Abstract
Climate change models predict that the snowpacks of temperate forests will develop later and be shallower resulting in a higher propensity for soil freezing. In the northern most island of Japan, Hokkaido, snowpack depth decreases from west to east. This snowpack depth gradient provided a unique opportunity to test the effects of variable snowpack and soil freezing on N biogeochemistry. The Shibecha Northern Catchment in Shibecha Experimental Forest, eastern Hokkaido had deciduous trees and a mean annual snowpack of 0.7 m while the M3 catchment in Uryu Experimental Forest, western Hokkaido had mixed deciduous and coniferous tree species and a mean annual snowpack of 2.0 m. We conducted a field study (October 2004–April 2005) to determine if differences in Shibecha and Uryu soil extractable N, N mineralization, and nitrification were controlled by the variability in soil freezing during winter or tree species composition that affected the quality of the forest floor. The mixed deciduous and coniferous trees forming the Uryu forest floor had a higher C:N ratio (25.0 vs. 22.4 at Shibecha), higher lignin:N ratio (15 vs. 8.8), and higher lignin concentrations (0.28 vs. 0.18 g lignin g−1). These differences in forest floor quality contributed to higher net N mineralization and nitrification in Shibecha compared to Uryu. In Shibecha, soil remained frozen for the entire study. For Uryu, except for an early period with cold temperatures and no snow, the soil generally remained unfrozen. As a result of the early winter cold period and soil freezing, extractable soil NH +4 did not change but NO −3 increased. Reciprocal 0–5 cm mineral soil transplants made between Shibecha and Uryu and incubated during winter at 0, 5, and 30 cm suggested that soil freezing resulted in greater net N mineralization yet lower nitrification regardless of the soil origin. The effect of soil freezing should be considered when evaluating differences in N dynamics between temperate ecosystems having a propensity for soil freezing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change may result in marked changes in the structure and function of temperate forested ecosystems including the altering of elemental cycling (Vitousek 1994; Mitchell et al. 1996; Tilman 1998). Although climatic models of north temperate ecosystems predict greater amounts of winter precipitation in the future (Hosaka et al. 2005; Hayhoe et al. 2006), reductions in snowpack are likely to occur due to the increased importance of thaws, sleet, and rain-on-snow events (Goodrich 1982; Hayhoe et al. 2006; Huntington et al. 2004). Without an insulating snowpack, soil ice formation at the onset of winter will persist throughout the winter and/or more frequent freeze-thaw cycles will occur (Goodrich 1982; Edwards and Cresser 1992).
Despite harsh winter conditions and cold temperatures, rates of microbial activity are high in winter, comprising a significant portion of nutrient cycling and loss (Brooks et al. 1996; Hobbie and Chapin 1996; Campbell et al. 2005). According to several laboratory studies, when soils freeze, root and microbial mortality results in a release of labile organic carbon (C) and nitrogen (N) to soil (Edwards and Cresser 1992; Schimel and Clein 1996; Groffman et al. 2001a; Neilsen et al. 2001) similar to what has been observed during wetting and drying cycles of soils (Birch 1958; Soulides and Allison 1961; Lund and Goksøyr 1980; Marumoto et al. 1982; Kieft et al. 1987; Davidson et al. 1998). The majority of the initial released organic N and C upon thawing comes from cellular material released from death and lysis of microbial biomass (in the form of simple sugars and amino acids) rather than a release from disrupted organic structure (Schimel and Clein 1996; Ryan et al. 2000; Hermann and Witter 2002). Increased net mineralization and nitrification occur during thawing as surviving organisms use the killed cells as a substrate (DeLuca et al. 1992; Neilsen et al. 2001) resulting in increased leaching of NO −3 to surface waters (Callesen et al. 2007). Increases in soil NO −3 levels also have been shown to be linked to increased fine-root mortality, resulting in lower plant N uptake (Groffman et al. 2001b).
Rates of nitrogen deposition input to forests in the northeastern USA are among the highest in the country, far exceeding natural nitrogen inputs (Boyer et al. 2002, Driscoll et al. 2003) yet outputs of N are often much less than inputs (Likens et al. 1996; Driscoll et al. 2003; Goodale et al. 2003). Over-winter processes may influence temporal variation in N retention in forested north temperate systems (Mitchell et al. 1996; Brooks et al. 1998). Some studies have suggested that over-winter activities influence nutrient losses during the subsequent growing season and can explain the year to year variability in NO −3 export from catchments (Mitchell et al. 1996; Brooks et al. 1998; Williams et al. 1998; Fitzhugh et al. 2001; Callesen et al. 2007). Understanding the role of the snowpack depth and duration as a factor influencing soil freeze/thaw and soil N dynamics is critical for evaluating the effect of climate change on ecosystem N biogeochemistry. However, there have been very few measurements of in situ biogeochemical processes during winter in temperate forests (Groffman et al. 2001b) and very little analysis of the influence of soil freezing on winter N dynamics.
Recent studies also have suggested that variation in tree species composition controls the variability of litter quality, N mineralization and nitrification rates, and therefore, N retention in temperate forested systems (Christopher et al. 2006; Pastor et al. 1984; Lovett et al. 2002, 2004; Lovett and Mitchell 2004) . Tree species composition drives differences in litter quality characteristics such as C:N ratio and lignin:N ratio among sites (Lovett et al. 2002, 2004). Lower C:N and lignin:N result in higher rates of NO −3 production (i.e. net nitrification) (Finzi et al. 1998; Gundersen et al. 1998; Lovett and Rueth 1999; Lovett et al. 2004; Melillo et al. 1982). Soil organic matter quality, which is strongly influenced by tree species composition, also has been shown to explain the variability in soil microbial response to freeze-thaw perturbations (Schimel and Clein 1996).
