Abstract
We present 42 dual-isotope nitrate analyses of fresh water samples collected in the St. Lawrence River between June 2006 and July 2008. Measured δ15N–NO3 − and δ18O–NO3 − values correlate negatively, while δ18O–NO3 − displays no negative correlation with nitrate concentration. This suggests that nitrate uptake and/or elimination by denitrification is not the main driver of observed variations in nitrate concentration and isotopic signature in the St. Lawrence River. In addition, δ18O–NO3 − is negatively correlated with the seasonally variable δ18O of ambient water, indicating that the variation in the isotopic signature of nitrate is barely modulated by in-stream nitrate regeneration (nitrification). It rather is constrained by along-river changes in the external sources of nitrate. Given the distinct nitrogen (N) and oxygen (O) isotopic signature of atmospheric nitrate, we argue that observed seasonal variations of δ15N–NO3 − and δ18O–NO3 − in the St. Lawrence River are due to variable contributions of snowmelt-derived water. Based on a N and O isotope mass balance, we show that total nitrate loading in the St. Lawrence River is dominated by a N input from the Great Lakes (47 ± 28 %) and from nitrate regeneration of both internal and external N (48 ± 22 %). While temporal nitrate N and O isotope dynamics in the St. Lawrence River are mainly influenced by the atmospheric N input fluctuations, with an increase in atmospheric loading during spring, atmospheric N plays overall a rather insignificant role with regards to the N budget (5 ± 4 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Riverine transport and processing of nitrogen (N) are major links between terrestrial and marine/estuarine nitrogen cycles, and exert a strong influence on productivity and biodiversity of coastal marine ecosystems (Galloway et al. 2004). Estuaries are particularly sensitive to terrestrial organic matter (OM) and nutrient inputs (Gearing and Pocklington 1990), which can affect primary productivity, OM deposition, and OM remineralization rates. As a major nutrient, fixed (i.e., bio-available) N is partly responsible for estuarine eutrophication, negatively affecting the health of coastal marine ecosystems (Nixon et al. 1996; Howarth 1998; Howarth et al. 2006). Anthropogenic N inputs from agricultural and fossil fuel sources have increased tenfold globally since the late nineteenth century, adding bio-available N to coastal waters (Galloway et al. 2004).
Recent research in the Laurentian Channel has shown that an hypoxic zone of approximately 1,300 km2 has developed in the bottom waters of the Lower St. Lawrence Estuary (LSLE) over the last decades (Gilbert et al. 2005). Climate-driven changes in ocean circulation patterns, which determine the partitioning of water masses that enter the Gulf of St. Lawrence from the North Atlantic through Cabot Strait, may have contributed to the historic decline in dissolved oxygen (DO) (Gilbert et al. 2005; Thibodeau et al. 2010a). However, eutrophication and increased organic particle fluxes may also have caused a decline in DO resulting from enhanced microbial respiration, mostly within the sediments (Benoit et al. 2006; Thibodeau et al. 2006; Lehmann et al. 2009). The observed decline in oxygen content in the LSLE bottom waters appears to coincide with a 70 % increase of fertilizer sold within the St. Lawrence watershed between 1970 and 1988 (Thibodeau et al. 2006). Hence, it is reasonable to assume that anthropogenic inputs of N, as well as climatic/oceanographic constraints, both play a role in the generation and maintenance of hypoxic conditions in the LSLE (Thibodeau et al. 2010b). While we know that the major sources of nitrate in large catchments are generally from agricultural areas (chemical fertilizers and manure), soils, and atmospheric precipitation (reviewed by Kendall et al. 2007), the contributions of the various N sources to the St. Lawrence system are poorly understood, and their relative contribution can be expected to vary with time. Sufficient measures to preserve the integrity of an aquatic system such as the St. Lawrence River (SLR) and estuary require identification of the main fixed-N sources and the fate of each nitrogen source within this system (i.e., in-stream cycling through assimilation, denitrification, remineralization or nitrification).
Nitrate N isotopes (δ15N–NO3 −; for definition see method section), and more recently nitrate O isotopes (δ18O–NO3 −) have proven to be excellent tracers of nitrate sources. The dual isotope approach is based on the fact that nitrate from different origins have distinct isotopic signature ranges (see Kendall et al. 2007 for a review). For example, inorganic nitrate fertilizers show significantly higher δ18O values compared to most other nitrate sources, whereas their δ15N value is generally quite low. In contrast, nitrate derived from organic sources tend to exhibit elevated δ15N values, but comparatively low δ18O values (Amberger and Schmidt 1987). For simple point-source analyses, these generalizations are useful. However, in riverine systems the situation may be more complex. The isotopic composition of SLR nitrate, for example, is not necessarily only modulated by variable nitrate source loading, but may also depend on isotopic fractionation during in-stream removal processes such as denitrification, assimilation, and in-stream nitrification (i.e., regeneration of nitrate with a different isotopic signature than nitrate from external sources). The dual isotopic analysis of nitrate allows the identification and assessment of coincidental processes such as (1) N-elimination processes (i.e., denitrification and assimilation), (2) nitrification and (3) input from multiple external sources that may have a cancelling effect on the nitrate concentration and δ15N–NO3 − alone (Burns and Kendall 2002; Mayer et al. 2002; Lehmann et al. 2003, 2004; Kendall et al. 2007; Burns et al. 2009).
