Abstract
Existing research on nutrients (nitrogen and phosphorus) in the Upper Mississippi River (UMR) can be organized into the following categories: (1) Long-term changes in nutrient concentrations and export, and their causes; (2) Nutrient cycling within the river; (3) Spatial and temporal patterns of river nutrient concentrations; (4) Effects of elevated nutrient concentrations on the river; and (5) Actions to reduce river nutrient concentrations and flux. Nutrient concentration and flux in the Mississippi River have increased substantially over the last century because of changes in land use, climate, hydrology, and river management and engineering. As in other large floodplain rivers, rates of processes that cycle nitrogen and phosphorus in the UMR exhibit pronounced spatial and temporal heterogeneity because of the complex morphology of the river. This spatial variability in nutrient processing creates clear spatial patterns in nutrient concentrations. For example, nitrate concentrations generally are much lower in off-channel areas than in the main channel. The specifics of in-river nutrient cycling and the effects of high rates of nutrient input on UMR have been less studied than the factors affecting nutrient input to the river and transport to the Gulf of Mexico, and important questions concerning nutrient cycling in the UMR remain. Eutrophication and resulting changes in river productivity have only recently been investigated the UMR. These recent studies indicate that the high nutrient concentrations in the river may affect community composition of aquatic vegetation (e.g., the abundance of filamentous algae and duckweeds), dissolved oxygen concentrations in off-channel areas, and the abundance of cyanobacteria. Actions to reduce nutrient input to the river include changes in land-use practices, wetland restoration, and hydrological modifications to the river. Evidence suggests that most of the above methods can contribute to reducing nutrient concentration in, and transport by, the UMR, but the impacts of mitigation efforts will likely be only slowly realized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Upper Mississippi River (UMR), like many large rivers, serves multiple natural and societal functions. It provides habitat for a diversity of organisms; supports commercial navigation; dilutes, processes, and transports wastes; is an important commercial and recreational fishery; and is a popular source of outdoor recreation (Fremling, 2005). Many aspects of nutrient input and cycling can affect these functions, but research on nutrients in large rivers has emphasized factors affecting nutrient loading and the delivery of nutrients by rivers to coastal areas (e.g., Turner & Rabalais, 1994; Humborg et al., 1997; Dagg & Breed, 2003). Recently, impacts of excessive nutrient inputs on river conditions and processes have received increased attention (e.g., Lung & Larson, 1995; Mainstone & Parr, 2002; Hilton et al., 2006; Dodds et al., 2009). More specifically, existing research on nutrients (nitrogen and phosphorus) in the UMR can be organized into the following categories: (1) Long-term changes in nutrient concentrations and export, and their causes; (2) Nutrient cycling within the river; (3) Spatial and temporal patterns of river nutrient concentrations; (4) Effects of elevated nutrient concentrations on the river; and (5) Actions to reduce river nutrient concentrations and flux. Our objectives are to briefly review existing research on these topics, summarize our understanding of nutrients in the UMR based on this research, and to suggest areas in which additional research would most improve our understanding of nutrient loading and cycling in the UMR and other large rivers.
Strictly speaking, the UMR extends from the source of the Mississippi River at Lake Itasca to the confluence of the Ohio and Mississippi Rivers near Cairo, Illinois. The research reviewed here includes work conducted on the UMR from its confluence with the Minnesota River (near the Minneapolis/St. Paul Minnesota) to the confluence with the Ohio River, and related research on the Lower Mississippi River (LMR) which begins at the confluence with the Ohio River (near Cairo, Illinois) and flows to the Gulf of Mexico. We generally limit the scope of our review and synthesis to research conducted on the Mississippi River or its floodplain and findings concerning the effects of land use at the UMR catchment scale or larger. There has been extensive research in many of the sub-basins within the UMR catchment addressing factors that affect nutrient export to many headwater streams and tributary rivers that, in the interest of brevity, we do not discuss here (e.g., Carey et al., 2001; Kloiber, 2006; Wollheim et al., 2008).
Long-term changes in nutrient concentrations in the Mississippi River and their causes
Nutrient concentration and flux in the Mississippi River have increased substantially over the last century because of changes in land use, climate, hydrology, and river management and engineering (e.g., Turner & Rabalais, 1991; Goolsby & Battaglin, 2001; McIsaac et al., 2001). The increase in nutrient flux from the Mississippi River has profoundly affected the Gulf of Mexico, increasing primary production, changing phytoplankton community composition, and increasing the temporal and spatial extent of hypoxia in the Gulf (e.g., Rabalais et al., 1996; Dagg & Breed, 2003).
