Abstract
Factors regulating the persistence of lignin in terrestrial ecosystems are highly debated, and recently lignin has been proposed to have a low residence time in soils. The catalytic efficiency and activation energy of the oxidoreductase enzymes regulate the residence time of lignin in soils. We characterized the spatial and seasonal changes in the apparent Michaelis–Menten kinetics and activation energy of soil peroxidases in three ecosystems of differing litter chemistries- pine forest, deciduous forest, and agricultural ecosystem. Peroxidases rarely follow true Michaelis–Menten kinetics, hence we measured the apparent K m (App K m ) and apparent V max (App V max ) of soil peroxidases under potentially H2O2 non-limiting conditions using 3,3′,5,5′-tetramethylbenzidine as the reducing substrate. The App V max and App K m measured in this study, when used independently, exhibited only a weak relationship with total quantity and composition of lignins. Hence, we adopted the ratio of these two parameters (App V max /App K m ) to define the apparent catalytic efficiency (App CE) of peroxidases. Across the three ecosystems and seasons, the App CE of peroxidase was ecosystem specific, and exhibited a strong correlation with the monolignol composition of the resident plant species. The App CE of peroxidase was higher in agricultural soils that had lower lignin content. Pine soils with a higher relative proportion of vanillyl units that contribute to more recalcitrant inter-unit linkages in lignins exhibited the highest App V max and App K m , resulting in the lowest App CE. This decoupling of App CE of peroxidases from chemistry of the native lignin observed in our assay-system could indicate a relatively longer persistence of lignin in ecosystems receiving recalcitrant litter inputs. Even though the apparent activation energy (App Ea) of peroxidases varied by depth and seasons across ecosystems, the App Ea did not relate to the chemistry or quantity of lignins, probably due to the substrate-saturated assay conditions. Our study captures the apparent kinetics of peroxidases in soils, which was ecosystem specific, and in part regulated by the composition of monolignols, thus providing a preliminary linkage between lignin chemistry and enzyme properties in natural systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignin, the second most abundant biopolymer on Earth (Gleixner et al. 2001), is traditionally thought to have a high biosequestration potential for atmospheric CO2 due to its lower susceptibility to undergo decomposition (Berg and McClaugherty 2003). However, recent investigations have convincingly shown that lignins turnover rapidly in soils than previously anticipated, with a turnover rate <10 years (Rasse et al. 2006; reviewed by Dungait et al. 2011; Schmidt et al. 2011), indicating that the factors regulating lignin decomposition in soils are less understood. One of the main factors that could control the rate of biodegradation of a polymeric substrate is the abundance and activity of the depolymerizing enzyme (Wallenstein et al. 2011; Davidson et al. 2012). Substrate abundance also plays a major role in determining the catalytic efficiency of enzymes. For example, the substrate availability regulates the production of hydrolases in soils, which in turn moderates the rate of degradation of polysaccharides (German et al. 2011). However, a similar relationship connecting the kinetics and activation energy of oxidoreductases to soil lignin dynamics remains largely unexplored.
The linkages connecting monolignols within the polymeric lignin units contribute to the stability of lignin, and is one of the primary factors that govern its rate of degradation (Hedges and Mann 1979; Campbell and Sederoof 1996; Boerjan et al. 2003; Grabber 2006; Talbot et al. 2012; Suseela et al. 2013, 2014). Lignin is comprised of three phenylpropanoid units: guaiacyl (G; vanillyl), syringyl (S), and p-hydroxyphenyl (H), which differ in the degree of methoxy substitution on the aromatic ring (Vanholme et al. 2010). Gymnosperm lignins are predominantly composed of G units, whereas angiosperm lignins are composed of approximately equivalent mixtures of G and S units, and lignins of grasses are composed of mixture of G, S, and H units (reviewed by Boerjan et al. 2003). The relative contribution of each lignin unit affects the relative abundance of the structural linkages in lignin. Since the C5 of the aromatic ring is available for coupling reactions in G unit, the gymnosperm lignins are abundant in β-5, and 5-5 inter-unit linkages, which are less prone to cleavage due to the C–C bonds. Contrary to this, the inter-unit linkages connecting S–S and S–G units are dominated by β-O-4 (β-aryl ether) bonds that are more susceptible to chemical cleavage (Bahri et al. 2006). Due to the above differential susceptibility of the monomeric linkages to degradation, the type and abundance of the phenylpropanoid units that vary across ecosystems (Hedges and Mann 1979; Otto and Simpson 2006; Thevenot et al. 2010) could regulate the residence time of lignin in soils (Hedges and Mann 1979; Bahri et al. 2006). Thus, as observed for hydrolases (German et al. 2011), the dynamics of lignin chemistry could also partially regulate the production and activity of oxidoreductases. However, it is less understood how lignin dynamics, namely the composition and abundance of lignin units in soils is related to the abundance and activity of oxidoreductases.
A second factor that could affect the rate of lignin decay is the catalytic efficiency of the oxidative enzymes (Bach et al. 2013). The enzyme characteristics is described by the Michaelis–Menten kinetics, and activation energy (Davidson and Janssens 2006; Wallenstein et al. 2011). In this study, the kinetic constants are defined as (i) the maximum rate of enzyme activity (V max ), and (ii) the substrate concentration at which 50 % V max is achieved (Michaelis constant −K m ). Conceptually, the K m , which is the ratio of the rate of dissociation of enzyme-substrate complex to the rate of association of enzyme and substrate, represents the substrate affinity of the enzyme under non-saturating substrate concentrations (Fig. S1a), whereas V max defines the rate of reaction under saturating substrate concentrations. Combined, the enzyme that achieves a higher V max at a lower K m can be considered more efficient. Alternatively, when comparing two enzymes with the same K m but with different V max (Marx et al. 2005), the enzyme with the higher V max can be considered more efficient as it can achieve a higher rate of product formation at K m (Fig. S1). From an ecosystem perspective, the Michaelis–Menten kinetics of hydrolases has been shown to vary by soil depth, and seasons (Marx et al. 2005; Bell et al. 2010; Brzostek and Finzi 2012). Given that the interaction of Michaelis–Menten kinetic parameters and activation energy ultimately govern the rate of substrate depolymerization (Bengtson and Bengtsson 2007; Davidson et al. 2012), linking the apparent kinetic behavior of oxidoreductases to the litter chemistry is pivotal to our understanding of lignin decomposition.