In Hokkaido, Japan, the climate is classified as temperate to sub-arctic with heavy snow during the winter. Snowmelt runoff in spring results in high discharge to surface waters (Shibata et al. 2002). Thus it is important to understand over-winter soil processes in Hokkaido that may affect the leaching of NO −3 to surface water. Although temperatures are similar across Hokkaido, the snowpack decreases markedly from west to east with western Hokkaido having much deeper (2–3 m depths; Shibata et al. 1997) snow compared to eastern Hokkaido (0.5–1 m depths; Takeuchi 1980; 1981). Maximum soil freezing depths of 35–50 cm have been noted in eastern Hokkaido due to lack of snow cover (Takeuchi 1980, 1981). Thus, this east to west snowpack gradient provided a unique opportunity to conduct a field experiment testing the effects of soil freezing on N biogeochemistry. We identified two study sites in Hokkaido. The Shibecha northern catchment (SN) Catchment, located in eastern Hokkaido (Fig. 1) is dominated by deciduous tress species while the M3 catchment, in northwestern Hokkaido, (Fig. 1) contains mixed coniferous and deciduous tree species. We conducted a field investigation to address the following questions:
-
1.
How does forest floor and soil quality affect the response of N mineralization and nitrification rates in Uryu versus Shibecha regardless of soil freezing? Non-frozen soils buried in situ in Uryu versus soils buried in Shibecha were compared.
-
2.
What is the effect of soil freezing on total dissolved soil nitrogen concentration (TDN, including NH +4 , NO −3 , and dissolved organic nitrogen-DON)? Monthly 0–5 cm soils sampled in situ in Uryu and Shibecha were compared throughout the study period.
-
3.
What is the effect of soil freezing on N mineralization and nitrification? Uryu soil buried in situ was compared to Uryu soil buried in Shibecha, and visa versa, to examine the response of each site to freezing and non freezing environments. In addition, at each site, soils from 0–5 cm soil incubated in situ at the surface were compared with 0–5 cm soil incubated in situ at 5 and 30 cm to examine differences in freezing (0 and 5 cm) and non-freezing environments (30 cm).
Methods
Site description
The M3 Catchment (3.5 ha), located in Uryu Experimental Forest (UREF), Hokkaido University (44°21′44″ N, 142°15′55″ E), hereafter referred to as Uryu, has a mean annual stream NO −3 concentration of 0.1 mg N l−1. Precipitation and air temperature have been monitored since 1969 at a meteorological station located approximately 1 km north of the study site, at the UREF headquarters. The mean annual air temperature at UREF is 3.1°C and the mean annual precipitation is 1390 mm (1969–1998, Yoshida et al. 2005), with approximately 50 % falling as snow between late November and early May. Mean annual maximum snow depth was 2.0 m from 1970–1989.
The bedrock in Uryu is Neogene andesite of Tertiary age, and soils are Inceptisols (Typic Dystrocrepts). Vegetation consists of natural mixed hardwood and conifer species mainly represented by Sakhalin fir (Abies sachalinensis Masters), Glehn’s spruce (Picea glehnii Masters), Mongolian oak (Quercus crispula Blume), Japanese Manchurian ash (Fraxinus mandshurica var. japonica Maxim.), Erman’s birch (Betula ermanii Cham.), painted maple (Acer mono Maxim.) and Japanese elm (Ulmus davidiana var. japonica Nakai). The forest understory is almost exclusively dominated by Chishima dwarf bamboo (Sasa kurilensis) except for some riparian zones. A detailed description of the vegetation in each sampling plot is given in the “Results” section.
The SN Catchment, located in the Shibecha Experimental Forest, Kyoto University (43°19′ N, 144°37′ E), hereafter referred to as Shibecha, has a mean annual stream NO −3 concentration of 2 mg N l−1. The mean annual air temperature and precipitation is 5.7°C and 1157 mm (1971–2000), respectively and have been continually monitored at the Field Science Education and Research Center (FSERC), the headquarters for the Experimental Forest. Snowfall occurs from December to March, but is less than 30% of the annual precipitation. The mean maximum snow depth was 0.71 m from 1988–2005. Soil freezing has been observed from December to May with maximum freezing depth varying from 35 to 50 cm and generally occurring in early March (Takeuchi 1980, 1981).
The bedrock in Shibecha is Abukuma Granitoids including basalt and chert. Soils are Humic Andosols derived from volcanic ash of Masyu (Ma-f). The deciduous broad-leaved canopy is dominated by Japanese elm, Japanese Manchurian ash, and Mongolian oak, and is discussed in more detail in the “Results” section. The understory vegetation in Shibecha is dominated also by a dwarf bamboo species (Sasa nipponica).
Temperature, snow depth, and soil frost
Two rectangular plots (approximately 10 m × 30 m) were established at two landscape positions (upper and lower slope) in each catchment. During the study period, October 2004-April 2005, soil temperature was measured with dataloggers at three depths (0, 5, and 30 cm) in each catchment plot at hourly intervals (the accuracy for our instruments was ± 0.4°C). Snow depth also was measured in each plot at ten locations during monthly visits and air temperature was continuously monitored by the field stations of UREF and FSERC. Frozen soil was measured using a ruler at five burial sites, described below, in each catchment plot during monthly visits.
Sampling and analyses
Soil and forest floor sampling and bulk density
In situ buried bag experiments were established at each of the two plots established in both catchments. Five, 0–5 cm mineral soil samples, weighing ∼1 kg, along a center line in each plot, were collected at approximately 5 m intervals, sieved to 5 mm, mixed, and ∼100 g of mixed soil was inserted in polyethylene bags. The polyethylene bags were then inserted into plastic bottles containing “air” holes to prevent animal chewing. We buried the 0–5 cm mineral soil samples in the plastic bottles at 0 cm, 5 cm, and 30 cm depths at five sites, approximately 5 m apart in each of the two catchment plots. At each burial site reciprocal 0–5 cm mineral soil bottle transplants were made between the Shibecha and Uryu sites at each depth. Thus, we buried 0–5 cm mineral soil at 2 plots × 5 burial sites in each plot x 3 depths at each burial site × 2 catchments (reciprocal soil transplants for each soil bottle buried) for a total of 60 buried samples in each catchment. It is important to note that we used the same initial soil for the in situ field incubations and reciprocal soil transplants, for each catchment. Mineral soil samples were incubated for the entire winter (end of October 2005–mid-April 2006) and collected in mid April, just before spring snowmelt. Five mixed 0–5 cm mineral soil samples, in each of the two catchment plots were returned to the laboratory in coolers with ice packs for determining pre-incubation extractable NO −3 and NH +4 . Soil samples were stored in 1°C incubators until extractions were conducted within three days of collection.