Denitrification refers to the dissimilatory reduction of NO3 − to gaseous products (N2, N2O, or NO) and usually occurs when O2 concentrations are lower than 20 μM (Kendall et al. 2007). Denitrification causes the δ15N and δ18O values of the residual nitrate pool to increase exponentially as nitrate concentration decreases (Kendall et al. 2007). Measured nitrate isotope enrichment factors are variable and range from 10 to 40 ‰, with a nitrate N:O isotope enrichment ratio (Δδ18O/Δδ15N) of 0.5–1 (Böttcher et al. 1990; Cey et al. 1999; Lehmann et al. 2003; Granger et al. 2004a; Panno et al. 2006). To some parts, the extent of measured fractionation depends on environmental conditions. For example, strong nitrate isotope fractionation is associated with water column denitrification (up to 40 ‰), expressed in the high N and O isotopic composition of residual NO3 −, whereas denitrification within sediments (0–3 ‰) is limited by diffusive substrate supply so that the expression of the biological N isotope fractionation in the environment is significantly suppressed (Lehmann et al. 2004, 2007; Sigman et al. 2005; Alkhatib et al. 2012). While denitrification in the water column should not play a role in the SLR at given DO concentrations, closed system conditions are often prevalent in anaerobic soil compartments along the groundwater flow path in aquifers and riparian zones, and occasionally in river sediments (Kellman and Hillaire-Marcel 1998; Sebilo et al. 2003). Nitrate that has undergone partial denitrification in these watershed compartments should, therefore, have elevated δ15N and δ18O values compared to input values. Another removal process that is known to discriminate between nitrogen (and O) isotopes is the assimilation of inorganic nitrogen compounds into living organisms via biosynthesis (Altabet et al. 1999). As with dissimilative nitrate reduction by denitrifiers, the nitrate assimilation results in a strongly coupled N and O fractionation (Granger et al. 2004b; Lehmann et al. 2005), with Δδ18O/Δδ15N of 1:1 in saltwater environments.
During the regeneration of nitrate by nitrification, δ15N and δ18O of new nitrate are affected in different ways (Sigman et al. 2005; Bourbonnais et al. 2009). The Ν-isotopic composition of nitrate produced in-stream by nitrification is principally constrained by the δ15N of the OM that is subjected to remineralization. A small isotope fractionation usually accompanies the aerobic mineralization of OM, and results in ammonium with a δ15N value 0–4 ‰ lower than the original bulk OM (Lehmann et al. 2002; Dijkstra et al. 2008). Nitrification of ammonium to nitrate is associated with large 15N isotope effects (14–38 ‰; Casciotti et al. 2003). This process may be difficult to observe in river systems, where remineralization and nitrification proceed nearly in parallel and to completion (i.e., with no ammonium accumulation) (Miyajima et al. 2009). Thus, we can expect that nitrate from nitrification will have a δ15N slightly lower or equal (of about 0–4 ‰) to the δ15N of the particulate OM. For a long time, it has been argued that up to 1/3 of the O atoms in regenerated nitrate derives from DO (Aleem et al. 1965; Hollocher 1984). However, more recent work has shown that ultimately (due to strong nitrifier-catalyzed nitrite-water exchange) the contribution of oxygen atoms in marine and freshwater nitrate from DO is minor or even nil (Casciotti et al. 2002; Wankel et al. 2006, 2007; Knapp et al. 2008; Bourbonnais et al. 2009). Irrespective of the exact mechanisms that determine the δ18O of newly-nitrified nitrate, seasonally-driven variation in the δ18O of river water should leave its imprint in the δ18O of nitrate that has been regenerated in-stream.
There are various potential external sources of nitrate in riverine systems: (1) nitrate derived from natural N2-fixation followed by remineralization and nitrification in watershed soils, (2) anthropogenic nitrate derived from manure, inorganic fertilizers, septic and animal waste, and domestic and industrial wastewaters and (3) nitrate derived from atmospheric deposition of nitrogen oxides in the watershed. Each pool of nitrate is characterized by generally distinct values of δ15N and δ18O. While nitrate derived from N2-fixation will carry a low-δ15N signature, nitrate originating from manure, septic or animal waste, and domestic waste typically display high δ15N values (+10 ‰ or higher; McClelland and Valiela 1998; Bedard-Haughn et al. 2003). Since nitrate from these sources are produced via nitrification in the soil, the corresponding δ18O should reflect the δ18O of the ambient soil water, which may be variably enriched in 18-O through evaporation. Synthetic fertilizers are characterized by low δ15N–NO3 − (0 to +5 ‰) and their δ18O–NO3 − depends on whether they are applied in reduced form (ammonium) or directly as nitrate. Nitrification of fertilizer ammonium will lead to a δ18O–NO3 −, that again reflects the δ18O of ambient water during nitrification (in the catchment or later in the river), while fertilizer in nitrate form will carry a δ18O–NO3 − that is significantly higher (~20 ‰) (Amberger and Schmidt 1987). However, nitrate fertilizers are used less frequently than ammonium fertilizers by farmers in Québec (Agriculture and Agri-Food Canada, Korol 2002) and are therefore less likely to play a major role in the seasonal variation of δ18O–NO3 −. Nitrate from atmospheric deposition is generally heavily enriched in 18O (δ18O > 60 ‰; Kendall et al. 2007), and δ18O measurements close to the study area (Vermont) averaged +83 ‰ for an annual cycle (Ohte et al. 2004). In the area surrounding the Great Lakes and the SLR watershed, measured δ15N–NO3 − values in precipitation samples ranged from −5.4 to +0.6 ‰ (reported in Kendall et al. 2007). Those estimates are consistent with time-series nitrate isotope data from a site within the Great Lakes watershed, with seasonal means of +65 and +90 ‰ and −6 to 0 ‰, for nitrate δ18O and δ15N, respectively, with the lower values observed in summer (Finlay et al. 2007). Since, on average, nitrate from precipitation displays significantly higher δ18O–NO3 −, and lower δ15N–NO3 −, than the nitrate isotope values observed in streams (Kendall et al. 2007), the leverage effect can be strong and a concomitant increase in δ18O–NO3 − and decrease in δ15N–NO3 − during the spring would suggest a sensitive response of the riverine nitrate pool to an elevated contribution of N from atmospheric precipitation due to the spring snowmelt.