However, Michaelis–Menten kinetics are more applicable to simple enzyme reactions that involve product formation following the binding of a single substrate to single catalytic site. As the kinetics of the reaction catalyzed by peroxidases are influenced by concentrations of both the reducing substrate and hydrogen peroxide, and as the saturation kinetics are rarely observed, these reactions seldom follow Michaelis–Menten kinetics (Chance 1943). Also, the free radical of the substrates resulting from the two consecutive one-step reduction of the oxidized enzyme undergo random coupling reactions to form mixtures of condensation products (Yu et al. 1994; Oudgenoeg et al. 2002), which confounds the measurement of the progression of the reaction (Wang et al. 2012). However, formation of stable Michaelis–Menten complex between horseradish peroxidases and several reducing substrates has been reported under specific assay conditions (Childs and Bardsley 1975; Rodríguez-López et al. 2000; Goodwin and Hertwig 2003; Wang et al. 2012). In this study, we characterized the apparent Michaelis–Menten kinetics, and apparent activation energy of a class of oxidative enzymes functionally classified as peroxidases by varying the 3,3′,5,5′-tetramethylbenzidine (TMB) concentration under a constant, non-limiting, supply of hydrogen peroxide (pseudo-first-order). The low pH optima and high redox potential of peroxidases (Wong 2009) enables these enzymes to oxidize aromatic compounds more efficiently than phenoloxidases (Wong 2009; Lundell et al. 2010), and therefore are appropriate target enzymes to understand the dynamics of lignin degradation. We determined the apparent efficiency and apparent activation energy of soil peroxidases as influenced by the native lignin content and chemistry across a pine, deciduous, and agricultural ecosystems. We hypothesized that soils with a lower total lignin content and a higher relative proportion of guaiacyl units in lignin would be characterized by peroxidases that exhibit a higher apparent catalytic efficiency and activation energy, potentially enabling peroxidases to overcome the bottle-neck in degrading these relatively recalcitrant lignins.
Materials and methods
Collection and processing of soils
Three ecosystems (a pine forest, a deciduous forest, and a cultivated agricultural field) that received qualitatively and quantitatively different litter inputs were selected for this study (Table 1). The soils of deciduous and pine forests were classified as Typic Kanhapludult, described as a pacolet fine sandy loam soil. The dominant species of the deciduous forest were white oak (Quercus alba), red oak (Quercus rubra), and mockernut hickory (Carya tomentosa). The pine forest was dominated by viriginia pine (Pinus virginiana) with sparse understory vegetation. The agricultural soil is described as thermic, Typic Udifluvent, typical of the alluvial soils, which has been in cultivation for at least 8 years under a corn–soybeans rotation. The selected sites were within 25 km from each other and experienced similar climates (Fig. S2). All the sites were sampled in 2012 during the first week of May, last week of June, and first week of September to capture soil enzyme activities, representing spring, summer, and fall seasons, respectively. At each site, three plots (6 × 6 m) were randomly selected for soil sampling, each plot was at least 15 m apart from neighboring plots. Within each plot, three soil cores (10 cm diameter) were taken at 5 cm intervals to a depth of 15 cm. Soils from the same depth within a plot were pooled to create a total of three samples per depth, per site. The same plots were re-visited each season for sample collection. Upon collection, the soil samples were kept at 4 °C until arrival in the lab, passed through a 2 mm sieve, and stored at −20 °C until analysis.
CuO oxidation for lignin analysis
To determine the lignin chemistry we conducted CuO oxidation of lignins in the soil collected during the spring as described by Otto and Simpson (2006) and Kaiser and Benner (2012). Briefly, soil equivalent to approximately 25 mg soil C, was combined with 1 g CuO, 150 mg ferrous ammonium sulfate and 14 ml of 2 M NaOH that had been sparged for 20 min with argon in a 23 ml Teflon-lined acid digestion vessel. Further, each vessel was sparged with argon for an additional 15 min before closing. The samples were heated at 160 °C for 160 min and then allowed to cool to room temperature. Upon cooling, the supernatants were transferred to centrifuged tubes, and 100 µl of the internal standards (cinnamic acid and ethylvanillin, 200 µg ml−1 in 100 % methanol) were added to each sample, followed by 3 ml of 18 N H2SO4. The samples were placed at 4 °C for 10 min, followed by centrifugation to settle the humic precipitates. Ten ml of the supernatant was extracted with 4 ml of ethyl acetate, the ethyl acetate fraction was collected, and stored at −20 °C for further analysis.
In preparation for high pressure liquid chromatography (HPLC) analysis of depolymerized lignin (Sun et al. 2015), 400 µl of the ethyl acetate fraction was evaporated under N2 and reconstituted in 25 % methanol. The samples were analyzed using a HPLC system (Shimadzu Corporation, Kyoto, Japan) coupled to a photodiode array detector and compounds were separated on a the Kinetex column (C18, 100 × 4.6 mm; Phenomenex, Torrence, CA, USA). Each compound was analyzed at the wavelength that corresponded to their λmax (Table S1). The mobile phases consisted of 0.05 % formic acid, and 100 % methanol, and the quantitation of phenolic compounds was based on standard curve using respective authentic standards. The concentrations of phenolic compounds were normalized to µg g−1 of soil C and the total lignin concentration (the sum of the vanillyl (V), syringyl (S), and cinnamyl (C) units; SVC lignin) were calculated (Otto and Simpson 2006; Kaiser and Benner 2012). The variation in the total abundance lignin monomer units was captured by computing C/V, P/V [p-hydroxbenzoic (P)] and S/V ratios, as described by Wang et al. (2015). In addition to these ratios, we computed the V/SVC ratio that specifies the relative proportion of vanillyl units in the total SVC lignin.
