissolved inorganic N (DIN), dissolved organic N (DON), total dissolved N (TDN), and total N (TN). Outliers defined as values greater than 1.5 times the inner quartile range, are removed
The molar ratio of total nitrogen (TN) and total phosphorus (TP) in Ramsey (RA), Huachuca (HU), and Garden (GA) Canyon streams. Grey horizontal line represents 16:1, the Redfield ratio (1958)
Multivariate analyses
RDA was performed on 41 samples of eight chemical constituents (DIN, DON, TDN, TN, SRP, DOP, TDP, and TP). Six of the sampling points were removed prior to the analysis after being identified as univariate outliers. Collinearity was not detected in the explanatory variables (stream descriptor variables); therefore, all descriptors were included in the analysis (temperature, discharge, CaCO3 reaction rate, CaCO3 mass transfer, and SIcalcite). The analysis revealed a significant two-dimensional ordination in which the stream descriptor variables explained 86 % of the variance in stream nutrient chemistry (p < 0.001; Fig. 10). The angles between the arrows of stream nutrient chemistry revealed high intercorrelation among N species (DON, TDN, and TN) and P species (SRP, TDP, and TP). The negative correlation of DIN and SRP also suggests a stoichiometric ordination on the first axis (Fig. 10). Phosphorus species and CaCO3 deposition rate variables were highly, negatively correlated, supporting the hypothesis that coprecipitation of phosphate with CaCO3 deposition lowers P availability in the stream water in these ecosystems. On the other hand, organic matter concentrations were positively (DOC and DON) or negatively (DOP) correlated with temperature and discharge, indicating the importance of biological activity and hydrologic processes on organic matter cycling. Samples with negative loadings on RDA1 and RDA2 tended to be from the winter and summer, while samples with positive loadings on RDA1 tended to be from the monsoon season and fall (Fig. 10); however, the impact of seasonality was not strongly evident in the triplot, as there was overlap in the sample loadings by season.
RDA ordination plot of stream water chemistry samples across each season. Chemistry variables are shown with thin lines and explanatory variables (CaCO3 deposition rates, as mass transfer, “CaCO3.m”; and reaction rate, “CaCO3.rate”; discharge; temperature, “temp”; calcite saturation index, “SI”) are shown with thick arrows
We also used linear regression to determine if CaCO3 deposition was linked to the stoichiometric ordination on the RDA triplot (Fig. 11). For this test, we used the reaction rate of CaCO3 deposition as the proxy of CaCO3 deposition rates, as it was the descriptor with the highest loading on RDA1 (−0.71; Fig. 11). The N:P ratio, both in terms of DIN:SRP or TN:TP, was positively correlated with CaCO3 deposition rate (DIN:SRP: R2 = 0.51, p < 0.001 and TN:TP: R2 = 0.27, p < 0.001; Fig. 11). Furthermore, deposition of CaCO3 occurred at atomic N:P ratios greater than 16 (Fig. 11a) or 30 (Fig. 11b).
The natural log of the atomic ratio of a dissolved inorganic nitrogen (DIN) to soluble reaction phosphorus (SRP) and b total nitrogen (TN) to total phosphorus (TP) compared to the reaction rate of CaCO3 deposition across all streams. The horizontal line represents a ratio of a 16:1, the Redfield ratio, or b 30:1. Shading marks the region with net positive CaCO3 deposition
Discussion
While many studies of stream ecosystems have focused on understanding the dynamics of single elements (Mulholland and Webster 2010), our study considers how hydrologic and biogeochemical processes may couple or uncouple the supplies and transport of both N and P in streams. Despite similar geomorphology, riparian vegetation, and hydrology, the study streams exhibit significant differences in stream water P chemistry (Fig. 7). Nutrient concentrations were related to discharge and temperature, but, of the variables tested, P availability and nutrient stoichiometry had the most pronounced association with CaCO3 deposition rates. Furthermore, the lower molar ratio of Ca2+ to P in travertine compared to the stream water supports the role of travertine in scavenging P from the water column (House et al. 1986). While it is possible that differences in P concentrations could be due to other differences between the streams that we did not consider, lines of evidence in our study support the hypothesis that CaCO3 deposition reduces stream water P concentrations and thus exports of P from headwater streams to receiving ecosystems. This result expands the role of CaCO3 coprecipitation of phosphate in lowering P availability from nutrient-rich streams (Arvin and Jenkins 1985; Jarvie et al. 2005; Jenkins et al. 1971) to include oligotrophic headwater streams. We also found that the relative amount of P compared to N is lower when there is active CaCO3 deposition and that there is a positive correlation between N concentrations and CaCO3 deposition rate, exacerbating the stoichiometric imbalance of N and P availability related to PO4 coprecipitation.