We present 42 dual-nitrate isotope measurements of nitrate sampled from the SLR at its outlet (Québec City) to the estuary, beginning in June 2006 and ending July 2008. We hypothesize that nitrate in the St. Lawrence at Québec City likely derives from three main sources (Great Lakes, atmospheric deposition and external sources ultimately nitrified in-stream or in the catchment) and that variation in the isotopic composition of nitrate are mostly constrained by seasonal changes in the partitioning of theses sources. In order to test this hypothesis we first need to evaluate potential N-elimination and nitrification influence on the isotopic composition of nitrate on a seasonal timescale in the SLR, potentially masking external nitrate source signatures. We will compare the nitrate-δ18O data to water-δ18O data obtained from the same location during the same time interval, in order to assess if and how natural δ18O variations of the stream water affect variations in the isotopic composition of the riverine nitrate pool, and what, if any, information on in-stream-nitrate regeneration can be gained from these observations. Finally we will quantitatively validate our hypothesis using an isotopic mass balance model to asses the partitioning between the various N sources that contribute to the St. Lawrence N loading.
Methods
Dissolved nitrate concentrations were determined at the Geochemistry and Geodynamics Research Center (GEOTOP) using a Braan and Luebbe autoanalyzer, with a detection limit of ~0.1 μmol L−1 (Strickland and Parsons 1972). Stable N and O isotope ratios of dissolved nitrate (δ15N, δ18O; where δ = [(R sample/R standard) − 1] * 1,000, and R refers to the 15N/14N or 18O/16O ratios) were measured using the denitrifier method (Sigman et al. 2001; Casciotti et al. 2002). Sample nitrate (and nitrite) was converted to nitrous oxide (N2O) by denitrifying bacteria that lack N2O reductase activity (Pseudomonas chlororaphis ATCC #43928 or ATCC #13985). The N2O was purged from the sample vial with helium carrier gas, cryo-concentrated, and purified using a Micromass TraceGas™ inlet. The N and O isotopic composition was determined using a Micromass Isoprime™ universal triple collector isotope ratio mass spectrometer in continuous flow mode. The target sample size was always 20 nmol. Blank contribution was generally lower than 0.3 nmol (~1.5 % of the sample). For δ18O isotope analyses, only P. chlororaphis ATCC #13985 (subspecies aureofaciens) was used. Oxygen isotope exchange with the ambient water during N2O production resulted in a δ18O-scale compression, which was corrected according to Casciotti et al. (2002). The O isotope exchange was never higher than 5 %. Based on replicate measurements of laboratory standards and samples (intra- and inter-run), the reproducibility (1σ) of δ15N and δ18O were better than ±0.3 and ±0.5 ‰, respectively. Isotopic values were normalized using IAEA-N3, which has a δ15N value of +4.72 ± 0.13 ‰ relative to atmospheric N2 (Gonfiantini et al. 1995 ) and a δ18O value of +25.6 ± 0.2 ‰ relative to V-SMOW (Bohlke et al. 2003). All isotope measurements are reported in the delta notation in permil (‰), relative to atmospheric N2 (AIR) for δ15N and VSMOW for δ18O.
The oxygen isotopic composition of water was analyzed at the GEOTOP research center. Water samples were collected in 60 mL plastic bottles, with no headspace, and stored at 4 °C. Unfiltered water (200 μL) was equilibrated with 200 μL of CO2 at 40 °C. The water/CO2 equilibration progressed for 7 h, after which the CO2 was sampled and measured on a Micromass Isoprime™ universal triple collector isotope ratio mass spectrometer in dual inlet mode coupled to an Aquaprep™ system. The resulting isotopic values were drift and temperature-corrected using two internal laboratory standards previously calibrated on the VSMOW-SLAP scale. The overall analytical uncertainty of the water O-isotope analysis was ±0.05 ‰. All reported correlation coefficients (R 2) and two-tailed P values were estimated using the Spearman nonparametric correlation between measured variables using the software Prism 6©.