The longest data records for nutrient concentrations in the Mississippi River are for nitrogen (N) concentrations in the LMR, which indicate large increases in N export from the Mississippi River Basin (MRB) over the last 100 years. Predevelopment nitrate (NO3 −) and total nitrogen (TN) concentrations in the LMR are estimated to have been about 0.1 and 1.24 mg l−1, respectively (Goolsby & Battaglin, 2001). By the 1990s, NO3 − had increased by a factor of more than 10 and TN had nearly doubled compared to predevelopment concentrations; this increase has occurred primarily since the 1970s (Goolsby & Battaglin, 2001). There has been a corresponding decline in silica concentration (~50%) from 1950 to the mid-1980s (Turner & Rabalais, 1991). The resulting change in atomic ratio of silica to NO3 − from about 4:1 to about 1:1 has led to important changes in coastal phytoplankton communities (Rabalais et al., 1996). The long-term changes in phosphorus (P) in the Mississippi River are less well documented because P data have only been collected since 1972 (Rabalais et al., 1996). However, paleolimnological evidence indicates that historical (pre-European settlement) total phosphorus (TP) concentration in Lake Pepin (a natural impoundment of the UMR near Lake City, Minnesota) was about 50 μg l−1 whereas average TP concentration is now about 200 μg l−1, a four-fold increase (Engstrom et al., 2009). In recent decades (1976–2004) total phosphorus concentrations in the inflow to Lake Pepin have declined somewhat, whereas nitrate concentrations continue to increase (Lafrancois et al., 2009).
The dominant cause of nutrient flux to the UMR is agricultural activities in the basin which contribute about 70% of N and P input to the Gulf of Mexico (Alexander et al., 2008). Corn and soybean fields are the largest source of N input (52%) followed by atmospheric deposition (16%). Among agricultural land uses, the largest P input was from pasture and range lands (animal manure; 37%) followed by corn and soybeans (25%). In contrast to N, urban sources (12%) made a significant contribution of P to the UMR (Alexander et al., 2008).
In the MRB, N export has been more intensively studied than P export and the increase in N flux over the last century has been attributed to a combination of factors including increased land area used for corn, soybean, and wheat cultivation (Donner, 2003; Donner et al., 2004), increased agricultural fertilizer application (McIsaac et al., 2001), drainage of wetlands and installation of artificial drainage systems (Mitsch et al., 2001; McIsaac & Hu, 2004; Royer et al., 2006) and increased precipitation and runoff in the basin (Donner et al., 2004). Nitrogen and P export from UMR tributaries is positively correlated with agricultural and urban land use (Wasley, 2000). Stable isotope analysis has shown that the main sources of N in the UMR adjacent to Illinois are synthetic fertilizers and soil organic N indicating the importance of agricultural sources of N (Panno et al., 2006). N export rate appears to be very sensitive to fertilizer application rate, especially in years with high precipitation (Donner & Kucharik, 2003).
To improve agricultural yields, vast areas of Illinois, Iowa, Ohio, and Minnesota have been drained by installing drainage networks to efficiently remove water. These drainage networks are the primary route of NO3–N export from many watersheds (Randall & Mulla, 2001; McIsaac & Hu, 2004; Royer et al., 2006). Drainage networks are also a contributing source for dissolved P, but overland flow is the dominant source of particulate P inputs to the river (Royer et al., 2006).
Climate and hydrology are important factors affecting nutrient export to the UMR. Donner et al. (2002) estimated that 25% of the increase in N export by the entire MRB between 1966 and 1994 was due to an increase in water runoff, the remaining increase was due to increases in N inputs, primarily fertilizer application. Variability among years in NO3 − flux is generally controlled by climate and river discharge (McIsaac et al., 2001; Donner & Kucharik, 2003). Furthermore, Royer et al. (2006) found that extreme discharge conditions (≥90th percentile) accounted for more than 50% of the NO3 − export and more than 80% of the P export.
A small proportion of the MRB exports most of the nutrients that reach the Gulf of Mexico. The majority of N is transported to the Gulf originates in the agricultural basins of southern Minnesota, Iowa, Illinois, Indiana, and Ohio (e.g., Goolsby & Battaglin, 2001; Donner et al., 2004). It has been estimated that the UMR contributes about 32% of the NO3 − load (Turner & Rabalais, 2004) and 35% of the TP load (Alexander et al., 2004) to the Gulf of Mexico.