Michaelis–Menten kinetics
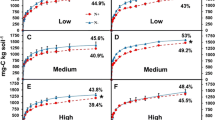
Compared to other soil enzyme assays, the determination of the apparent activity of peroxidases in soils is susceptible to multiple interferences, a subject that has been extensively and elegantly reviewed by Bach et al. (2013). The l-3,4-dihydroxyphenylalanine (l-DOPA) substrate is commonly used for the measurement of potential activity of soil oxidoreductases. However, unlike other o-diphenol substrates, l-DOPA rarely exhibits Michaelis–Menten kinetics with horseradish peroxidase (Rodríguez-López et al. 2000). Thus, for characterization of the apparent Michaelis–Menten kinetics (App V max and App K m ) of soil peroxidase 3,3′,5,5′-tetramethylbenzidine (TMB) was used as the substrate (Josephy and Mason 1982; Johnsen and Jacobsen 2008). The stock solution (2.5 mM) of the substrate was prepared fresh by dissolving the TMB in 20 mM citrate buffer (pH 3.0). Warming the solution to 45 °C while stirring facilitated a faster dissolution of TMB. Lower pH aids in the dissolution of TMB in water by protonating the molecule (pKa of 4.3 and 3.3). Citric acid exhibits a very low radical-quenching capacity (Scalzo 2008) compared to ascorbic acid that quench the substrate radical to maintain a pseudo-first order condition in peroxidase assays (Goodwin et al. 1995; Rodríguez-López et al. 2000). By chelating metals (Pizzocaro et al. 1993) that facilitates Fenton’s reaction (Perron and Brumaghim 2009) citric acid could reduce the rate of hydroxyl-radical formation, and thus prevent the non-enzymatic oxidation of TMB. Direct reaction of citric acid with peroxidase in the absence of TMB was not detected in our assay system (mass spectrometric studies, data not shown) however non-optimized levels of citrate could chelate with the metal center of peroxidases resulting in a lower catalytic efficiency. The TMB stock was serially diluted using citrate buffer, and was kept away from direct light to prevent any potential photo-oxidation. The TMB sock solutions were colorless, indicating absence of any auto-oxidation, at the start of all experiments. Excess amounts of both substrate radical (Huang et al. 2005) as well as H2O2 (Arnao et al. 1990) is shown to hamper the catalytic efficiency of peroxidases. Based on our initial experiments that optimized for H2O2 concentration and incubation time, the soil slurries were prepared by blending 200 mg of soil with 100 ml of 50 mM acetate buffer (pH 5.0) for 1 min. To measure Michaelis–Menten kinetics, 200 µl of the respective TMB standards were combined with 50 µl of H2O2 (0.3 %) and 200 µl of the soil slurry in 2 ml micro-centrifuge tubes to obtain a final TMB concentration of 0, 38, 62, 125, 250, 375, 500, 750, 1000 µM, under identical background matrix. Citric acid reduced pH of the final assay to 4.8, closer to the mean pH across the study soils (pH 5.0; Table 1). The sample blank contained the soil slurry combined with the H2O2 and 200 µl of acetate buffer but with no substrate, and the substrate blank contained all reagents except for the soil slurry. The soil slurries did not cause measurable oxidation of TMB in the absence of H2O2 during the assay period (Fig. S7). The samples were incubated at 25 °C on a rotary shaker (26 rpm) for 30 min. The reaction was terminated with the addition of 1 ml of 5 % H2SO4, followed by centrifugation and the absorbance of supernatant was measured at 450 nm on a UV–Vis spectrophotometer.
The maximal enzyme activity was calculated using the molar extinction coefficient of the yellow chromophore (representing two-electron oxidized TMB) 5.9 × 104 M−1 cm−1 (Josephy and Mason 1982). This was followed by nonlinear regression analysis to the Michaelis–Menten equation to acquire the App K m and App V max constants using GraphPad (La Jolla, CA, USA):
where [S] is the substrate concentration.
Initial experiments confirmed the Michaelis–Menten kinetic responses of soil peroxidase using TMB as the substrate in all soils (Fig. 1). The agricultural soils showed a steady V max , while the V max of the pine and deciduous soils were less distinct, potentially indicating the necessity of optimizing the assay conditions for each soil-type. Majority of the biological immunoassays that track physiological peroxidase activity use TMB as reducing substrates due to its high oxidation susceptibility (redox potential of +250 and +400 mV for two electron oxidation at pH 5.0, Volpe et al. 1998) and remarkably higher sensitivity. For example, the molar extinction coefficient of the two electron oxidation product of TMB is 5.9 × 104 mol−1 cm−1 (E450nm; Josephy and Mason 1982) compared to 4.2 for pyrogallol, 7.9 for l-DOPA, and 73 for ABTS. Thus for the same rate of oxidation, TMB offers approximately 14,000, 8400, and 800 fold higher sensitivity of oxidation measurements than pyrogallol, l-DOPA and ABTS, respectively. Also, the rate of oxidation is faster for TMB thus requiring a lower incubation time (30 min as compared to 2 h for most other peroxidase substrates). However, similar to other regularly used peroxidase substrates Bach et al (2013), TMB is very hydrophobic and has a very low water solubility at 20 °C, and hence could be forced out from the solution on to soil particles when this compound is used as substrate in soil enzyme assays, especially at pH >5.0. Apart from the above physical sorption, the protonated TMB formed at lower pH (<4.3) could chemisorb on to the anionic sorption sites in soils and could lead to substrate limitation. However, due to the higher sensitivity of the TMB-based peroxidase assays, the soil slurries could be proportionally diluted (even up to 1:2000 soil to buffer depending on soil; w/w) so as to reduce the amount of soil in the final reaction mixture, thus partially avoiding any potential interferences. In this study, excess of non-oxidized TMB in the reaction medium at the saturation kinetics of the assay was confirmed by mass spectrometric measurements (Fig. S3). Because of the limited aqueous solubility of TMB, the excess concentration of the substrate in the reaction mixture at the steady-state kinetics should be ensured by reducing either the volume of soil slurry or the reaction time as required.
Catalytic efficiency of peroxidase
For in vitro assays of purified enzymes K m and V max are independent parameters within the Michaelis-Menten model and subject to different selection pressures, and hence are less corelated. However, in environmental samples K m and V max could be generally correlated for reasons related to microbial homeostasis, selection and assay methodology (Sinsabaugh et al. 2015). Traditionally in soil enzyme assays, the Michaelis–Menten constants are statistically analyzed as separate variables. Thus it is difficult to assess the overall enzyme efficiency when both App V max and App K m are positively related. Also, multiple V max and K m values makes the comparison across a dataset difficult. Hence we computed the apparent catalytic efficiency (App CE) of peroxidase as (App V max /App K m ) × 1000, thereby incorporating both the catalytic parameters into one, which has been widely used to measure performance of enzymes (Fox and Clay 2009). Thus, though specific to the substrate characteristics of the assay system, this apparent efficiency term integrates the net effect of kinetic constants of peroxidases in soil systems (Fig. S1). This parameter is different from the traditional parameter K cat /K m (K cat = V max /E t ; E t represents the concentration of catalytic sites of the enzyme in the reaction mixture) used to define the catalytic efficiency of purified enzyme. The non-traditional approach was adopted as it is difficult to quantify the absolute concentration of soil enzymes, since only part of the enzymes can be extracted in their active form from soils (Baldrian 2009). The ratio of V max /K m adopted in this study has been frequently used across diverse fields to assess the catalytic efficiency of enzymes (Fersht 1977; Radika and Northrop 1984; Kamdem et al. 2005; Motabar et al. 2012; Nobre et al. 2013; Zheng et al. 2013; Grkovic et al. 2014; Rosini et al. 2014), especially under assay conditions where the true concentration of enzymes/catalytic-site (E t ) is unknown.
Apparent activation energy (App Ea)
To determine the temperature sensitivity of the peroxidase activity, the soil samples were tested at 10, 20, 30, and 40 °C. Briefly, the soil slurries were prepared as 100 mg soil in 100 ml acetate buffer, and 200 µl slurry was aliquoted to 2 ml tubes, and pre-incubated at the designated temperature in a shaker at 100 rpm for 30 min. Following this 50 µl of H2O2 (0.30 %) was added and the samples were supplied with 200 µl of TMB (200 µg ml−1) to obtain a working concentration of 1000 µM. The reaction was terminated by adding 1 ml of 5 % H2SO4 after 30 min and the absorbance was measured at 450 nm. The absorbance values were converted to µM of the TMB oxidation product using the molar extinction coefficient, normalized to rate velocities µM min−1 g−1 soil and analyzed for the activation energies (App Ea) according to the
Arrhenius equation:
where k is the rate constant (µM min−1 g−1 soil), A is the pre-exponential factor, Ea (J mol−1) is the activation energy, R (8.134 Jk mol−1) is the gas constant, and T is the temperature in Kelvin. Log transformation of the Arrhenius equation (Eq. 2), expresses the equation as:
The activation energy is the slope of the ln k (rate constant) plotted by (1/T) (Parham and Deng 2000). The Q 10 of peroxidase was also analyzed (see Methods S1; Table S2).