Regional settings
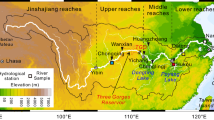
All samples were collected from a water filtration plant located on the south shore of the SLR, near its outlet to the upper St. Lawrence Estuary, close to Québec City (see Hélie and Hillaire-Marcel 2006 for methods). Here, the SLR water represents a mixture of various water sources originating from the Great Lakes and northern tributaries, such as the Ottawa River (Fig. 1; Table 1). The discharge from Lake Ontario does not display significant seasonal variations. In contrast, discharge from tributaries can be strongly modulated by the seasonal hydrological cycle. During spring snowmelt, outflow rates are three times higher than the mean annual discharge (Environment Canada, unpublished data). Hence, the proportional contribution from Great Lakes water to the total discharge at Québec City varies from >70 % in summer to <55 % in spring (Environment Canada, unpublished data). Base-flow conditions can be assumed for the summer months. The watershed is composed of different eco- and land use zones, i.e., boreal and mixed forests, as well as agricultural and urban areas, which are located primarily around the Great Lakes and the SLR (Fig. 1).
Results
Nitrate concentrations in the SLR exhibited strong annual cyclicity, with minimal concentrations (<15 μmol L−1) in fall and maximum concentrations (>30 μmol L−1) in winter/spring (Fig. 2). The δ15N–NO3 − values increased during the summer and climaxed in the fall (>7 ‰), then decreased during the winter months to a minimum in April (<4.5 ‰; Fig. 2). Values for δ18O–NO3 − parallel the nitrate concentration data, with a minimum (<3 ‰) at the end of the summer (August) and a maximum (>6 ‰) in April (Fig. 2). As a logical consequence, δ18O–NO3 − and δ15N–NO3 − show a negative correlation (R 2 = 0.60, P < 0.0001; Fig. 3). The observed correlation between δ18O–NO3 − and δ15N–NO3 − is similar for every season except fall, when no trend is apparent. Strong intra-seasonal variability is observed in the spring but seems to be of less importance during other seasons (Table 2). Mean [NO3 −], δ15N–NO3 − and δ18O–NO3 − show distinct, discernible signatures for each season except between winter and spring, which may be due to the gradual (but irregular) input of snowmelt, as illustrated by the larger standard deviation of δ18O–NO3 − data in spring. The δ18O–H2O at Québec City obeys a seasonal cycle, with 18O-depleted values during the spring during snowmelt, and more 18O enriched values in the fall (Fig. 4).
Discussion
In-stream nitrate removal processes
The present dataset is characterized by a significant negative correlation between δ15N–NO3 − and δ18O–NO3 −, yet the correlation of δ18O–NO3 − and nitrate concentration is positive (R 2 = 0.32, P < 0.0001). These observations suggest that the isotopic variations throughout the year cannot be attributed solely to seasonally variable removal processes within the stream water. While the lack of negative correlation between nitrate concentration and the δ18O–NO3 − could be due to denitrification occurring under diffusion-limiting conditions (i.e., in the sediment), which would likewise limit isotopic fractionation (Lehmann et al. 2007), the negative δ18O–NO3 versus δ15N–NO3 − correlation speaks against denitrification in general as the dominant modulator of nitrate concentrations and isotope ratios. Denitrification within the system cannot totally be excluded, yet, if present at all, the isotopic fractionation linked to nitrate consumption processes appears to be overprinted by stronger signals possibly due to external N loading.
In analogy to the argument above, the negative correlation between δ18O–NO3 − and δ15N–NO3 − suggests that either nitrate assimilation is not important or, more likely, that its N and O isotopic signature is overprinted by (1) other fractionating N reactions or (2) the input of nitrate sources, which would likely decouple a parallel evolution of the nitrate δ15N and δ18O. Hélie and Hillaire-Marcel (2006) demonstrated that particulate organic carbon at the sampling station was mainly of autochthonous origin, which confirms the importance of primary productivity and, in turn, implies assimilation of nitrate by algae. Therefore, we conclude that although assimilation is present, its fractionation signature has been overprinted by stronger signals either from in-stream production of nitrate or external inputs. Below we will further consider these two aspects.
In-stream production of nitrate
The observed seasonal pattern in δ18O–H2O (with 18O-depleted values during the spring due to snowmelt compared to high-δ18O water during the fall; Fig. 4) is driven by variations observed in the δ18O–H2O of precipitation, which is due to the temperature-dependent fractionation occurring during condensation of water in air masses (Dansgaard 1964). Snowmelt events represent an input of 18O-depleted precipitation that was sequestered on land during winter (Myre 2006). Assuming that the δ18O of nitrified nitrate is controlled only by the δ18O of the ambient water, we would predict it to follow seasonal variations as shown in Fig. 4. In turn, a positive relationship between the δ18O–H2O and the measured δ18O–NO3 − is expected if nitrate from nitrification is a dominant source for the St. Lawrence riverine nitrate pool. There is a statistically significant negative correlation (R 2 = 0.24, P = 0.0016) between δ18O–H2O and the δ18O–NO3 − (Fig. 5), which essentially excludes in-stream nitrification as the major factor driving the variation in δ18O–NO3 (and the incorporation of water O atoms in the regenerated nitrate). Moreover, δ18O–O2 data (collected in 2000–2001 by Hélie, unpublished results) were mostly constant throughout the year (+22.0 ± 0.9 ‰), and the variations in δ18O–NO3 originating from nitrification would still follow the seasonal variations of the δ18O–H2O, even when considering potential incorporation of O atoms from O2 in the nitrate molecules during nitrification. Hence, irrespective of the O-atom source during nitrate regeneration, the observed variations in δ18O–NO3 are not modulated by nitrification. While we thus exclude in-stream nitrate production as an important constraint on observed δ18O–NO3 − variations, we do not argue that nitrification does not occur at all in the river. Its isotopic traces may simply be overprinted by variations in the external nitrate sources, which in fact may to a large extent originate from nitrification in the catchment, as we will discuss subsequently.