Discharge and nutrient availability
The multivariate analysis of the study streams suggests that discharge is linked to N concentrations, but, unexpectedly, does not strongly influence P concentrations except in terms of organic matter. There are several potential reasons for this apparent hydrologic decoupling of N and particulate or inorganic P concentrations. First, increases in N or DOC during higher flows may be caused by discharge-induced sediment suspension (Casey and Farr 1982) and because coprecipitated P is bound in CaCO3 deposits that cement the stream bottom (House 2003), P might not easily be suspended in increased flow events. However, as one of the study streams did not exhibit CaCO3 deposition (Ramsey Canyon), sediment P suspension during higher flow events should have been detected in the dataset. Alternatively, watershed P inputs to the streams may be low; therefore, changes in lateral transport may lead to an increase in N, but a dilution of DOP. Another explanation may be that the sampling dates did not occur directly during or after a storm event (Fig. 2) and, thus, we may have missed an increase in nutrient concentrations following spates (Grimm 1992; McDiffett et al. 1989; Meyer et al. 1988). Closer monitoring of these streams will help differentiate whether the mechanism behind the relationships between discharge and nutrient concentrations are due to differences in nutrients in the watersheds or due to the low-frequency sampling scheme.
Biological processes and nutrient cycling
Biological processes are often seasonal, generating predictable lows in nutrient concentrations in temperate streams in the spring and fall when biological demand is greatest due to low canopy cover (Meyer and Likens 1979; Mulholland 2004). The riparian zone of our study streams is dominated by sycamore, maple, and other deciduous trees (Brown 1994); therefore, seasonality in biological processes due to changes in light availability should be similar to streams in temperate forests. Field observations of our study streams suggested that autotrophic growth was seasonal, with periphyton biomass greatest in the spring or summer and lowest in the winter. Thus, the lack of a strong effect of seasonality on stream nutrient concentrations in the multivariate analysis (Fig. 10) may be because other processes that vary with, e.g., temperature, were able to capture the effects of seasonality on nutrient cycling. Indeed, temperature was an important variable for explaining N concentrations in the study streams. The positive relationship between temperature and TN (Fig. 10) suggests that warmer temperatures stimulated decomposition and N mineralization, thereby increasing streamwater N concentrations (Chergui and Pattee 1990; Ferreira and Chauvet 2011).
The riparian zones of streams can also influence nutrient dynamics via microbial mediated organic matter transformations or plant nutrient uptake (e.g., Hill 1996; Bernal et al. 2015). However, as the riparian vegetation and geomorphology was similar across the streams, we consider it unlikely that riparian processes would drive the strong gradients in nutrient chemistry that we observed between streams.
CaCO3 deposition and nutrient availability
The combination of methods used to estimate CaCO3 deposition rates agree with preliminary observations that CaCO3 deposition rates are highest in Garden Canyon and lowest (or not occurring) in Ramsey Canyon. The positive saturation indices and presence of some travertine in Ramsey (Figs. 4, 6) suggest that CaCO3 deposition rates in this stream may have been greater in the past. Observations of periphyton growth on travertine in Garden and Huachuca Canyon streams suggest biologically induced changes to the microenvironment (e.g., due to photosynthesis) may play an important role in influencing deposition patterns (Bissett et al. 2008); this hypothesis deserves further consideration.
The results provide strong support for the role of CaCO3 coprecipitation of phosphate in lowering stream phosphate concentrations. This relationship extends to other forms of P as well, based on both the inverse relationship between various P concentrations and stream identity (Fig. 7) and the grouping of SRP, DOP, TDP, and TP in the multivariate analysis (Fig. 10). These results suggest that retention of phosphate due to coprecipitation influences other aspects of in-stream P cycling, changing the form and quantity of P transported to downstream ecosystems (Withers and Jarvie 2008). The strong spatial variability of CaCO3 deposition rates within a stream (Fig. 5) also indicates that there are “hot spots” of P retention that may be overlooked by coarse-level sampling.