Approximately monthly soil samples at each burial site in each catchment (2 plots × 5 burial sites = 10 total sites for each catchment) also were collected between October 2004 and April 2005 at 5 cm depth and analyzed for NO −3 , NH +4 , and DON. The first samples collected in October were collected on the same day that we collected the pre-incubation soil; therefore, October samples and pre-incubation soil was assumed to have similar characteristics. All soil N solute concentrations, pH, and soil moisture data reported for soil collected in Uryu on November 29, are referred to as having been collected in December to maintain consistency with Shibecha data, which was collected on December 2. During the October sampling date only, litter samples from the surface of the forest floor were collected at the soil sampling sites and analyzed for total lignin, carbon, and nitrogen. Bulk density for the 0–5 cm mineral soil was determined using the core method (Blake and Hartge 1986). Five bulk density cores (5 cm diameter) were collected from each of the two catchment plots (2 plots × 5 samples = 10 total samples for each catchment) and returned to the laboratory. Dry bulk density was calculated, after the entire cores were dried at 70°C, and corrected for root density.
Vegetation inventory
We sampled vegetation in the two plots established for each catchment. At each soil sampling site, diameter at breast height (DBH) and species of trees greater than 10 cm DBH were recorded within 5 m of the sampling site, parallel to the center line. The basal area was calculated by species and per unit catchment area (m2 ha−1).
Chemical and moisture determination
Soil samples were extracted with 2 M KCl. The extracts were filtered (0.7 μM pore size) and analyzed for NH +4 , NO −3 , and TDN using continuous flow colorimetry (AACS-4, BLTEC Co Ltd., Osaka). DON was calculated by subtracting dissolved inorganic nitrogen (NH +4 + NO −3 ) from TDN. Subsamples of each soil sample were oven dried for 2–3 days at 70°C, until they remained at a consistent mass, to determine the moisture content during each sampling date. Additional subsamples also were separated to analyze for pH using a ratio of 1 to 2.5 of soil to 0.01 M DI water. Net N mineralization rates during the incubation period were calculated by subtracting initial soil NH +4 + NO −3 content from incubated soil NH +4 + NO −3 content. Net nitrification rates were calculated by subtracting initial soil NO −3 content from incubated soil NO −3 content. Net mineralization and nitrification rates were converted to an areal basis (g N m−2) using mineral soil depth (5 cm) and bulk density values. Litter samples were ground and analyzed for total C and N content on a Perkin-Elmer CNH autoanalyzer. An alcohol-benzene soxhlet extraction was performed on the litter samples to separate lignin compounds from other organic species. Lignin content was analyzed using the acetyl bromide method and spectrophotometry (Johnson et al. 1961).
Statistical analyses
A randomized complete analysis of variance (ANOVA) was performed to detect differences in nitrification, mineralization, and nitrification/mineralization for each landscape position and depth between catchments and switched soil within a catchment (i.e., Uryu soil buried in Uryu, Uryu soil buried in Shibecha, Shibecha soil buried in Shibecha, and Shibecha soil buried in Uryu). ANOVA also was used to assess differences in depth at each landscape position for Shibecha soil buried in Shibecha, Uryu soil buried in Uryu, Shibecha soil buried in Uryu and Uryu soil buried in Shibecha. Significant differences between means were identified using the Student–Neuman–Keuls procedure. A randomized split-plot ANOVA with repeated measures was performed to detect differences in NO −3 , NH +4 , DON, soil moisture, and pH by landscape position between catchments and months as well as by catchment between landscape positions and months. Differences in C, N, C:N, lignin, lignin:N, and bulk density between catchments and landscape position also were assessed using a 2-way randomized complete ANOVA. Within our study, all statistical comparisons were assessed at α = 0.05 using the Statistical Analysis System software (SAS 2004).
Results
Snow depth, soil temperature, sand soil freezing
A snowpack of ∼20 cm formed on November 29 in Uryu and increased to a maximum depth of 194 cm by early March. By late April, ∼150 cm of snow still remained at Uryu (Fig. 2). No snowpack developed in Shibecha in late November/early December; however; by February, there was ∼55 cm of snow. A maximum snow depth of 87 cm that was observed in Shibecha in early March was higher than the average maximum at this catchment. By late April, no snowpack was observed in the Shibecha upper slopes, while ∼20 cm of snow still covered Shibecha lower slopes.
The mean daily air temperature in Uryu, during the study period, was −3.1°C with a maximum daily air temperature of 19.5°C on October 7 and minimum daily air temperature of −34.3°C on January 26 (Fig. 3a). In Shibecha, the mean daily air temperature was −0.6°C during the study period. The maximum daily air temperature that occurred on October 7 was 23.7°C while the minimum daily air temperature was −25.9°C on March 1 (Fig. 3b). Temperature fluctuated concomitantly with air temperature in those soils without snowcover. In contrast, soils covered with snow remained at ∼0°C. In Shibecha, the sudden drop in air temperature between November 28 and December 3 resulted in diurnal soil temperature cycles at the 0 and 5 cm soil depths with lower temperature (−5 to −8°C for 0 cm and −3 to −4 for 5 cm) observed at night and temperatures closer to 0°C observed during the day (Fig. 3d, f). Soil freezing occurred in Shibecha soil during the early December temperature drop to mean depths of 5 and 3 cm, respectively, in the lower and upper slopes (Fig. 4). Soil freezing occurred for the February, March, and April sample dates in Shibecha with deeper freezing in the upper (2–8 cm) versus the lower (2–3 cm) slopes. Soil temperatures in Uryu did not fall very far below 0°C except during the beginning of the cold period when the snowpack was just starting to form in Uryu (November 29) and air temperatures plummeted to a minimum of −17°C. There was relatively little soil freezing observed in Uryu soil (Fig. 3c, e), though we may have missed any freezing that would have occurred November 28–29, before the development of a snowpack in Uryu.