Partitioning of the N-sources
Nitrate in the SLR at Québec City likely derives from three different main sources: (1) input from the Great Lakes reservoir, (2) from atmospheric deposition (3) and from external N sources in the catchment (mainly from soils, ammonium fertilizer, manure, etc.). As discussed in the previous section, N loading from the catchment likely reflects the δ18O of ambient water during nitrification (in the catchment, or later in the river). We can thus consider that external reduced N, if not already oxidized to nitrate during the transfer, is later transformed to nitrate by nitrification occurring in the river between the Great Lakes and Québec City. As discussed previously, while nitrification does not modulate the seasonal O isotopic variations it can still be a significant source of NOx. The nitrified nitrate pool at Québec City would thus originate from in-stream nitrification in the SLR, as well as nitrate from nitrification of N-inputs from the northern tributaries and within the catchment soils. A dual isotope, three-end-member linear mixing model can be formulated from the following mass balance equations:
where the subscripts refer to the isotopic composition of nitrate from the different sources (Great Lakes, atmosphere and nitrification) and the observed nitrate isotopic composition at the sampling location (QC; Québec City). The source partitioning is represented by the respective partitioning coefficients f. Calculations for each season were performed according to Phillips and Gregg (2001).
The Great Lakes end-member δ18Ο–NO3 − and δ15Ν–NO3 − values were estimated to be around +7 and +9 ‰, respectively (Ostrom et al. 2006; Table 3). Atmospheric δ18Ο–NO3 − and δ15Ν–NO3 − values are variable thorough the year (+65 to +90 ‰ and −6 to +6 ‰, respectively) (Finlay et al. 2007). Obviously, the N isotopic composition of the various sources of nitrified nitrate likely covers a relatively large range so that it is difficult to pinpoint the mean N isotopic composition with one single δ15N value (Fig. 6), so a range of 0 to +6 ‰ was used. The theoretical spectrum of possible δ15N values extents to higher δ15N when considering sewage and manure. However, δ15N value higher than +6 ‰ yielded negative f Atm, which suggests that it is not a possible solution. Thus, the 0 to +6 ‰ range for δ15N represent the largest possible range to solve our mass balance equations. The δ18O of the nitrified δ18O, however, is relatively well constrained, defined by the relatively small range of water δ18O between −8 and −12 ‰ (average ~−9 ‰) in the SLR and in regional catchment (~−10 ‰; Spoelstra et al. 2007). Albeit moderate seasonal variations (Fig. 4), a single average nitrate δ18O was assumed based on our SLR data for nitrified nitrate. Given the relatively large difference between the nitrate δ18O of the other nitrate sources (Great Lakes and atmospheric precipitation), this simplification does not induce a large error in our isotope budget calculations.
Despite a relatively large uncertainty introduced by the large possible range of δ15N values for nitrified nitrate, our results indicate that nitrate input from Great Lakes (47 ± 28 %) and nitrification (48 ± 22 %) are the dominant N-source during all seasons (Table 4). In summer, atmospheric-N only contributes about 0–4 % to the total N-load, while in the spring about 4–11 % of the total N load derives from atmospheric deposition. These numbers are lower than estimates from previous studies, which reported that between 12 and 19 % of the total N loading in the SLR have its ultimate origin in atmospheric deposition (Howarth et al. 1996; Nixon et al. 1996; Prospero et al. 1996). The discrepancy may partly be explained by the fact that our calculation ignores that Great Lakes water feeding the St. Lawrence stream may already contain a relatively large amount of N from the atmosphere and only considered the atmospheric deposition in the catchment between the Great Lakes and Québec City. Deposition of atmospheric N has been estimated to contribute up to 27 % to the total N loading to the Great Lakes (Sterner et al. 2007). Still, while the atmospheric N deposition seems be the main controlling factor with regards to the nitrate isotope composition in the river, dominating observed seasonal isotope dynamics because of its very high δ18O, it does not play a prime role in the SLR N budget in this portion of the SLR.