Data analysis
Within each season, the apparent enzyme efficiency, App Ea, App K m , and App V max , were analyzed by a restricted maximum likelihood analysis of variance (REML) with ecosystem and depth as the fixed effects, and replicate core within site as the random effect. This was followed by post hoc Tukey’s HSD test to compare between means. To compare the seasonal effects for each ecosystem independently, the fixed effects of season, soil depth, and their interaction were tested with the replicate core within site as the random effect, followed by post hoc Tukey’s HSD test. Within site variation was allowed to accommodate observed heteroscedasticity. The total SVC lignin concentrations were analyzed by a two-way ANOVA using the fixed effects of ecosystem and depth, followed by post hoc Tukey’s HSD test. The apparent catalytic efficiency was linearly regressed against the V/SVC, C/V, and P/V ratios of lignin to discern relationships. The statistics were performed with SAS 9.2 (SAS Institute Inc. Cary, NC) and the linear regressions were generated with Sigma Plot 12.0.
Results
Site characteristics
The top 0–5 cm of pine and deciduous site exhibited a higher C and N content compared to the lower depths (Table 1). Due to tillage, the agricultural field site exhibited similar % C and % N across all soil depths. The soil pH remained constant across the seasons, however across the ecosystems, the soil pH was highest in the agricultural soil (ca. 5.5), followed by the two forest soils (Table 1). The soil moisture at all sites varied with depth and seasons. In the deciduous and pine soils, the gravimetric moisture content was greatest in the spring and declined in the summer and fall. Meanwhile, in the agricultural soil, the soil moisture was greatest in the fall. Overall, the total precipitation in this region was greatest in the summer (250 mm), followed by the spring (200 mm), and fall (150 mm), respectively (Fig. S2).
Lignin profile
The relative lignin concentrations of the soils was dependent upon an ecosystem × depth interaction (Fig. 2a; F4,4 = 17.98; P < 0.001). The lignin content, normalized to soil C concentration, was higher in 0–5 cm soil profile across pine and deciduous soils, whereas the lignin content of agricultural soils was similar across the depths and was lower (Fig. 2a). The SVC lignin of the top 0–5 cm accounted for 22.5 and 17.5 % of the soil C content in deciduous and pine sites, respectively, and was approximately four times higher than that of the lower soil depths. Lignin composition was similar across the seasons.
The concentration of total lignins in soils and the relationship of apparent catalytic efficiency of peroxidase with the composition of monolignols in soils. The total soil lignin concentrations (µg g−1 soil C; the bars represent mean, (n = 3) ±SE (a). The data was analyzed by a two-way ANOVA using ecosystem, depth, and ecosystem × depth as the effects followed by a post hoc Tukey’s HSD test. Similar letters across the depths within each ecosystem (x,y,z), across the three ecosystems at 0–5 cm depth (A,B,C), at 5–10 cm depth (a,b,c) and at 10–15 cm depth (p,r,q) represent no significant difference (α = 0.05). Linear regression of apparent catalytic efficiency (App CE) of peroxidase with relative abundance of vanillyl units in lignin (b), cinnamyl-to-vanillyl ratio (c), and hydroxybenzoic-to-vanillyl ratio (d)
Apparent catalytic efficiency (App CE)
In general, the SVC ratios exhibited a stronger correlation with App CE than with either App V max or App K m alone (Figs. 2, 3). Across the seasons the App CE of peroxidase remained negatively associated with the V/SVC ratio (Fig. 2b; R2 = 0.79; P < 0.001), and positively associated with C/V and P/V ratios (Figs. 2, 3, S4). Across the seasons and soil depths the App CE of peroxidase was higher in agricultural soils than pine and deciduous soils (Table 2). The App CE decreased with depth in pine soils across the three seasons, while it increased with depth in deciduous soils, both in spring and summer. The agricultural soil exhibited similar App CE across the three depths during spring and summer. The App CE in fall varied with the ecosystems (Table 2; F3,8 = 87.6; P < 0.001), and the App CE of the agricultural soil was 3.8-, and 4.0-fold greater than that of the deciduous, and pine soils, respectively (P < 0.001).
The linear regression of peroxidase kinetic parameters to the lignin chemistry. The regression of the apparent catalytic efficiency of peroxidase in summer (a) and fall (b) to the relative abundance of vanillyl units in lignin (V/SVC ratio). The linear regression of App V max (c), and App K m (d) to the V/SVC ratio
Apparent K m of soil peroxidase (App K m )
Across the seasons, the agricultural soils and pine soils exhibited the highest and lowest App K m, respectively (Table 3). The deciduous and pine soils exhibited 4- and 5-fold greater App K m than that of the agricultural soils (P < 0.001). In spring season the App K m decreased with soil depth in deciduous soils, increased with depth in pine soils, and remain unaffected by depth in agricultural soils. This variation in App K m in spring season was not related to ambient substrate concentrations in these soils measured by permanganate oxidizable carbon or total lignin (SVC) content (Fig. S5). In deciduous soils, compared to 0–5 cm, the App K m decreased by 53 and 68 % at 5–10 and 10–15 cm, respectively, whereas, compared to 0–5 cm, the App K m of 5–10 and 10–15 cm soil depth in pine soils increased by approximately 50 %. While a similar pattern of App K m across soil depth continued on to summer season in deciduous soils, this trend reversed for pine soils during summer, and the App K m decreased with soil depth. The App K m remained similar across soil depths across the three ecosystems during fall season (Table 3).
Apparent V max of soil peroxidase (App V max )
The agricultural soils exhibited a lower App V max (Table 4), which was more pronounced during the spring season, and was half as that of pine and deciduous soils, resulting in an ecosystem × depth interaction (Table 4; F4,12 = 3.98; P = 0.028). In the deciduous soils during spring, the App V max in the 0–5 cm was 36 and 84 % higher than in the 5–10 cm (P = 0.04) and 10–15 cm depths, respectively (P = 0.002). In the deciduous soils during summer, the App V max of the top soils was 31 and 70 % greater than the 5–10 cm (P = 0.002) and 10–15 cm depths (P < 0.001), respectively, and a similar trend in App V max was present in the pine soils. Across the ecosystems at 0–5 cm, the App V max of deciduous soils were 25 % (P = 0.015) and 155 % (P < 0.001) greater than that of the pine and agricultural soils, respectively, and this trend continued in the 5–10 cm depths.