Mean daily air temperature in Uryu (a) and Shibecha (b); soil temperature at the lower slopes of Uryu (c) and Shibecha (d); and soil temperature at the upper slopes of Uryu (e) and Shibecha (f). The inset graphs in (d) and (f) are hourly soil temperatures during a cold snap before snowpack development in Shibecha
Vegetation
Tree species composition was different between the M3 catchment in Uryu and the SN catchment in Shibecha (Table 1). The Uryu catchment contained mixed deciduous species. Erman’s birch and Japanese linden dominated the lower slope while Glehn’s spruce dominated the upper slope. The Shibecha site contained only deciduous species with Japanese elm dominating both the lower and upper slopes.
Forest floor characteristics
There were no marked differences in total C (0.5 g C g−1, P = 0.6) and N (0.02 and 0.02 g N g−1, respectively, P = 0.07) between the forest floor in Uryu and Shibecha (Table 2). The C:N ratio, however, was slightly higher (25.0, P = 0.05) at Uryu compared to Shibecha (22.4). Lignin concentrations (0.3 and 0.2 g lignin g−1, P = 0.001) and lignin to N ratios (15 and 8.8, P = 0.006) were significantly higher for Uryu compared to Shibecha, respectively.
Soil nitrogen solute concentrations, soil moisture, and pH
DON was the dominant (40–90%) N solute in both Shibecha and Uryu 5 cm soil (Fig. 5). At the lower slope, NO −3 -N concentrations were significantly greater (P = 0.01) in Shibecha versus Uryu. Nitrate concentrations in the upper slope of Shibecha soil were significantly greater than in Uryu soil in October (P = 0.0003), December (P < 0.0001), February (P < 0.0001), and March (P < 0.0001) though the disparity of catchment means differed among months. DON concentrations were significantly greater in Uryu versus Shibecha only in October (P = 0.006) and in the upper slope. There were no differences in NH +4 -N concentrations between the catchments in the lower or upper slopes.
DON, NO −3 , and NH +4 concentration (mg N kg−1) in October, December, February, March, and April 2004–2005 in the lower slopes (a) and upper slopes (b) of Uryu and Shibecha. There was no difference in NH +4 between the catchments in the upper and lower slopes nor was there any difference in DON between the catchments in the lower slope. Letters that differ within each month for NO −3 -N (top row of letters for the upper slope) and DON (bottom row of letters for the upper slope) indicate significant differences between catchments at the 0.05 level
A marked decrease in TDN concentrations was observed from October to December in both catchments, most noticeably in the upper slopes though this shift was only significant (P = 0.0002) in the upper slopes of Uryu (Fig. 5, differences not shown). During this October to December shift, for Uryu and Shibecha, there was a marked decrease in DON concentrations in the lower (36.8–23.1 g N kg−1 and 44.1–31.6 g N kg−1, respectively) and upper slopes (96.7–33.7 and 58.8–21.6 g N kg−1, respectively), though only the decrease in DON in Uryu in the upper slopes was significant (P < 0.0001). An increase in NO −3 -N concentrations from October to December in Uryu (0.0–1.4 g N kg−1 in the lower slopes and 0.3–1.9 g N kg−1 in the upper slopes) and Shibecha (9.0–16.7 g N kg−1 in the lower slopes and 20.3–29.6 g N kg−1in the upper slopes) also was noted, though none of these changes were significant. Ammonium concentrations remained relatively unchanged between October and December in both catchments. A marked increase in Shibecha in the upper and lower slopes in DON and NO −3 -N concentrations was observed from February to March in Shibecha though these shifts were not significant.
Shibecha soil moisture was significantly greater than Uryu only in the lower slopes (P = 0.02) regardless of the month (Fig. 6). In the upper slopes Shibecha soil moisture was greater than Uryu only in March (P < 0.0001). There were no differences in soil moisture between the upper and lower slopes in Shibecha (Table 3). In Uryu, upper slope soil moisture was greater than lower slope in October (P = 0.002) and December (P = 0.01) while lower slope soil moisture was greater than upper in March (P = 0.04).
The pH was significantly higher in Shibecha (5.4) versus Uryu (4.6) in the upper slope (P = 0.002, data not shown). In the lower slope, the same trend occurred (mean pH was 6.2 and 4.9 in Shibecha and Uryu, respectively) but there were differences in the magnitude of catchment differences in monthly means.
Comparison of N processes in Uryu versus Shibecha non-frozen soil
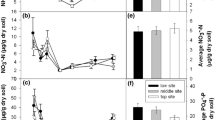
There were markedly different N mineralization and nitrification rates between Uryu versus Shibecha soil under conditions without soil freezing (soil buried at 30 cm depth). In the lower and upper slopes, net N mineralization in 30 cm soil, was significantly greater (P < 0.0001 for both landscape positions) in Shibecha versus Uryu (Fig. 7). Nitrification was higher than mineralization in Shibecha (104% of mineralized N was nitrified, P = 0.002 and 100% of mineralized N was nitrified, P < 0.0001, in the lower and upper slopes, respectively) versus Uryu (87% and 57% of mineralized N was nitrified in the lower and upper slopes, respectively), suggesting that a significantly greater percentage of mineralized ammonium was nitrified in Shibecha versus Uryu in both lower and upper slopes.
Mean net nitrification and N mineralization (mg N m−2 d−1, left axis) and nitrification/mineralization (right axis) (+SE of the grouped means) in the lower slopes (a) and upper slopes (b) of Uryu and Shibecha. Means are of catchment 0–5 cm mineral soil buried at 30 cm in situ + transplanted (N = 10). Letters that differ within each N soil process indicate significant differences between catchments at the 0.05 level
Comparison of N processes between frozen and non-frozen soil
The effects of soil freezing were most pronounced in Uryu soil buried in Shibecha at 0 cm and 5 cm depths (Fig. 8). For example, lower slope Uryu soil at 0 and 5 cm depths that was transferred to the lower slopes of Shibecha had significantly greater net N mineralization (P = 0.0004 and <0.0001, respectively) compared to lower slope Uryu soil buried in Uryu at the same depths (Fig. 8a). However, the fraction of N mineralization that resulted in nitrification was significantly lower in Uryu lower slope soil that was buried in Shibecha at 0 and 5 cm depths (P < 0.001 for both depths). Similarly, upper slope Uryu soil at 0 and 5 cm depths that was transferred to the upper slopes of Shibecha had greater net N mineralization (P < 0.0001) but a lower percentage of total mineralization that was nitrified (P < 0.0010) compared to upper slope Uryu soil buried in Uryu at the same depths (Fig. 8b). Soil freezing effects on N soil cycling also were more prominent in soil buried at 0 cm versus soil buried deeper and was especially apparent in Uryu soil buried in Shibecha where 0 cm soil had greater net N mineralization yet a lower percentage of mineralized NH +4 , which was subsequently nitrified, compared to soil buried at 5 and 30 cm (Table 4).