By using multivariate analysis, we found an unexpected positive relationship between CaCO3 deposition and N concentrations. That is, CaCO3 deposition was associated with low P concentrations and high N concentrations. Thus, while temperature was likely an important driver for N concentrations in the streams (Fig. 10), it was not the only process influencing N availability. If it were, N concentrations would have been greatest in Huachuca Canyon, the stream with the highest temperature (Fig. 3), but they were not (Fig. 8). Instead, it seems that greatest variability in nutrient concentrations is driven by CaCO3 deposition.
The positive correlation between N concentrations and CaCO3 deposition may be an indirect outcome of biological responses to reduced P availability. CaCO3 deposition was strongly related to N:P ratios (Fig. 11) and, when CaCO3 deposition was detectable, molar N:P ratios in the water were generally greater than 30, a threshold indicative of potential P limitation of primary producers (Downing and McCauley 1992). A recent study of nutrient cycling in different montane headwater streams suggests that stoichiometric constraints on biological nutrient uptake can be detected from ecosystem-scale measurements of nutrient cycling (Schade et al. 2011). Schade and co-authors showed that streams dominated by heterotrophic processes exhibit a strong coupling of N and P uptake. Hence, under P limitation, the ability of the biota, particularly heterotrophic microbial communities, to sequester and process N may be reduced allowing N to reach higher concentrations in the water column. High N:P ratios have been documented in other CaCO3-depositing streams (Elser et al. 2005; Marks et al. 2006) and wetlands (Noe et al. 2001); we suggest that, in all of these systems with high rates of CaCO3 deposition, biological N demand and, therefore, biological N uptake, are lower because the organisms are P-limited.
Comparisons with other CaCO3-depositing streams
CaCO3-depositing streams are found across the world (Pentecost 2005), including in Australia (e.g., Drysdale et al. 2002), Asia (e.g., Liu et al. 2010; Lu et al. 2000), Europe (e.g., Auqué et al. 2013; House and Denison 1997; Merz-Preiß and Riding 1999), and North America (e.g., Herman and Lorah 1987; Jacobson and Langmuir 1970; Malusa et al. 2003). Compared with other headwater streams, CaCO3 deposition rates found in the study streams of the Huachuca Mountains are low (Figs. 5, 6). For instance, in Falling Springs Creek, VA, USA, and Fossil Creek, AZ, USA, calculated CaCO3 deposition rates range over a magnitude of order higher (3.3–93 μg CaCO3 L−1 s−1, Herman and Lorah 1987; 1.2–39 μg CaCO3 L−1 s−1, Malusa et al. 2003) than those from Garden Canyon (e.g., 0.5 μg CaCO3 L−1 s−1; Fig. 6). Therefore, we expect that the stoichiometric feedback between CaCO3 deposition and nutrient cycling may be even stronger in other CaCO3-depositing streams.
CaCO3 deposition is potentially related to many ecological processes in streams. For example, CaCO3 encases leaf litter, which is thought to decrease rates of detrital decomposition in CaCO3-depositing reaches of streams (Casas and Gessner 1999; Martínez et al. 2014). CaCO3 deposition is also related to increased rates of leaf litter decomposition as travertine dams increase turbulence and therefore physical breakdown of leaf material (Carter and Marks 2007; Milisa et al. 2010). CaCO3-depositing reaches have also been found to host higher biodiversity of macro-organisms (Carter and Marks 2007; Marks et al. 2006), but lower overall macroinvertebrate abundance (Carter and Marks 2007; Martínez et al. 2014), although the reason is unknown. Additionally, some organisms interact directly with CaCO3 deposits. Amphipods in a stream in southern Germany use CaCO3 as body armor to reduce predation pressure (Ruff and Maier 2000). Microbes, as well, have long interacted with CaCO3 deposits, both promoting and inhibiting lithification (Dupraz and Visscher 2005). When biofilms interact with CaCO3 deposits, phosphate coprecipitation rates may be lower than expected, suggesting that CaCO3 formation may not always result in decreased P bioavailability (Jarvie et al. 2002). Microbial metabolic processes in a biofilm may also lead to diurnal dissolution of CaCO3 deposits, rendering P available for biological uptake (Hagerthey et al. 2011). In future research, it will be important to distinguish under what conditions CaCO3 deposition acts as a permanent versus temporary sink of P.