Summary and concluding remarks
The negative correlation between δ15N–NO3 − and δ18O–NO3 − observed in the SLR at Québec City suggests that the isotopic fractionation associated with removal processes such as in-stream nitrate uptake and denitrification, are not the principal drivers of seasonal variations in the isotopic composition of nitrate in the river. Moreover, given that nitrate from nitrification should reflect the δ18O of the ambient water, the decoupling between observed seasonal variations in δ18O–H2O and δ18O–NO3 − indicates that, although nitrification within the stream must occur to some extent, its O isotopic signature within the nitrate pool is masked by other processes. Nitrate isotope variations observed in the SLR system are mostly influenced by nitrate sources that have not been regenerated within the stream. Fluctuations in the isotopic composition of nitrate in the SLR at the Québec station can be best explained by mixing of variable “external” sources of nitrate, and seasonal variations in the relative nitrate source partitioning modulates the nitrate isotope dynamics in the SLR. The SLR receives greater portions of high-δ18O/low-δ15N nitrate in the spring compared to summer (i.e., during baseflow conditions). Spring is characterized by intense snowmelt, and atmospheric nitrate stocked in snowpack added to the riverine nitrate pool can explain the observed nitrate isotope trends. During baseflow conditions, in-stream δ15N–NO3 − and δ18O–NO3 − values reflect the isotopic signatures of soil nitrate, or a mixture of nitrate from agricultural and urban areas. Our N and O isotope mass balance considerations revealed a sixfold increase in the atmospheric N input during spring compared to summer. Yet with less than 10 % of the total N loading, atmospheric precipitation does not play a major role in the St. Lawrence N budget, leaving the N input from the Great Lakes, as well as the N regeneration as the most important fixed N sources. Unfortunately, our approach does not allow more detailed information on the actual source of N that is regenerated, be it from soil-derived or agricultural N, or from in-stream OM remineralization.
Nitrate source tracing using nitrate dual-isotope measurements can be hampered if microbial immobilization and transformation processes have an overprinting effect on the bulk nitrate isotope composition (Aravena et al. 1993; Mengis et al. 2001). Our study demonstrates that in the SLR, in-stream N transformations have a minor impact on observed nitrate isotope dynamics, and nitrate isotope measurements thus allow valuable quantitative insight into temporal variation and N source partitioning in a riverine system. The nitrate isotopic composition in the SLR seems particularly sensitive to the input of atmospheric nitrate.
References
Aleem MI, Hoch GE, Varner JE (1965) Water as source of oxidant and reductant in bacterial chemosynthesis. Proc Natl Acad Sci USA 54(3):869–873. doi:10.1073/pnas.54.3.869
Alkhatib M, Lehmann MF, del Giorgio PA (2012) The nitrogen isotope effect of benthic remineralization-nitrification-denitrification coupling in an estuarine environment. Biogeosciences 9(5):1633–1646. doi:10.5194/bg-9-1633-2012
Altabet MA, Pilskaln C, Thunell R, Pride C, Sigman D, Chavez F, Francois R (1999) The nitrogen isotope biogeochemistry of sinking particles from the margin of the eastern North Pacific. Deep Sea Res Part I 46(4):655–679
Amberger A, Schmidt HL (1987) The natural isotope content of nitrate as an indicator of its origin. Geochim Cosmochim Acta 51(10):2699–2705
Aravena R, Evans ML, Cherry JA (1993) Stable isotopes of oxygen and nitrogen in source identification of nitrate from septic systems. Ground Water 31(2):180–186
Bedard-Haughn A, van Groenigen JW, van Kessel C (2003) Tracing 15N through landscapes: potential uses and precautions. J Hydrol 272(1–4):175–190
Benoit P, Gratton Y, Mucci A (2006) Modeling of dissolved oxygen levels in the bottom waters of the Lower St. Lawrence Estuary: coupling of benthic and pelagic processes. Mar Chem 102:13–32
Bohlke JK, Mroczkowski SJ, Coplen TB (2003) Oxygen isotopes in nitrate: new reference materials for 18O:17O:16O measurements and observations on nitrate-water equilibration. Rapid Commun Mass Spectrom 17(16):1835–1846
Böttcher J, Strebel O, Voerkelius S, Schmidt HL (1990) Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer. J Hydrol 114(3–4):413–424
Bourbonnais A, Lehmann MF, Waniek JJ, Schulz-Bull DE (2009) Nitrate isotope anomalies reflect N2 fixation in the Azores Front region (subtropical N-E Atlantic). J Geophys Res 114. doi:10.1029/2007JC004617
Burns DA, Kendall C (2002) Analysis of δ15N and δ18O to differentiate NO3 − sources in runoff at two watersheds in the Catskill Mountains of New York. Water Resour Res 38(5):91–912
Burns DA, Boyer EW, Elliott EM, Kendall C (2009) Sources and transformations of nitrate from streams draining varying land uses: evidence from dual isotope analysis. J Environ Qual 38(3):1149–1159
Casciotti KL, Sigman DM, Hastings MG, Böhlke JK, Hilkert A (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74(19):4905–4912