Mean net nitrification and N mineralization (mg N m−2 d−1, left axis) and nitrification/mineralization (right axis) (+SE of the grouped means) in the lower slopes (a) and upper slopes (b) of Uryu and Shibecha at 0, 5, and 30 cm. Letters that differ among catchment burial sites (Uryu, Shibecha in Uryu, Uryu in Shibecha, and Shibecha) indicate significant differences at the 0.05 level
Discussion
How does forest floor and soil quality affect the response of net N mineralization and nitrification rates in Uryu versus Shibecha?
There were some differences in forest floor characteristics between the Uryu and Shibecha sites that could partly explain the overall greater rates of net nitrification and N mineralization in Shibecha versus Uryu. Higher rates of net nitrification and N mineralization also could explain the greater NO −3 concentrations in soil extractions and the higher stream NO −3 concentration in Shibecha versus Uryu. The forest floor C:N and lignin:N ratios (indicators of forest floor quality) were significantly higher in Uryu versus Shibecha. Differences in these ratios were likely driven by the differences in tree species composition between the two sites; the coniferous species in Uryu contributed to higher ratios of C:N and lignin:N. Many studies have suggested that deciduous species such as sugar maple (Acer saccharum Marsh.), eastern hophornbeam (Ostrya virginiana (Mill.) K. Koch), and white ash (Fraxinus Americana L.) are associated with soil having low C:N ratios while soil under species such as red oak (Quercus rubra L.), American beech, and various conifer species have higher C:N ratios in litter (Finzi et al. 1998; Lovett and Mitchell 2004; Lovett et al. 2004; Mitchell et al. 1992, 2003; Pastor et al. 1984). The relationship between vegetation and litter C:N ratios are likely driven by lignin:N ratios (Lovett and Mitchell 2004). Lower lignin:N ratios in soil often result in higher net carbon mineralization (Melillo et al. 1982; Pastor and Post 1986; McGee et al. 2007) that can result in lower C:N ratios. Lower C:N ratios and the associated higher N mineralization and nitrification rates result in elevated soil NO −3 concentrations and export to surface waters (Christopher et al. 2006; Finzi et al. 1998; Gunderson et al. 1998; Lovett and Rueth 1999; Lovett et al. 2004; Melillo et al. 1982).
What is the effect of soil freezing on extractable N?
The cold period in late November/early December resulted in marked changes in soil TDN concentrations from the October to the December sampling dates, most noticeably in the upper slopes of Uryu and Shibecha. At each sampling site, there was a decrease in DON, an increase in NO −3 -N, and very little change in NH +4 -N. Greater N mineralization likely resulted in a release of labile NH +4 to soil from cellular material released from death and lysis of microbial cells (Edwards and Cresser 1992; DeLuca et al. 1992; Boutin and Robitaile 1995; Hermann and Witter 2002; Schimel and Clein 1996; Ryan et al. 2000; Neilsen et al. 2001) in these catchments that were subjected to extreme cold before a snowpack had developed. Although there were no increases in NH +4 -N concentrations, NO −3 -N concentrations increased at both catchments possibly suggesting that decreased competition for NH +4 -N between nitrifiers and heterotrophs and/or nitrifiers and root-mycorrhizal complexes, stimulated nitrification. In a snow manipulation experiment in Hubbard Brook, N.H., USA (Fitzhugh et al. 2001), NO −3 -N was the dominant N species in soil solution under snow-less plots containing mostly sugar maple trees (Acer saccharum Marsh.) and was attributed to a decreased competition for available NH +4 -N. Similarly, in Quebec, Canada, another snow manipulation study attributed increases in NO −3 -N concentration to lack of root uptake of NH +4 -N thereby decreasing competition of NH +4 -N for nitrifiers (Boutin and Robitaille 1995).
During the December and February sampling dates, TDN (including NH +4 -N, NO −3 -N, and DON) remained relatively unchanged. In March, there was a sudden increase in TDN at Shibecha that coincided with the melting of the snowpack and the mobilization of solutes. This is further supported by the observed increase in soil moisture in Shibecha during March (Fig. 6). Mobilization of N species during snowmelt has been shown to be an important mechanism of N export in forest catchments (Hinton et al. 1997; Creed and Band 1998; Frank et al. 2000; Inamdar et al. 2006; Mitchell et al. 1996; Piatek et al. 2005).
What is the effect of soil freezing on net N mineralization and nitrification?
Rates of net N mineralization during the entire winter incubation were different for frozen soil compared to unfrozen soil. Freezing effects were most noticeable with Uryu soil buried in 0 cm and 5 cm depths in Shibecha. Greater net N mineralization (NH +4 production + nitrification) was found in frozen soil compared to the same soil not subjected to freezing while the fraction of mineralized N that was nitrified was smaller in frozen compared to unfrozen soils. A laboratory study conducted with soil from the Hubbard Brook Experimental Forest in N.H. suggested that increased N mineralization in the Oa and A horizons subject to low temperatures and freezing (−13°C) was a result of reductions of available C following freezing in these horizons (Neilsen et al. 2001). However, N mineralization was unaffected by freezing at higher temperatures (−1 to −4°C) in laboratory experiments (Neilsen et al. 2001) and field experiments (Groffman et al. 2001b) at Hubbard Brook. Schimel et al. (2004) suggested that increased N mineralization in arctic soils subject to freezing at moderately low temperatures (∼ < 0°C > −7) was a result of C limitation occurring when microbes shift their substrate use pattern from C rich material (when soils are thawed) to using more N rich material (when soils are frozen). Schimel et al. (2004) suggested there are two possible reasons why this would occur: (1) a relative increase in the availability of N rich substrates such as from a release from organic matter (Edwards and Cresser 1992) or release from damaged or filled roots and microbes (DeLuca et al. 1992; Brooks and Williams 1999; Larsen et al. 2002) or (2) relative decrease in the availability of C rich substrate from C rich plant detritus as soils freeze (Schimel and Mikan 2005). Schimel and Mikan (2005) also observed a shift in substrate use from detrital material toward dissolved material and recycled microbial biomass at temperature just above 0°C as microbes prepare physiologically for freezing conditions.