Conclusion
The total and relative amounts of nutrients available in an ecosystem have important consequences for numerous ecological processes. Different hydrologic and biogeochemical processes are associated with changes in nutrient concentrations in streams (Alexander et al. 2007) and our results indicate that CaCO3 deposition can regulate the N:P stoichiometry of stream water nutrients. Furthermore, while growth of primary producers in streams is often found to be co-limited by N and P (Dodds and Welch 2000; Elser et al. 2007; Francoeur 2001), our results suggest CaCO3 deposition may be a geochemical scenario in which primary P limitation may prevail. These influences are important because streams with active CaCO3 deposition are geographically widespread (Pentecost 2005). However, stream beds are not pure CaCO3 substrates, and the presence of other minerals, organic matter, or biofilms may alter expected relationships between CaCO3 deposition rates and phosphate coprecipitation (Jarvie et al. 2002). Therefore, kinetic experiments with sediment sorption capacity may be useful in translating these results to other streams (Demars 2008; Jarvie et al. 2005). These data add to accumulating research on CaCO3-depositing streams that document the biogeochemical effects of CaCO3 on ecosystems and highlight the potential for managing CaCO3 to minimize downstream P flows, but not N flows, to receiving environments.
References
Alexander RB, Boyer EW, Smith RA, Schwarz GE, Moore RB (2007) The role of headwater streams in downstream water quality. J Am Water Resour Assoc 43:41–59
APHA (2005) Standard methods for the examination of water and wastewater. APHA-AWWA-WEF, Washington, DC
Arvin E, Jenkins D (1985) Biological removal of phosphorus from wastewater. Crit Rev Environ Sci Technol 15:25–64
Aspila KI, Agemian H, Chau ASY (1976) A semi-automatic method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Auqué L, Arenas C, Osácar C, Pardo G, Sancho C, Vázquez-Urbez M (2013) Tufa sedimentation in changing hydrological conditions: the River Mesa (Spain). Geol Acta 11:85–102
Austnes K, Evans C, Eliot-Laize C, Naden P, Old G (2010) Effects of storm events on mobilisation and in-stream processing of dissolved organic matter (DOM) in a Welsh peatland catchment. Biogeochemistry 99:157–173. doi:10.1007/s10533-009-9399-4
Avilés A, Rodero J, Amores V, de Vicente I, Rodríguez MI, Niell FX (2006) Factors controlling phosphorus speciation in a Mediterranean Basin (River Guadalfeo, Spain). J Hydrol 331:396–408. doi:10.1016/j.jhydrol.2006.05.024
Bernal S, Lupon A, Ribot M, Sabater F, Martí E (2015) Riparian and in-stream controls on nutrient concentrations and fluxes in a headwater forest stream. Biogeosciences 12:1941–1954
Bissett A, de Beer D, Schoon R, Shiraishi F, Reimer A, Arp G (2008) Microbial mediation of stromatolite formation in karst-water creeks. Limnol Oceanogr 53:1159–1168
Borovec J, Sirová D, Mošnerová P, Rejmánková E, Vrba J (2010) Spatial and temporal changes in phosphorus partitioning within a freshwater cyanobacterial mat community. Biogeochemistry 101:323–333. doi:10.1007/s10533-010-9488-4
Boyer M, Wheeler B (1989) Vegetation patterns in spring-fed calcareous fens: calcite precipitation and constraints on fertility. J Ecol 77:597–609
Brown DE (ed) (1994) Biotic communities: southwestern United States and northwestern Mexico. University of Utah Press, Salt Lake City
Carter CD, Marks JC (2007) Influences of travertine dam formation on leaf litter decomposition and algal accrual. Hydrobiologia 575:329–341
Casas J, Gessner M (1999) Leaf litter breakdown in a Mediterranean stream characterised by travertine precipitation. Freshw Biol 41:781–793
Casey H, Farr I (1982) The influence of within-stream disturbance on dissolved nutrient levels during spates. Hydrobiologia 91:447–462
Chave KE (1965) Carbonates: association with organic matter in surface seawater. Science 148:1723–1724
Chergui H, Pattee E (1990) The influence of season on the breakdown of submerged leaves. Arch Hydrobiol 120:1–12