Casciotti KL, Sigman DM, Ward BB (2003) Linking diversity and stable isotope fractionation in ammonia-oxidizing bacteria. Geomicrobiol J 20(4):335–353
Cey EE, Rudolph DL, Aravena R, Parkin G (1999) Role of the riparian zone in controlling the distribution and fate of agricultural nitrogen near a small stream in southern Ontario. J Contam Hydrol 37(1–2):45–67
Dansgaard W (1964) Stable isotopes in precipitation. Tell 16:436–468
Dijkstra P, Laviolette CM, Coyle JS, Doucett RR, Schwartz E, Hart SC, Hungate BA (2008) 15N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function. Ecol Lett 11(4):389–397
Finlay JC, Sterner RW, Kumar S (2007) Isotopic evidence for in-lake production of accumulating nitrate in lake superior. Ecol Appl 17(8):2323–2332. doi:10.1890/07-0245.1
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vöosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70(2):153–226
Gearing JN, Pocklington R (1990) Organic geochemical studies in the St. Lawrence Estuary. In: El-Sabh MI, Silverberg N (eds) Oceanography of a large-scale estuarine system, the St.-Lawrence Coastal and estuarine studies, vol 39. Springer, New York, pp 170–201
Gilbert D, Sundby B, Gobeil C, Mucci A, Tremblay GH (2005) A seventy-two-year record of diminishing deep-water oxygen in the St. Lawrence estuary: the northwest Atlantic connection. Limnol Oceanogr 50(5):1654–1666
Gonfiantini R, Stichler W, Rozanski K (1995) Standards and intercomparison materials distributed by the International Atomic Energy Agency for stable isotope measurements. In: Proceedings of a consultants meeting Vienna, Austria, 1–3 Dec 1995. International Atomic Energy Agency, pp 13–29
Granger J, Sigman DM, Lehmann MF, Tortell PD (2004a) Nitrogen and oxygen isotope effects associated with nitrate assimilation and denitrification by laboratory cultures of marine plankton. Eos Trans AGU 85 (47):Fall Meet Suppl, Abstract H51E–O52
Granger J, Sigman DM, Needoba JA, Harrison PJ (2004b) Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnol Oceanogr 49(5):1763–1773
Hélie JF, Hillaire-Marcel C (2006) Sources of particulate and dissolved organic carbon in the St Lawrence River: isotopic approach. Hydrol Process 20(9):1945–1959
Hollocher TC (1984) Source of the oxygen atoms of nitrate in the oxidation of nitrite by Nitrobacter agilis and evidence against a PON anhydride mechanism in oxidative phosphorylation. Arch Biochem Biophys 233(2):721–727
Howarth RW (1998) An assessment of human influences on fluxes of nitrogen from the terrestrial landscape to the estuaries and continental shelves of the North Atlantic Ocean. Nutr Cycl Agroecosyst 52(2–3):213–223
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch P, Zhu ZL (1996) Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35(1):75–139
Howarth RW, Swaney DP, Boyer EW, Marino R, Jaworski N, Goodale C (2006) The influence of climate on average nitrogen export from large watersheds in the North-eastern United States. Biogeochemistry 79(1–2):163–186
Kellman L, Hillaire-Marcel C (1998) Nitrate cycling in streams: using natural abundances of NO3 −δ15N to measure in situ denitrification. Biogeochemistry 43(3):273–292
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell, Oxford, p 592
Knapp AN, DiFiore PJ, Deutsch C, Sigman DM, Lipschultz F (2008) Nitrate isotopic composition between Bermuda and Puerto Rico: implications for N2 fixation in the Atlantic Ocean. Global Biogeochem Cycles 22(3). doi:10.1029/2007GB003107
Korol M (2002) Canadian fertilizer consumption, shipments and trade 2001–2002. Agriculture and AgriFood Canada Strategic Policy Branch, Ottawa, Canada
Lehmann MF, Bernasconi SM, Barbieri A, McKenzie JA (2002) Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochim Cosmochim Acta 66(20):3573–3584
Lehmann MF, Reichert P, Bernasconi SM, Barbieri A, McKenzie JA (2003) Modelling nitrogen and oxygen isotope fractionation during denitrification in a lacustrine redox-transition zone. Geochim Cosmochim Acta 67(14):2529–2542
Lehmann MF, Sigman DM, Berelson WM (2004) Coupling the 15N/14N and 18O/16O of nitrate as a constraint on benthic nitrogen cycling. Mar Chem 88(1–2):1–20
Lehmann MF, Sigman DM, McCorkle DC, Brunelle BG, Hoffmann S, Kienast M, Cane G, Clement J (2005) Origin of the deep Bering Sea nitrate deficit: constraints from the nitrogen and oxygen isotopic composition of water column nitrate and benthic nitrate fluxes. Global Biogeochem Cycles 19(4). doi:10.1029/2005GB002508
Lehmann MF, Sigman DM, McCorkle DC, Granger J, Hoffmann S, Cane G, Brunelle BG (2007) The distribution of nitrate 15N/14N in marine sediments and the impact of benthic nitrogen loss on the isotopic composition of oceanic nitrate. Geochim Cosmochim Acta 71(22):5384–5404
Lehmann MF, Barnett B, Gélinas Y, Gilbert D, Maranger RJ, Mucci A, Sundby B, Thibodeau B (2009) Aerobic respiration and hypoxia in the lower St. Lawrence Estuary: stable isotope ratios of dissolved oxygen constrain oxygen sink partitioning. Limnol Oceanogr 54(6):2157–2169
Mayer B, Boyer EW, Goodale C, Jaworski NA, Van Breemen N, Howarth RW, Seitzinger S, Billen G, Lajtha K, Nadelhoffer K, Van Dam D, Hetling LJ, Nosal M, Paustian K (2002) Sources of nitrate in rivers draining sixteen watersheds in the north-eastern US.: isotopic constraints. Biogeochemistry 57–58:171–197