In our study, it is uncertain why there was a net decrease in NO −3 production in frozen versus unfrozen soils over the study period. One possibility is that soil freezing disrupts the tight coupling between NH +4 production and subsequent nitrification (Pawluk 1988; Miller and Jastrow 1990). Though surviving nitrifiers were able to produce NO −3 in soil subject to the short freezing episode observed in late November/early December, their activity was could have been depressed when soil was subjected to prolonged freezing over the entire winter, such as in Shibecha 0 and 5 cm soil depths, compared to the same soil subjected to unfrozen (e.g., at Uryu) environments. This is consistent with other studies that have suggested prolonged freeze-thaw cycles kill portions of the microbial populations (Clein and Schimel 1995; Schimel and Clein 1996; Sulkava and Huhta 2003) such that their ability to metabolize C and N is diminished. An increase in soil NH +4 but no increase in NO −3 also was observed in a mesocosm study done in Swedish arctic soils frozen to ∼ −4 (Grogan et al. 2004). Another possibility why there was a net decrease in NO −3 in frozen versus unfrozen soils over the study period could have been due to losses from denitrification. Neilson et al. (2001) suggested that the observed increase in NH +4 following freezing in the Hubbard Brook, N.H. soils (Oa horizon) did simulate nitrification especially since they did not observe a decrease in microbial biomass. They hypothesized that denitrification also increased such that a significant portion of the excess NO −3 produced after freezing was denitrified rather than leached. Several researchers have suggested that the increase in N2O flux following freezing is due to increases in denitrification rates (Nyborg et al. 1997; Kaiser et al. 1998; Röver et al. 1998).
In the current study, it is unclear why effects of soil freezing on nitrification and mineralization were more pronounced in Uryu soils both for Uryu soil buried in Shibecha (frozen) versus Uryu soil buried in situ (non-frozen) and in 0 cm Uryu soil buried in Shibecha (frozen) versus 30 cm Uryu soil buried in Shibecha (non-frozen). Shibecha soils had higher soil moisture than Uryu soils, at least in the lower slopes (Fig. 6). Soils with higher soil moisture have a higher heat capacity and therefore reduced soil thermal sensitivity. Hence, Shibecha soils were likely more buffered from the cold, resulting in less marked response in N dynamics. Similarly, in the Hubbard Brook snow manipulation study, sugar maple plots froze after yellow birch (Betula alleghaniensis Britt.) plots since the sugar maple soil had higher moisture content and more buffering from latent heat of fusion (Hardy et al. 2001), which partially explained the variability in N dynamics under these different tree species (Groffman et al. 2001b).
Over-winter activity and climate change
Our study raises questions concerning impacts of changes in snowpack depth and duration in affecting soil freezing and N biogeochemistry. In the current study, soil frost helped explain differences in N mineralization and nitrification rates between frozen and unfrozen soils. Our study ended before snowmelt; therefore, we did not measure N mineralization and nitrification rates during spring/summer. We were unable to observe any changes in the net nitrification rate in soils after the over-winter period. For the snow removal experiment at Hubbard Brook (Groffman et al. 2001b) the researchers suggested that increased fine-root mortality reducing plant N uptake did not result in immediate increases in stream water NO −3 , but rather the effect was observed later in the summer. Similarly, the snow manipulation experiment in Quebec, Canada did not observe increase in NO −3 after plots were subjected to deep frost until the summer (Boutin and Robitaille 1995). Further evaluation of N mineralization and nitrification rates as well as soil extractable NO −3 is needed throughout the entire year at Uryu and Shibecha.
Conclusions
There were marked differences in soil net N mineralization and nitrification rates between the M3 catchment in Uryu and the SN catchment in Shibecha. Shibecha had much greater net N mineralization, net nitrification, and stream water NO −3 versus Uryu soil, partially a function of differences in tree species composition. A marked drop in temperature in late November/early December resulted in unchanged extractable NH +4 yet higher extractable NO −3 . Pro-longed soil freezing over the entire winter resulted in greater net N mineralization yet lower net nitrification. Although this study was only conducted during winter, it raises important questions about the effect of climate change on N biogeochemistry. Soil freezing in temperate forested systems is likely to become more common if snowpacks develop later and melts occur earlier as predicted to occur as the climate warms. Several studies conducted to evaluate the effect of freezing on microbial communities and N cycling have been done in arctic and alpine soils (Brooks et al. 1997, 1998; Larsen et al. 2002; Sulkava and Huhta 2003; Grogan et al. 2004; Schimel et al. 2004; Schimel and Mikan 2005), in mostly mesocosm experiments and few in temperate soils where freezing is less severe (Groffman et al. 1999; 2001b; Neilsen et al. 2001). The effect of soil freezing should be considered when evaluating differences in N dynamics between temperate ecosystems that have different propensity for soil freezing. Furthermore, it will be important to evaluate changes in snowpack depth especially in sites such as Uryu, where soils rarely freeze, but have the potential for large changes in snowpack regimes resulting in soil freezing and marked alteration of over-winter biogeochemistry processes.