Cohen MJ, Kurz MJ, Heffernan JB, Martin JB, Douglas RL, Foster CR, Thomas RG (2013) Diel phosphorus variation and the stoichiometry of ecosystem metabolism in a large spring-fed river. Ecol Monogr 83:155–176
Dahm CN, Baker MA, Moore DI, Thibault JR (2003) Coupled biogeochemical and hydrological responses of streams and rivers to drought. Freshw Biol 48:1219–1231
Demars BO (2008) Whole-stream phosphorus cycling: testing methods to assess the effect of saturation of sorption capacity on nutrient uptake length measurements. Water Res 42:2507–2516. doi:10.1016/j.watres.2008.02.010
Dodds WK, Oakes RM (2008) Headwater influences on downstream water quality. Environ Manag 41:367–377
Dodds WK, Welch EB (2000) Establishing nutrient criteria in streams. J N Am Benthol Soc 19:186–196
Dorioz J, Pilleboue E, Ferhi A (1989) Phosphorus dynamics in watersheds: role of trapping processes in sediments [Dynamique du phosphore dans les bassins versants: importance des phenomenes de retention dans les sediments]. Water Res 23:147–158
Downing JA, McCauley E (1992) The nitrogen:phosphorus relationship in lakes. Limnol Oceanogr 37:936–945
Drysdale RN, Taylor MP, Ihlenfeld C (2002) Factors controlling the chemical evolution of travertine-depositing rivers of the Barkly karst, northern Australia. Hydrol Process 16:2941–2962
Dupraz C, Visscher PT (2005) Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol 13:429–438. doi:10.1016/j.tim.2005.07.008
Elser JJ et al (2005) Effects of phosphorus enrichment and grazing snails on modern stromatolitic microbial communities. Freshw Biol 50:1808–1825. doi:10.1111/j.1365-2427.2005.01451.x
Elser JJ et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. doi:10.1111/j.1461-0248.2007.01113.x
Ferreira V, Chauvet E (2011) Future increase in temperature more than decrease in litter quality can affect microbial litter decomposition in streams. Oecologia 167:279–291. doi:10.1007/s00442-011-1976-2
Fisher SG, Sponseller RA, Heffernan JB (2004) Horizons in stream biogeochemistry: flowpaths to progress. Ecology 85:2369–2379
Francoeur SN (2001) Meta-analysis of lotic nutrient amendment experiments: detecting and quantifying subtle responses. J N Am Benthol Soc 20:358–368
Geisser S, Greenhouse SW (1958) An extension of box’s results on the use of the F distribution in multivariate analysis. Ann Math Stat 29:885–891
Grimm NB (1992) Biogeochemistry of nitrogen in Sonoran Desert streams. J Ariz Nev Acad Sci 26:139–155
Grimm NB, Fisher SG (1986) Nitrogen limitation in a Sonoran Desert stream. J N Am Benthol Soc 5:2–15
Hagerthey SE, Bellinger BJ, Wheeler K, Gantar M, Gaiser E (2011) Everglades periphyton: a biogeochemical perspective. Crit Rev Environ Sci Technol 41:309–343. doi:10.1080/10643389.2010.531218
Hamilton SK, Bruesewitz DA, Horst GP, Weed DB, Sarnelle O (2009) Biogenic calcite–phosphorus precipitation as a negative feedback to lake eutrophication. Can J Fish Aquat Sci 66:343–350. doi:10.1139/f09-003
Herman JS, Lorah MM (1987) CO2 outgassing and calcite precipitation in Falling Spring Creek, Virginia, U.S.A. Chem Geol 62:251–262. doi:10.1016/0009-2541(87)90090-8
Hill AR (1996) Nitrate removal in stream riparian zones. J Environ Qual 25:743–755
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
House W (1990) The prediction of phosphate coprecipitation with calcite in freshwaters. Water Res 24:1017–1023
House WA (2003) Geochemical cycling of phosphorus in rivers. Appl Geochem 18:739–748
House WA, Denison FH (1997) Nutrient dynamics in a lowland stream impacted by sewage effluent: Great Ouse, England. Sci Total Environ 205:25–49
House WA, Donaldson L (1986) Adsorption and coprecipitation of phosphate on calcite. J Colloid Interface Sci 112:309–324
House WA, Casey H, Smith S (1986) Factors affecting the coprecipitation of inorganic phosphate with calcite in hardwaters—II. Water Res 20:923–927
Hu YB, Dieckmann GS, Wolf-Gladrow DA, Nehrke G (2014) Laboratory study on coprecipitation of phosphate with ikaite in sea ice. J Geophys Res Oceans 119:7007–7015
Jacobson RL, Langmuir D (1970) The chemical history of some spring waters in carbonate rocks. Groundwater 8:5–9
Jaeger K, Olden J (2012) Electrical resistance sensor arrays as a means to quantify longitudinal connectivity of rivers. River Res Appl 28:1843–1852