Apparent activation energy of soil peroxidase (App Ea)
In general, compared to App V max and App K m the magnitude of the variation of App Ea was less pronounced across the seasons and ecosystems (Table 5). Similar to the kinetic parameters, the App Ea of agricultural soils did not exhibit significant difference across seasons and depths. In spring, the App Ea in the 5–10 cm depth of the deciduous soils was 14.8 and 7.7 % higher than that of the 0–5 cm (P < 0.001) and 10–15 cm depths (P = 0.003), respectively. The pine soils exhibited a similar trend, the App Ea of the 5–10 cm depth was 25 and 42 % greater than that in the 0–5 cm (P = 0.008) and 10–15 cm depths (P < 0.001). The App Ea in the summer varied by depth across the ecosystems (Table 5; F4,12 = 8.20; P = 0.002). In the deciduous soil, the App Ea in the 0–5 cm depth was 10 % greater than the App Ea of the 5–10 cm (P = 0.004) and 10–15 cm (P = 0.006) depths. Meanwhile, in the pine soil the App Ea at 5–10 cm was 15 % lower than that in the 0–5 and the 10–15 cm depths (P < 0.001).
Discusssion
Given that peroxidases facilitate the degradation of lignin that contributes to 30 % of organic C in the biosphere (Boerjan et al. 2003), the characterization of soil peroxidase kinetics and activation energy could assist in predicting the rate of carbon and nitrogen cycling in ecosystems. The App V max and App K m reported in this study is within the range of K m and V max of plant (Guida et al. 2011) and fungal (Bourbonnais et al. 1988; Wang et al. 2012) peroxidases, and similar to the K m and V max of horseradish peroxidase obtained using different o-diphenols as reducing substrates (Rodríguez-López et al. 2000). We adopted an approach that incorporates both App V max and App K m into a single parameter that calculates the catalytic efficiency (App CE) of peroxidase. This parameter, though less recommended in industrial biotransformations involving high concentration of engineered enzymes (Fox and Clay 2009), is frequently used to monitor the efficiency of reactions catalyzed by enzymes present at ambient concentrations across diverse fields (Radika and Northrop 1984; Kamdem et al. 2005; Motabar et al. 2012; Nobre et al. 2013; Zheng et al. 2013; Grkovic et al. 2014; Rosini et al. 2014). Although the App V max was higher in lignin-rich pine soils, the associated increase in App K m resulted in a lower App CE in these soils; whereas the lignin-poor agricultural soils that had the lowest App V max and App K m exhibited the highest App CE. Thus, the integrative parameter of V max and K m better described the potential efficiency of the soil enzymes in our study sites, highlighting the usefulness of App CE in capturing the potential enzyme activity. Overall, the App CE of peroxidases across the depths and seasons was higher in soils with low lignin content, which partly supports our hypothesis, and is in agreement with the substrate-dependent activity of hydrolases (German et al. 2011). However, contrary to our hypothesis, the App CE of peroxidases was lower in soils with higher proportion of relatively recalcitrant vanillyl units. The decoupling of the catalytic efficiency of peroxidases from the native lignin chemistry observed in our assay system indicates that the residence time of lignins in soils could be highly ecosystem specific (Tamura and Tharayil 2014) and is supported by the observation that lignin phenolics dominate in soils under gymnosperms that produce lingnin with higher proportion of recalcitrant inter-unit linkages (Otto and Simpson 2006; Talbot et al. 2012).
Response of Michaelis–Menten kinetic constants of soil peroxidase and App CE across ecosystems
We hypothesized that the degree of peroxidase activity would vary with the quantity and chemical identity of lignin monomers, and hence with the vegetation history of a site. For example, pine litter is composed predominantly of vanillyl units that form C–C inter-unit linkages that are difficult to enzymatically breakdown (Tai et al. 1983). Hence, we expected the peroxidases in pine ecosystem to exhibit a higher App CE. In addition to the vanillyl units, the deciduous trees and grasses also contain syringyl and cinnamyl monomers that are linked through C–O–C bonds that are more labile. Therefore, we expected a lower App CE in deciduous and agricultural soils. As expected, the quantity of lignin and relative abundance of vanillyl units were higher in pine soils, followed by deciduous and agricultural soils (Fig. 2a). Across the three ecosystems the App V max was greater in pine and deciduous sites (Table 4), indicating a higher abundance of the peroxidase enzymes. However, the observed negative relationship of the App CE with V/SVC and positive relationship with the C/V, and P/V across the three ecosystems (Figs. 2, 3) suggests that the increase in the relative abundance of vanillyls was accompanied by a corresponding decrease in App CE. This lower App CE in pine soils, despite a higher App V max was contributed by a higher App K m (Table 3). According to the Michaelis–Menten model the rate of formation and dissociation of enzyme-substrate complex, and hence the K m , is independent of enzyme concentration, thus the higher K m in pine and deciduous soils is not directly associated with the higher V max observed in these soils. A greater abundance of ambient substrates including lignins (Fig. 2a) could potentially compete with TMB during the assay, and could result in the observed higher App K m in pine soils (Sinsabaugh et al. 2014). However, the content of the measured ambient substrates (lignin and permanganate oxidizable carbon) did not correlate with the App K m (Fig. S5) in our study. Tannins that are potentially abundant in pine and deciduous soils could complex with the enzymes (Tharayil et al. 2011) and/or quench the peroxide radical (Triebwasser et al. 2012), which would also result in the observed higher App K m in these soils.
This observed disconnect between K m and the ambient substrate content could be contributed by the inability of analytical techniques to recover the complex heteropolymers in a recognizable form from soil-matrices, which significantly underestimates their true concentration in soils (Hernes et al. 2013). Though rapidly disappearing out of the analytical window, the phenolic heteropolymers are shown to retain their chemical and biological reactivity in soil matrices (Tharayil et al. 2011).