References
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10:9–31
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis. Part I. Physical and mineralogical methods. Agronomy Society of America and Soil Science Society of America, Madison, WI, pp 363–376
Boutin R, Robitaille G (1995) Increased soil nitrate losses under mature sugar maple trees affected by experimentally induced deep frost. Can J For Res 25:588–602
Boyer EW, Goodale CL, Jaworski NA, Howarth RW (2002) Anthropogenic nitrogen sources and relationships to riverine nitrogen export in the northeastern USA. Biogeochem 57:137–169
Brooks PD, Williams MW (1999) Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrol Process 13:2177–2190
Brooks PD, Williams MW, Schmidt SK (1996) Microbial activity under alpine snowpacks, Niwot Ridge, Colorado. Biogeochem 32:93–113
Brooks PD, Schmidt SK, Williams MW (1997) Winter production of CO2 and N2O from alpine tundra: environmental controls and relationship to inter-system C and N fluxes. Oecologia 110:403–413
Brooks PD, Williams MW, Schmidt SK (1998) Inorganic nitrogen and microbial biomass dynamics before and during snowmelt. Biogeochem 43:1–15
Callesen I, Borken W, Kalbitz K, Matzner E (2007) Long-term development of nitrogen fluxes in a coniferous ecosystem: does soil freezing trigger nitrate leaching? J Plant Nutr Soil Sci 170:189–196
Campbell JL, Mitchell MJ, Groffman PM et al (2005) Winter in northeastern North America: a critical period for ecological processes. Front Ecol Environ 3:314–322
Christopher SF, Page BD, Campbell JL (2006) Contrasting stream water NO −3 and Ca2+ in two nearly adjacent catchments: the role of soil Ca and forest vegetation. Global Change Biol 12:364–381
Clein JS, Schimel JP (1995) Microbial activity of tundra and taiga soils at sub-zero temperatures. Soil Biol Biochem 27:1231–1234
Creed IF, Band LE (1998) Export of nitrogen from catchments within a temperate forest: evidence for a unifying mechanism regulated by variable source area dynamics. Water Resour Res 34:3105–3120
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Global Change Biol 4:217–227
DeLuca TH, Keeney DR, McCarty GW (1992) Effect of freeze-thaw events on mineralization of soil nitrogen. Biol Fertil Soils 14:116–120
Driscoll CT, Whitall D, Aber JD et al (2003) Nitrogen pollution in the Northeastern US: sources, effects, and management options. Bioscience 53:357–374
Edwards AC, Cresser MS (1992) Freezing and its effects on chemical and biological properties of soil. Adv Soil Sci 18:59–79
Finzi AC, Van Breemen N, Canham CC (1998) Canopy Tree-soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecological Applications 8:440–446
Fitzhugh RD, Driscoll CT, Groffman PM et al (2001) Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochem 56:215–238
Frank HP, Schleppi WP, Hannes F (2000) Export of dissolved organic carbon and nitrogen from Gleysol dominated catchments—the significance of water flowpaths. Biogeochem 50:137–161
Goodale CL, Aber JD, Vitousek PM (2003) An unexpected nitrate decline in New Hampshire streams. Ecosystems 6:75–86
Goodrich LE (1982) The influence of snow cover on the ground thermal regime. Can Geotech J 19:421–432
Groffman PM, Hardy JP, Nolan S et al (1999) Snow depth, soil frost and nutrient loss in a northern hardwood forest. Hydrol Process 13:2275–2286
Groffman PM, Driscoll CT, Fahey TJ et al (2001a) Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochem 56:135–150
Groffman PM, Driscoll CT, Fahey TJ et al (2001b) Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochem 56:191–213
Grogan P, Michelsen A, Ambus P et al (2004) Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol Biochem 36:641–654
Gundersen P, Callesen I, de Vries W (1998) Nitrate leaching in forest ecosystems is related to forest floor C/N ratios. Environ Pollut 102:403–407
Hardy JP, Groffman PM, Fitzhugh RD, et al (2001) Snow depth manipulation and its influence on soil frost and water dynamics in a northern hardwood forest. Biogeochem 56:151–174
Hayhoe C, Wake CP, Huntington TG et al (2006) Past and future changes in climate and hydrological indicators in the US Northeast. Clim Dyn doi: 10.1007 /s00382-006-0187-8
Herrmann A, Witter E (2002) Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biol Biochem 34:1495–1505
Hinton MJ, Schiff SL, English MC (1997) The significance of storms for the concentration and export of dissolved organic carbon from two Precambrian Shield catchments. Biogeochem 36:67–88
Hobbie SE, Chapin FS (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochem 35:327–338
Hosaka M, Nohara D, Kitoh A (2005) Changes in snow cover and snow water equivalent due to global warming by a 20 km-mesh global atmospheric model. Sola 1:93–96
Huntington TG, Hodgkins GA, Keim BD et al (2004) Changes in proportion of precipitation occurring as snow in Northeast (1949 to 2000). J Clim 17:2626–2636
Inamdar SP, O’Leary NO, Mitchell MJ, Riley JT (2006) The impact of storm events on solute exports from a glaciated forested watershed in western New York, USA. Hydrol Process 20:3423–3429
Johnson DB, Moore WE, Zank LC (1961) The spectrophotometric determination of lignin in small wood samples. Tappi 44:793–798
Kaiser E-A, Kohrs K, Kücke M et al (1998) Nitrous oxide release from arable soil: importance of N-fertilization, crops and temporal variation. Soil Biol Biochem 30:1553–1563
Kieft TL, Soroker E, Firestone MK (1987) Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol Biochem 19:119–126
Larsen KS, Jonasson S, Michelsen (2002) Repeated freeze-thaw and their effects on biological processes in two arctic ecosystem types. Appl Soil Ecol 21:187–195
Likens GE, Driscoll CT, Buso DC (1996) Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272:244–245
Lovett G, Mitchell MJ (2004) Sugar maple in nitrogen cycling in the forests of eastern North America. Front Ecol Environ 2:81–88
Lovett GM, Rueth H (1999) Soil nitrogen transformations in beech and maple stands along a nitrogen deposition gradient. Ecol Appl 9:1330–1344
Lovett GM, Weathers KC, Arthur MA (2002) Control of nitrogen loss from forested watersheds by soil carbon: nitrogen ratio and tree species composition. Ecosystems 5:712–718