Jäger P, Röhrs J (1990) Phosphorfällung über Calciumcarbonat im eutrophen Wallersee (Salzburger Alpenvorland, Österreich). [Coprecipitation of phosphorus with calcite in the eutrophic Wallersee (Alpine Foreland of Salzburg, Austria)]. Int Rev gesamten Hydrobiol Hydrogr 75:153–173
Jarvie H et al (2002) Phosphorus uptake into algal biofilms in a lowland chalk river. Sci Total Environ 282:353–373
Jarvie HP, Jürgens MD, Williams RJ, Neal C, Davies JJ, Barrett C, White J (2005) Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye. J Hydrol 304:51–74
Jarvie HP, Neal C, Withers PJ (2006) Sewage-effluent phosphorus: a greater risk to river eutrophication than agricultural phosphorus? Sci Total Environ 360:246–253. doi:10.1016/j.scitotenv.2005.08.038
Jenkins D, Ferguson JF, Menar AB (1971) Chemical processes for phosphate removal. Water Res 5:369–389
Kleiner J (1988) Coprecipitation of phosphate with calcite in lake water: a laboratory experiment modelling phosphorus removal with calcite in Lake Constance. Water Res 22:1259–1265
Liu Z, Sun H, Baoying L, Xiangling L, Wenbing Y, Cheng Z (2010) Wet–dry seasonal variations of hydrochemistry and carbonate precipitation rates in a travertine-depositing canal at Baishuitai, Yunnan, SW China: implications for the formation of biannual laminae in travertine and for climatic reconstruction. Chem Geol 273:258–266. doi:10.1016/j.chemgeo.2010.02.027
Lorah MM, Herman JS (1988) The chemical evolution of a travertine-depositing stream: geochemical processes and mass transfer reactions. Water Resour Res 24:1541–1552
Lu G, Zheng C, Donahoe R, Berry Lyons W (2000) Controlling processes in a CaCO3 precipitating stream in Huanglong Natural Scenic District, Sichuan, China. J Hydrol 230:34–54
Malusa J, Overby ST, Parnell RA (2003) Potential for travertine formation. Appl Geochem 18:1081–1093. doi:10.1016/s0883-2927(02)00241-x
Marks JC, Parnell R, Carter C, Dinger EC, Haden GA (2006) Interactions between geomorphology and ecosystem processes in travertine streams: implications for decommissioning a dam on Fossil Creek, Arizona. Geomorphology 77:299–307. doi:10.1016/j.geomorph.2006.01.008
Martínez A, Pérez J, Molinero J, Sagarduy M, Pozo J (2014) Effects of flow scarcity on leaf-litter processing under oceanic climate conditions in calcareous streams. Sci Total Environ 503–504:251–257
Mattsson T, Kortelainen P, Räike A, Lepistö A, Thomas DN (2015) Spatial and temporal variability of organic C and N concentrations and export from 30 boreal rivers induced by land use and climate. Sci Total Environ 508:145–154
McDiffett WF, Beidler AW, Dominick TF, McCrea KD (1989) Nutrient concentration–stream discharge relationships during storm events in a first-order stream. Hydrobiologia 179:97–102
McGarigal K, Cushman S, Stafford SG (2000) Multivariate statistics for wildlife and ecology research. Springer, New York
Merz-Preiß M, Riding R (1999) Cyanobacterial tufa calcification in two freshwater streams: ambient environment, chemical thresholds and biological processes. Sediment Geol 126:103–124. doi:10.1016/S0037-0738(99)00035-4
Meyer JL, Likens GE (1979) Transport and transformation of phosphorus in a forest stream ecosystem. Ecology 60:1255–1269
Meyer JL et al (1988) Elemental dynamics in streams. J N Am Benthol Soc 7:410–432
Milisa M, Belancic A, Kepčija RMk, Sertic-Peric M, Ostojic A, Habdija I (2010) Calcite deposition in karst waters is promoted by leaf litter breakdown and vice versa. Ann Limnol 46:225–232
Mooshammer M et al (2012) Stoichiometric controls of nitrogen and phosphorus cycling in decomposing beech leaf litter. Ecology 93:770–782
Mulholland PJ (2004) The importance of in-stream uptake for regulating stream concentrations and outputs of N and P from a forested watershed: evidence from long-term chemistry records for Walker Branch Watershed. Biogeochemistry 70:403–426
Mulholland PJ, Hill WR (1997) Seasonal patterns in streamwater nutrient and dissolved organic carbon concentrations: separating catchment flow path and in-stream effects. Water Resour Res 33:1297–1306