McClelland JW, Valiela I (1998) Linking nitrogen in estuarine producers to land-derived sources. Limnol Oceanogr 43(4):577–585
Mengis M, Walther U, Bernasconi SM, Wehrli B (2001) Limitations of using δ18O for the source identification of nitrate in agricultural soils. Environ Sci Technol 35(9):1840–1844. doi:10.1021/es0001815
Miyajima T, Yoshimizu C, Tsuboi Y, Tanaka Y, Tayasu I, Nagata T, Koike I (2009) Longitudinal distribution of nitrate δ15N and δ18O in two contrasting tropical rivers: implications for instream nitrogen cycling. Biogeochemistry 95:1–18
Myre A (2006) Isotopic monitoring (2H and 18O) of the St. Lawrence and Ottawa rivers from 1997 to 2003: linkages with seasonal and interannual hydroclimatic variability. Thesis Dissertation, Université du Québec à Montréal, Montreal
Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Boicourt WC, Boynton WR, Church TM, Ditoro DM, Elmgren R, Garber JH, Giblin AE, Jahnke RA, Owens NJP, Pilson MEQ, Seitzinger SP (1996) The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35(1):141–180
Ohte N, Sebestyen SD, Shanley JB, Doctor DH, Kendall C, Wankel SD, Boyer EW (2004) Tracing sources of nitrate in snowelt runoff using a high-resolution isotopic technique. Geophys Res Lett 31. doi:10.1029/2004GL020908
Ostrom NE, Elsbury K, McLaughlin K, Kendall C, Paytan A (2006) Stable isotope signatures of nitrate and phosphate: potential tools for determining sources and cycling of nutrients in Lake Erie. Lake Erie millennium network conference, Windsor, ON, Canada, 28 Feb–2 Mar 2006
Panno SV, Hackley KC, Kelly WR, Hwang HH (2006) Isotopic evidence of nitrate sources and denitrification in the Mississippi River, Illinois. J Environ Qual 35(2):495–504
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127(2):171–179. doi:10.1007/s004420000578
Prospero JM, Barrett K, Church T, Dentener F, Duce RA, Galloway JN, Levy Ii H, Moody J, Quinn P (1996) Atmospheric deposition of nutrients to the North Atlantic Basin. Biogeochemistry 35(1):27–73
Sebilo M, Billen G, Grably M, Mariotti A (2003) Isotopic composition of nitrate-nitrogen as a marker of riparian and benthic denitrification at the scale of the whole Seine River system. Biogeochemistry 63(1):35–51
Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Böhlke JK (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73(17):4145–4153
Sigman DM, Granger J, DiFiore PJ, Lehmann MM, Ho R, Cane G, van Geen A (2005) Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin. Global Biogeochem Cycles 19(4). doi:10.1029/2005gb002458
Spoelstra J, Schiff SL, Hazlett PW, Jeffries DS, Semkin RG (2007) The isotopic composition of nitrate produced from nitrification in a hardwood forest floor. Geochim Cosmochim Acta 71(15):3757–3771
Sterner RW, Anagnostou E, Brovold S, Bullerjahn GS, Finlay JC, Kumar S, McKay RML, Sherrell RM (2007) Increasing stoichiometric imbalance in North America’s largest lake: nitrification in Lake Superior. Geophys Res Lett 34(10):L10406. doi:10.1029/2006gl028861
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res Board Can 167:207–211
Thibodeau B, de Vernal A, Mucci A (2006) Recent eutrophication and consequent hypoxia in the bottom waters of the Lower St. Lawrence Estuary: micropaleontological and geochemical evidence. Mar Geol 231(1–4):37–50
Thibodeau B, de Vernal A, Hillaire-Marcel C, Mucci A (2010a) Twentieth century warming in deep waters of the Gulf of St. Lawrence: a unique feature of the last millennium. Geophys Res Lett 37. doi:10.1029/2010GL044771
Thibodeau B, Lehmann MF, Kowarzyk J, Mucci A, Gélinas Y, Gilbert D, Maranger R, Alkhatib M (2010b) Benthic nutrient fluxes along the Laurentian Channel: impacts on the N budget of the St. Lawrence marine system. Estuar Coast Shelf Sci 90(4):195–205. doi:10.1016/j.ecss.2010.08.015
Wankel SD, Kendall C, Francis CA, Paytan A (2006) Nitrogen sources and cycling in the San Francisco Bay estuary: a nitrate dual isotopic composition approach. Limnol Oceanogr 51(4):1654–1664
Wankel SD, Kendall C, Pennington JT, Chavez FP, Paytan A (2007) Nitrification in the euphotic zone as evidenced by nitrate dual isotopic composition: observations from Monterey Bay, California. Global Biogeochem Cycles 21(2). doi:10.1029/2006GB002723
Acknowledgments
This work was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) through Allocation and Discovery Grants to MFL and Discovery Grants to C. Hillaire-Marcel. BT acknowledges Fonds Québécois de Recherche Nature et Technologie (FQRNT), GEOTOP Research Center and the Japan Society for the Promotion of Science (JSPS) for financial support. We thank S. Xiu Phuong for technical assistance during laboratory measurements, E. Rosa for the sampling, B. Williams and T. Miyajima for their comments on a earlier version of the manuscript. We are grateful to two anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thibodeau, B., Hélie, JF. & Lehmann, M.F. Variations of the nitrate isotopic composition in the St. Lawrence River caused by seasonal changes in atmospheric nitrogen inputs. Biogeochemistry 115, 287–298 (2013). https://doi.org/10.1007/s10533-013-9834-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9834-4