Lovett GM, Weathers KC, Arthur MA et al (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochem 67:289–308
Lund V, Goksøyr J (1980) Effects of water fluctuations on microbial mass and activity in soil. Microb Ecol 6:115–123
Marumoto T, Anderson JPE, Domsch KH (1982) Mineralization of nutrients from soil microbial biomass. Soil Biol Biochem 14:469–475
McGee GG, Mitchell MJ, Leopold DJ et al (2007) Relationships among forest age, composition and elemental dynamics of Adirondack northern hardwood forests. J Torr Bot Soc 134:253–268
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Miller RM, Jastrow JD (1990) Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol Biochem 22:579–584
Mitchell MJ, Foster NW, Shepard JP et al (1992) Nutrient cycling in Huntington Forest and Turkey Lakes deciduous stands: nitrogen and sulfur. Can J For Res 22:457–464
Mitchell MJ, Driscoll CT, Murdoch P et al (1996) Climatic control of nitrate loss from forested watershed in the northeast United States. Environ Sci Technol 30:2609–2612
Mitchell MJ, Driscoll CT, Inamdar S et al (2003) Nitrogen biogeochemistry in the Adirondack mountains of New York: hardwood ecosystems and associated surface waters. Environ Pollut 123:355–364
Neilsen CB, Groffman PM, Hamburg SP et al (2001) Freezing effects on carbon and nitrogen cycling in northern hardwood forest soils. Soil Sci Soc Am J 65:1723–1730
Nyborg M, Laidlaw JW, Solberg ED (1997) Denitrification and nitrous oxide emissions from a Black Chernozemic soil during spring thaw in Alberta. Can J Soil Sci 77:153–160
Pastor JJ, Post WM (1986) Influence of climate, soil moisture, and succession on forest carbon and nitrogen cycles. Biogeochem 2:3–27
Pastor J, Aber JB, McClaugherty CA et al (1984) Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65:256–268
Pawluk S (1988) Freeze-thaw effects on granular structure reorganization for soil materials of varying texture and moisture content. Can J Soil Sci 68:485–494
Piatek KB, Mitchell MJ, Silva SR, Kendall C (2005) Sources of nitrate in Adirondack surface water during dissimilar snowmelt events. Water, Air, and Soil Pollut 165:13–35
Röver M, Heinemeyer O, Kaiser E-A (1998) Microbial induced nitrous oxide emissions from an arable soil during winter. Soil Biol Biochem 30:1859–1865
Ryan MC, Kachanoski RG, Gillham RW (2000) Overwinter soil nitrogen dynamics in seasonally frozen soils. Can J Soil Sci 80:541–550
SAS Institute, Inc (2004) Statistical analysis software version 9.1.3 for microcomputers. SAS Institute Inc, Cary (NC)
Schimel JP, Clein JS (1996) Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol Biochem 28:1061–1066
Schimel JP, Mikan C (2005) Changing microbial substrate use in Arctic tundra soils through a freeze-thaw cycle. Soil Biol Biochem 37:1411–1418
Schimel JP, Bilbrough C, Welker JM (2004) Increased snow depth affects microbial activity and nitrogen mineralization in two arctic tundra communities. Soil Biol Biochem 36:217–227
Shibata H, Kirikae M, Tanaka Y et al (1997) Proton budgets of forest soils during the snowmelt process in Hokkaido, Northern Japan. In: Aoyama K, Katoh K, Murano T, Paces T, Taguchi Y (eds) Proceedings of international congress of acid snow and rain 1997, TACT Press, Niigata University, Niigata, Japan
Shibata H, Ichikawa K, Nomura M et al (2002) Elemental budgets of forested watershed at cold snowy region. J Jpn Assoc Hydrol Sci 32:49–56 (in Japanese with English abstract)
Soulides DA, Allison FE (1961) Effect of drying and freezing soils on carbon dioxide production, available mineral nutrients, aggregation, and bacterial populations. Soil Sci 91:291–298
Sulkava P, Huhta V (2003) Effects of hard frost and freeze-thaw cycles on decomposer communities and N mineralization in boreal forest soil. Appl Soil Ecol 22:225–239
Takeuchi M (1980) Studies on the freezing and thawing of the volcanic ash soils in eastern Hokkaido. I. The seasonal aspects of the freezing and thawing in a plowed field. Bull. Kyoto Univ. Forests 52:117–129, (in Japanese with English summary)
Takeuchi M (1981) Studies on the freezing and thawing of the volcanic ash soils in eastern Hokkaido. II. Soil freezing and thawing in a grassland (Sasa nipponica) and a brush cutting area. Bull. Kyoto Univ. Forests 53:205–215, (in Japanese with English summary)
Tilman GL (1998) Species composition, species diversity, and ecosystem processes: understanding the impacts of global change. In: Pace ML, Groffman PM (eds) Successes, limitations and frontiers in ecosystem science. Springer-Verlag, New York, pp 452–472
Vitousek PM (1994) Beyond global warming: ecology and global change. Ecology 75:1861–1876
Williams MW, Brooks PD, Seastedt T (1998) Nitrogen and carbon soil dynamics in response to climate change in a high-elevation ecosystem in the rocky mountains, U.S.A. Arctic Alpine Res 20:26–30
Yoshida T, Iga Y, Ozawa M et al (2005) Factors influencing early vegetation establishment following soil scarification in a mixed forest in northern Japan. Can J For Res 35:175–188
Acknowledgements
This research was supported by the Japanese Society for the Promotion of Science sponsoring the postdoctoral fellowship of the first author. Special thanks are given to the staff at Uryu Experimental Forest and Shibecha Experiment forest for assistance in fieldwork and accommodations. Thanks also to the staff and students at the Nayoro office of Hokkaido University for help with fieldwork and logistics (especially Mahoko Noguchi for aide with the vegetation sampling) and to Dr. Toshizumi Miyamoto and his staff for aiding with the lignin analyses. We appreciate also the thoughtful and thorough comments from two anonymous reviewers that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christopher, S.F., Shibata, H., Ozawa, M. et al. The effect of soil freezing on N cycling: comparison of two headwater subcatchments with different vegetation and snowpack conditions in the northern Hokkaido Island of Japan. Biogeochemistry 88, 15–30 (2008). https://doi.org/10.1007/s10533-008-9189-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-008-9189-4