The agricultural soils which had the lowest SVC-lignin concentrations exhibited a lower App V max and App K m resulting in a higher App CE. Thus, compared to pine and deciduous soils, the peroxidase-pool in agricultural soils were able to attain half their potential maximum rate of product formation at a lower substrate concentration, indicating a higher catalytic efficiency. The lower App V max could indicate a lower abundance of oxidative enzymes in agricultural soils, which could be expected due to the lower abundance of lignin substrates (Fig. 2a). Substrate scarcity could instigate microbes to produce enzymes that have a higher affinity for the substrates (lower K m ), thus increasing the overall catalytic efficiency of the enzymes (Stone et al. 2011), which might partly explain a higher App CE of peroxidase in soils with lower lignin concentrations. The App CE also robustly relates to the lignin vegetation index represented by the ratio parameters S/V and C/V (Fig. 4). The agricultural soil that had lower vanillyl units thus a higher S/V and C/V ratios had the highest App CE, followed by the deciduous soil that had equivalent amount of S and V units, and the App CE of pine soil that had a higher vanillyl content, thus lower S/V and C/V ratios, was the lowest. This relationship between the apparent enzyme activity and the chemistry of the resident-vegetation highlights the usefulness of this integrated parameter to meaningfully define enzyme efficiency at ecosystem level. It should be noted that properties of enzymes could be better associated with its catalytic functions under defined conditions as in single-substrate assays, and for peroxidases the kinetic constants derived using artificial substrates are highly assay-specific. As peroxidases exhibit non-specificity in substrate oxidation, the observed catalytic parameters could be prone to biasness due to the presence of ambient substrates that are ecosystem specific (Eichlerová et al. 2012). Moreover, along with the influence of potential cross linkages within lignin monomers discussed in this study, the incorporation of polymeric lignin within the tissue matrix through interspecific cross linkages with other homo-and heteropolymers (Suseela et al. 2013, 2014) will also influence the efficiency of peroxidases to undertake lignin degradation. Given the above complexities, this study forms an initial attempt to link lignin chemistry of the resident plant species to the properties of native soil peroxidases. A follow-up measurement of peroxidase kinetics in systems where the quantity and quality of lignin varies independently would be complimentary.
Apart from lignin concentration, the App CE could also be modulated by the nutrient availability of the ecosystem. For example, nitrogen deposition in forest ecosystems have shown to reduce the activity of oxidative enzyme (Waldrop et al. 2004; Waldrop and Zak 2006; Grandy et al. 2008), whereas nutrient deficiency and resource heterogeneity could induce peroxidase activity. In contrast, the addition of nitrogen to agricultural soils has been reported not to alter the activity of peroxidases (Henry et al. 2005; Stursova et al. 2006; Zeglin et al. 2007). However, in our study, the agricultural soils, which contained twice more nitrogen per unit of carbon (Table 1) maintained a higher App CE indicating that the effect of N on the peroxidase activity could be modulated by other environmental parameters that are ecosystem specific. The ecosystem specific function of peroxidases to microbial C and N demands is further corroborated by the lack of a relationship of App CE with labile C, described as the permanganate oxidizable C (Fig. S6a), and total soil N (Fig. S6b). The ecosystem specific responses of the App CE could also be the consequence of several abiotic factors. The pH and moisture have also been reported to mediate oxidoreductase activity in soil (Sinsabaugh et al. 2008; Sinsabaugh 2010; Fujii et al. 2013). However, in this study, while the pH and moisture varied across the ecosystems and depths (Table 1), they were not associated to the kinetic constants (data not shown), suggesting that these abiotic factors were not the primary drivers of the observed kinetics in our study system.
Spatiotemporal dynamics of the apparent peroxidase temperature kinetics
The activation energy (App Ea) and temperature sensitivity (Q 10 ) are important parameters that determine the stability of enzyme and enzyme-substrate complex (Farrell et al. 1994; Trasar-Cepeda et al. 2007; Wallenstein et al. 2011). However, given the complexity of the lignin chemistries that varies across ecosystems, it remains unexplored if the reaction kinetics of peroxidases is related to the soil lignin chemistry. Due to the structural complexity of lignin, we anticipated that the activation energy of the lignin oxidation reactions would be similar to those catalyzed by phenol oxidases that degrade phenolic compounds, but greater than the reactions catalyzed by hydrolases which hydrolyze relatively labile molecules. Moreover, due to the refractory nature of vanillyl units, we expected the peroxidases from the pine soils to exhibit higher activation energy compared to other two ecosystems that are low in vanillyl units. In our study, the App Ea (Table 5) and Q 10 values (Table S2) were within the ranges reported from other ecosystems (Trasar-Cepeda et al. 2007; Steinweg et al. 2013). However, contrary to our predictions, the App Ea (ca. 36 kJ mol−1) and Q 10 (Q 10 < 2) exhibited lower temperature sensitivities than reported for soil phenol oxidases and hydrolases (Steinweg et al. 2013; Wang et al. 2012). The low activation energies of peroxidases observed could suggest that peroxidases are less reliant upon external energy for its catalytic activity in our study systems. In comparison to hydrolases, this was unexpected given that the substrates targeted by hydrolases have lower chemical complexities (Davidson and Janssens 2006), resulting in low activation energies of the corresponding catalytic reactions. Similarly, the App Ea did not exhibit substantial variation across the ecosystems, which could imply that App Ea is less-driven by lignin monomer composition or the availability of lignins in the soil.
The observed responses of App Ea could be due to the saturating substrate concentration maintained in the lab assay, which masks the natural bottlenecks in substrate availability (rate of substrate diffusion) regulated by temperature-dependent sorption dynamics and the diffusion kinetics experienced in the field (Sinsabaugh and Follstad Shah 2012). Thus, substrate saturation may partly over-ride any facilitatory influence that temperature might have on the catalytic reaction. Although the TMB substrate can capture potential peroxidase kinetics, the structural simplicity of TMB could result in lower activation energy of this oxidation reaction, in comparison to the reactions that oxidize structurally complex lignins. Experiments using l-DOPA as a substrate also reflected a similar, lower App Ea values (Steinweg et al. 2013). Similarity in App Ea between beta-glucosidase, peroxidase and phenoloxidase has been also reported by Wang et al. (2012). Multiple steps involved in peroxidase catalysis could be differentially influenced by temperature. For example, the substrate oxidation caused by the peroxidase could be less temperature sensitive especially under substrate saturation conditions, and the activation energy would be more critical for the enzyme-substrate binding. However, lignin degradation is demonstrated to increase with increase in temperature (Feng et al. 2008; Conant et al. 2008; Suseela et al. 2013). This signifies a need for conducting assays with substrates that are structurally similar to lignin to capture the dynamics of soil peroxidases.
Conclusion
The velocity of enzyme activity and its corresponding temperature sensitivity, defined by the Michaelis–Menten kinetics and activation energy of soil enzymes, are considered to be key factors in the decomposition process (Davidson et al. 2012). We determined that the App CE, described as the integrative term of V max and K m , best associated the soil peroxidase activity to the relative composition of lignin. The App CE exhibited a stronger correlation with the content and composition of lignins, with App CE decreasing with an increase in total lignin content and decreasing with an increase in the proportion of vanillyl units that forms recalcitrant inter-unit linkages in lignin. The observed decoupling of App CE of peroxidases from chemistry of the native lignin could partly explain the longer persistence of lignins in ecosystems receiving recalcitrant litter inputs.