Mulholland PJ, Webster JR (2010) Nutrient dynamics in streams and the role of J-NABS. J N Am Benthol Soc 29:100–117
Neal C (1999) The potential for phosphorus pollution remediation by calcite precipitation in UK freshwaters. Hydrol Earth Syst Sci 5:119–131
Noe GB, Childers DL, Jones RD (2001) Phosphorus biogeochemistry and the impact of phosphorus enrichment: why is the Everglades so unique? Ecosystems 4:603–624
Oksanen J et al (2013) Vegan: community ecology package, R package version 2.1-10. http://vegan.r-forge.r-project.org/. Accessed 22 Aug 2014
Otsuki A, Wetzel RG (1972) Coprecipitation of phosphate with carbonates in a marl lake. Limnol Oceanogr 17:763–767
Parkhurst DL, Appelo CAJ (1999) Description of input and examples for PHREEQC (version 3)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Techniques and Methods 6–A43, U.S. Geological Survey, 519 pp
Pentecost A (2005) Travertine. Springer, Berlin
Peterson BJ et al (2001) Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Pinheiro J, Bates D, Debroy S, Sarkar D, Team RC (2014) nlme: linear and non-linear mixed effects models, R package version 3.1-117. http://CRAN.R-project.org/package=nlme. Acessed 22 Aug 2014
R Core Team (2014) R: a language and environment for statistical computing. R Core Team, Vienna
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Ruff H, Maier G (2000) Calcium carbonate deposits reduce predation pressure on Gammarus fossarum from salamander larvae. Freshw Biol 43:99–105
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect Plant Ecol Evol Syst 14:33–47
Schade JD et al (2011) The stoichiometry of nitrogen and phosphorus spiralling in heterotrophic and autotrophic streams. Freshw Biol 56:424–436. doi:10.1111/j.1365-2427.2010.02509.x
Smith VH (1983) Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221:669–671
Solorzano L, Sharp JH (1980) Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol Oceanogr 25:754–758
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Tabachnick BG, Fidell LS (2001) Using multivariate statistics, 5th edn. Allyn and Bacon, Inc., Needham Heights
ter Braak CJ, Prentice IC, Caswell H (1988) A theory of gradient analysis. Adv Ecol Res 34:235–282
Triska FJ, Jackman AP, Duff JH, Avanzino RJ (1994) Ammonium sorption to channel and riparian sediments: a transient storage pool for dissolved inorganic nitrogen. Biogeochemistry 26:67–83
Withers P, Jarvie H (2008) Delivery and cycling of phosphorus in rivers: a review. Sci Total Environ 400:379–395
WRRC (2014) Monthly climate summary, Fort Huachuca, Arizona. Western Regional Climate Center. http://www.wrcc.dri.edu/cgi-bin/cliMAIN.pl?az3120. Accessed 16 Nov 2014
Acknowledgments
The authors would like to thank Nicole Nevarez, Megan (Brundage) Freeman, Robin Greene, Chanelle Hope, Cathy Kochert, Roy Erickson, Krist Rouypirom, Joseph Rittenhouse, Elizabeth Gaige, and all other members of Traverteam for help with field and laboratory analyses. Thank you to Sheridan Stone (US Department of Defense, Fort Huachuca) and Brooke Gebow (The Nature Conservancy, Ramsey Canyon Nature Preserve) for logistical support. This work was supported in-kind by a Department of Defense Strategic Environment Research and Development Project (SERDP, RC-1726) and with funding to JRC from Science Foundation Arizona, the Achievement Rewards for College Scholars (ARCS) Program, the California Lake Management Society, and the ASU Graduate and Professional Student Association Graduate Research Support Program and to JJE from the US National Science Foundation (DEB-0950175) and the National Aeronautics and Space Administration (NASA) Astrobiology Program (NAI5-0018). This manuscript was much improved by feedback from Nancy Grimm, Michelle McCrackin, Daniel Childers, and three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: James Sickman.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Corman, J.R., Moody, E.K. & Elser, J.J. Stoichiometric impact of calcium carbonate deposition on nitrogen and phosphorus supplies in three montane streams. Biogeochemistry 126, 285–300 (2015). https://doi.org/10.1007/s10533-015-0156-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0156-6