References
Arnao MB, Acosta M, Del Rio JA, Varon R, Garcia-Canovas F (1990) A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide. Biochim Biophys Acta 1041:43–47
Bach CE, Warnock DD, Van Horn DJ, Weintraub MN, Sinsabaugh RL, Allison SD, German DP (2013) Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: effect of assay conditions and soil type. Soil Biol Biochem 67:183–191
Bahri H, Dignac M-F, Rumpel C, Rasse DP, Chenu C, Mariotti A (2006) Lignin turnover kinetics in an agricultural soil is monomer specific. Soil Biol Biochem 38:1977–1988
Baldrian P (2009) Microbial enzyme-catalyzed processes in soils and their analysis. Plant Soil Environ 55:370–378
Bell TH, Klironomos JN, Henry HAL (2010) Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen. Soil Sci Soc Am 74:820–828
Bending GD, Read DJ (1996) Effect of the soluble polyphenol tannic acid on the activities of ericoid and ectomycorrhizal fungi. Soil Biol Biochem 28:1595–1602
Bengtson P, Bengtsson G (2007) Rapid turnover of DOC in temperate forests accounts for increased CO2 production at elevated temperatures. Ecol Lett 10:783–790
Berg B, McClaugherty C (2003) Plant litter, decomposition, humus, Carbon Sequestration. Spring-Verlag, Berlin
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annual review. Plant Biol 54:519–546
Bourbonnais R, Paice MG (1988) Veratryl alcohol oxidases from the lignindegrading basidiomycete Pleurotus sajor-caju. Biochem J 255: 445–450
Brzostek ER, Finzi AC (2012) Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J Geophys Res 117. doi:10.1029/2011JG001688
Campbell MM, Sederoof RR (1996) Variation in lignin content and composition (mechanism of control and impliations for the genetic improvement of plants). Plant Physiol 110:3–13
Chance B (1943) The kinetics of the enzyme-substrate compound of peroxidase. J Biol Chem 151:553–577
Childs RE, Bardsley WG (1975) The steady-state kinetics of peroxidase with 2,2'-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J. 145:93–103
Conant RT, Steinweg JM, Haddix ML, Paul EA, Plante AF, Six J (2008) Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 89:2384–2391
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Davidson EA, Samanta S, Caramori SS, Savage K (2012) The dual Arrhenius and Michaelis–Menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob Change Biol 18:371–384
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2011) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Biol Change 18:781–1796
Eichlerová I, Šnajdr J, Baldrian P (2012) Laccase activity in soils: considerations for the measurement of enzyme activity. Chemosphere 88:1154–1160
Farrell RE, Gupta VVSR, Germinda JJ (1994) Effects of cultivation on the activity and kinetics of arylsulfatease in Saskatchewan soils. Soil Biol Biochem 26:1033–1040
Feng X, Simpson AJ, Wilson KP, Williams DD, Simpson MJ (2008) Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat Geosci 1:836–839
Fersht AR (1977) Enzyme structure and mechanism, 2nd edn. W. H. Freeman and Co., Reading
Fox RJ, Clay MD (2009) Catalytic effectiveness, a measure of enzyme proficiency for industrial applications. Trends Biotechnol 27:137–140
Fujii K, Uemura M, Hayakawa C, Funakawa S, Kosaki T (2013) Environmental control of lignin peroxidase, manganese peroxidase, and laccase activities in forest floor layers in humid Asia. Soil Biol Biochem 57:109–115
German DP, Chacon SS, Allison SD (2011) Substrate concentration and enzyme allocation can affect rates of microbial decomposition. Ecology 92:1471–1480
Gleixner G, Czimczik CJ, Kramer C, Luhker B, Schmidt MWIP (2001) Plant compounds and their turnover and stability as soil organic matter. In: E-D Schulze, M Heimann, S Harrison, E Holland, J.L Lloyd, C Prentice, D Schimel (Eds) Global Biogeochemical Cycles in the Climate System. Academic Press, San Diego, pp. 201–215
Goodwin DC, Hertwig KM (2003) Peroxidase-catalyzed oxidation of capsaicinoids: steady-state and transient-state kinetic studies. Arch Biochem Biophys 417:18–26
Goodwin DC, Ymazaki I, Aust SD, Grover TA (1995) Determination of rate constants for rapid peroxidase reactions. Anal Chem 231:333–338
Grabber JH (2006) How do lignin composition, structure and cross-linking affect degradability? a review of cell wall model studies. Crop Sci 45:820–831
Grandy AS, Sinsabaugh RL, Neff JC, Stursova M, Zak DR (2008) Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 91:37–49
Grkovic I, Bjelobaba I, Nedeljkovic N, Mitrovic N, Drakulic D, Stanojlovic M, Horvat A (2014) Developmental increase in ecto-5′-nucleotidase activity overlaps with appearance of two immunologically distinct enzyme isoforms in rat hippocampal synaptic plasma membranes. J Mol Neurosci 54:109–118
Guida V, Tamburino R, Parente A (2011) Purification and enzymatic properties of a peroxidase from leaves of Phytolacca dioica L. (Ombú tree). Biochem Mol Biol Reports 44: 64–69
Hedges JI, Mann DC (1979) The characterization of plant tissues by their lignin oxidation product. Geochimica et Cosmochimica Acta 43:1803–1807. doi:10.1016/0016-7037(79)90028-0
Henry HAL, Juarez JD, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob Change Biol 11:1808–1815
Hernes PJ, Kaiser K, Dyda RY, Cerli C (2013) Molecular trickery in soil organic matter: hidden lignin. Environ Sci Technol 47: 9077–9085. doi:10.1021/es401019n
Huang Q, Pinto RA, Griebenow K, Schweitzer-Stenner R, Weber WJ Jr. (2005) Inactivation of horseradish peroxidase by phenoxyl radical attack. J Am Chem Soc 127:1431–1437
Johnsen AR, Jacobsen OS (2008) A quick and sensitive method for the quantification of peroxidase activity of organic surface soil from forests. Soil Biol Biochem 40:814–821
Josephy PD, Eling T, Mason RP (1982) The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. J Biol Chem 257:3669–3675
Kaiser K, Benner R (2012) Characterization of lignin by gas chromatography and mass spectrometry using a simplified CuO oxidation method. Anal Chem 84:459–464
Kamdem LK, Streit F, Zanger UM, Brockmoller J, Oellerich M, Armstrong VW, Wojnowski L (2005) Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem 51:1374–1381
Lundell TK, Makela MR, Hilden K (2010) Lignin-modifying enzymes in filamentous basidiomycetes: ecological functional and phylogenetic review. J Basic Microbiol 50:1–16
Marx M, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol Biochem 37:35–48
Motabar O, Goldin E, Leister W et al (2012) A high throughput glucocerebrosidase assay using the natural substrate glucosylceramide. Anal Bioanal Chem 402:731–739
Nobre A, Empadinhas N, Nobre MF et al (2013) The plant Selaginella moellendorffii possesses enzymes for synthesis and hydrolysis of the compatible solutes mannosylglycerate and glucosylglycerate. Planta 237:891–901
Otto A, Simpson MJ (2006) Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil. Biogeochemistry 80:121–142
Oudgenoeg G, Dirksen E, Ingemann S et al (2002) Horseradish peroxidase-catalyzed oligomerization of ferulic acid on a template of a tyrosine-containing tripeptide. J Biol Chem 277:21332–21340
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosamindase activity in soil. Soil Biol Biochem 32:1183–1190
Perron NR, Brumaghim JL (2009) A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys 53:75–100
Pizzocaro F, Torreggini D, Gilardi G (1993) Inhibition of apple polyphenoloxidase by ascorbic acid, citric acid and sodium chloride. J Food Process Prot 17:21–30
Radika K, Northrop DB (1984) Correlation of antibiotic resistance with V max /K m ratio of enzymatic modification of aminoglycosides by kanamycin acetyltransferase. Antimicrob Agents Chemother 4:479–482
Rasse DP, Dignac M-F, Bahri H, Rumpel C, Mariotti A, Chenu C (2006) Lignin turnover in an agricultural field: from plant residues to soil-protected fractions. Eur J Soil Sci 57:530–538
Rodríguez-López JN, Gilabert MA, Tudela J, Thorneley RNF, Garcia-Canovas F (2000) Reactivity of horseradish peroxidase compound II toward substrates: kinetic evidence for a two-step mechanism. Biochemistry 39:13201–13209
Rosini E, Tonin F, Vasylieva N, Marinesco S, Pollegioni L (2014) Evolution of histamine oxidase activity for biotechnological applications. Appl Microbiol Biotechnol 98:739–748
Scalzo RL (2008) Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem 107:40–43
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner K, Lehmann L, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–342. doi:10.1146/annurev-ecolsys-071112-124414
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Steinweg JM, Jagadamma S, Frerichs J, Mayes MA (2013) Activation energy of extracellular enzymes in soil from different biomes. PLoS One 8:e59943
Sinsabaugh RL, Belnap J, Findlay SG, Shah JJF, Hill BH, Kuehn KA, Kuske CR et al (2014) Extracellular enzyme kinetics scale with resource availability. Biogeochemistry 121:287–304
Sinsabaugh RL, Shah JJF, Findlay SG, Kuehn KA, Moorhead DL (2015) Scaling microbial biomass, metabolism and resource supply. Biogeochemistry 122:175–190
Stone MM, Weiss MS, Goodale CL, Adams MB, Fernandez IJ, German DP, Allison SD (2011) Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob Change Biol 18:1173–1184
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–98
Sun L, Spencer RG, Hernes PJ, Dyda RY, Mopper K (2015) A comparison of a simplified cupric oxide oxidation HPLC method with the traditional GC‐MS method for characterization of lignin phenolics in environmental samples. Limnol Oceanogr Methods 13(1):1–8
Suseela V, Tharayil N, Xing B, Dukes JS (2013) Labile compounds in plant litter reduce the sensitivity of decomposition to warming and altered precipitation. New Phytol 200:122–133. doi:10.1111/nph.12376
Suseela V, Tharayil N, Xing B, Dukes JS (2014) Warming alters potential enzyme activity but precipitation regulates chemical transformations in grass litter exposed to simulated climatic changes. Soil Biol Biochem. 75:102–112
Tai D, Terasawa M, Chen C-L, Chang H-M, Kirch TK (1983) Biodegradation of guaiacyl and guaiacyl–syringyl lignins in wood by Phanerochaete chrysosporium. U.S.–Japan Cooperative Science Program. Abstract
Talbot JM, Yelle DJ, Nowick J, Tresedar KK (2012) Litter decay rates are determined by lignin chemistry. Biogeochemistry 108:279–295 doi:10.1007/s10533-011-9599-6
Tamura M, Tharayil N (2014) Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol 231:110–124. doi:10.1111/nph.12795
Tharayil N, Suseela V, Triebwasser DJ, Preston CM, Gerard PD, Dukes JS (2011) Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: climatic stress-induced tannins are more reactive. New Phytol 191:132–145
Thevenot M, Dignac M, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–1211
Trasar-Cepeda C, Gil-Sotres F, Leirós MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol Biochem 39:311–319
Triebwasser D, Tharayil N, Preston CM, Gerard P (2012) The susceptibility of soil enzymes to inhibition by leaf litter tannins is dependent on the tannin chemistry, enzyme class and vegetation history. New Phytol. doi:10.1111/j.1469-8137.2012.04346.x
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153:895–905
Volpe G, Compagnone D, Draisci R, Palleshi G (1998) 3,3′ 5,5′-Tetramethylbenzidine as electrochemical substrate for horseradish peroxidase based enzyme immunoassays. A comparative study. Analyst 123:1303–1307
Waldrop M, Zak D (2006) Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9:921–933
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451
Wallenstein M, Allison S, Ernakovich J, Steinweg JM, Sinsabaugh R (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil enzymology. Springer, Berlin, pp 245–258. doi:10.1007/978-3-642-14225-3_13
Wang G, Post WM, Mayes MA, Frerichs JT, Sindhu J (2012) Parameter estimation for models of ligninolytic and cellulolytic enzyme kinetics. Soil Biol Biochem 48:28–38
Wang J, Tharayil N, Chow AT, Suseela V, Zeng H (2015) Phenolic profile within the fine root branching orders of an evergreen species highlights a disconnect in root tissue quality predicted by elemental- and molecular-level carbon composition. New Phytologist (in press)
Wong DWS (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209. doi:10.1007/s12010-008-8279-z
Yu J, Taylor KE, Zou H, Biswas N, Bewtra JK (1994) Phenol conversion and dimeric intermediates in horseradish peroxidase-catalyzed phenol removal from water. Environ Sci Technol 28:2154–2160
Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL (2007) Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154:349–359
Zheng JL, Luo Z, Liu CX, Chen QL, Zhu QL, Hu W, Gong Y (2013) Differential effects of the chronic and acute zinc exposure on carnitine composition, kinetics of carnitine palmitoyltransferases I (CPT I) and mRNA levels of CPT I isoforms in yellow catfish Pelteobagrus fulvidraco. Chemosphere 92:616–625
Acknowledgments
The authors thank Drs. Petr Baldrian, Robert Sinsabaugh, Vidya Suseela, Matthew Wallenstein and two anonymous reviewers for their constructive and thoughtful comments that substantially improved the quality of the manuscript. This study was financially supported by NSF (DEB-1145993) and USDA (2009-35320-05042) grants to N.T. The NSF Graduate Research Fellowship to D.J.T-F. is greatly acknowledged. This is Technical Contribution No. 6136 of the Clemson University Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Melany Fisk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Triebwasser-Freese, D.J., Tharayil, N., Preston, C.M. et al. Catalytic kinetics and activation energy of soil peroxidases across ecosystems of differing lignin chemistries. Biogeochemistry 124, 113–129 (2015). https://doi.org/10.1007/s10533-015-0086-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0086-3