Nutrient processing, cycling, and transformation in the UMR
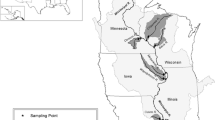
The complex morphology of large floodplain rivers strongly affects the rates and distribution of nutrient processing (Amoros & Bornette, 2002). The UMR is highly modified for navigation by a series of 29 low-head dams. The resulting inundation has increased the wetted perimeter of most of the navigation pools (river reach between successive locks and dams) with more of the floodplain that is wet more of the time now than prior to impoundment (Fremling, 2005; Theiling and Nestler, this volume). Each navigation pool can be described as having four basic types of aquatic area: main channel, side channels, impounded areas (directly upstream of the locks and dams), and backwaters, which vary in their hydrologic connectivity to channels (Fig. 1, Strauss et al., 2004). Generally, the proportion of surface area composed of backwaters and side channels decreases, and the proportion of main channel increases, downstream through the UMR. The typical characteristics of these different aquatic areas have been described in detail elsewhere and we briefly review these characteristics here (Wilcox, 1993; Soballe & Fischer, 2004; Strauss et al., 2004). The main channels and side channels are characterized as zones of high water velocity and kinetic energy and are generally erosional. Bed sediments tend to be inorganic and oxygenated except in channel irregularities (e.g., embayments) where eddies and small areas of deposition can occur. Backwaters are low flow zones of reduced kinetic energy, and are regions of deposition and accumulation of fine, carbon-rich sediments and dense populations of rooted and floating aquatic plants. Impounded areas are intermediate between channels and backwaters, where water velocity and sediment deposition vary depending on distance from defined channels.
Understanding the cycling and retention of nutrients in large floodplain rivers such as the UMR requires knowledge of the physical and chemical requirements of the various processes affecting nutrient cycling, as well as the spatial and temporal heterogeneity of the controlling environmental conditions. The N cycle in rivers consists of four main processes: nitrification, denitrification, ammonification, and N assimilation (Duff & Triska, 2000). Major processes in the cycling of P in rivers include electrochemical sorption and desorption, assimilation by biota, sedimentation and resuspension, and burial (Froelich, 1988; House & Warwick, 1998; Mainstone & Parr, 2002). Because of the nature of their respective dominant processes, N cycling is generally more affected by microbial processing whereas P cycling is more affected by chemical and physical processes.
Denitrification
Denitrification is the set of metabolic reactions performed by a large suite of facultatively anaerobic bacteria that use NO3 − as an electron acceptor in anoxic environments. For high rates of denitrification to occur, anoxia, sufficient organic carbon, and NO3 − are required. These requirements, combined with the spatial and temporal heterogeneity in environmental conditions in the UMR, result in highly variable rates of denitrification. Channel sediments tend to be oxygenated and contain relatively little organic matter resulting in poor conditions for denitrification (Alexander et al., 2000; Richardson et al., 2004; Strauss et al., 2004). Thus, ambient denitrification rates are usually low in channels, despite abundant NO3 −, and the potential for denitrification (measured as denitrification enzyme assay, DEA) is low relative to other aquatic areas (1–5 μg cm−2 h−1; Richardson et al., 2004). There are two exceptions to the general pattern of low ambient denitrification and denitrification potential in channels. Macrophyte beds along channel borders and beds of the invasive zebra mussel (Dreissena polymorpha) can be hotspots for denitrification because abundant organic material and anoxic sediments are present in these areas (Richardson et al., 2004; Bruesewitz et al., 2006).
Backwaters often exhibit ideal conditions for denitrification: highly organic, anoxic sediments with a thin (~1 to 2 mm), oxic surficial layer (Strauss et al., 2004). As a result, the potential for denitrification (measured as DEA) is generally high in backwaters (Table 1). However, ambient denitrification rates are typically low in backwaters, limited by NO3 − availability. NO3 − limitation of denitrification in backwaters is especially acute during low flow periods, particularly in late summer, when water temperatures and bacterial metabolic rates are high and backwater NO3 − concentrations are low. Because of the high denitrification potential and rapid assimilation by epiphytes and macrophytes, NO3 − uptake is rapid in backwaters (Richardson et al., 2004; James et al., 2008a). For example, NO3 − was depleted rapidly in a UMR backwater lake following a midsummer flood (Fig. 2). NO3 − in the backwater prior to flooding was low (~1 mg l−1), peaked during flooding (6.5 mg l−1), and declined to pre-flood concentrations after only 21 days. Impounded areas of the UMR tend to have relatively high rates of denitrification (Table 1) because of the occurrence of intermediate concentrations of sediment organic matter, some anoxia, increased hydraulic retention time (relative to channels), and an adequate supply of NO3 −.
Denitrification rates are affected by water temperature, leading to large seasonal differences in those rates. Highest potential denitrification rates occur during warm water conditions in non-winter months with rates generally 3 to 246 (impoundment) times higher than during winter (Richardson et al., 2004). Low rates of backwater denitrification potential in winter (0.05 to 4 μg cm−2 h−1) are due to low water temperatures and low NO3 − concentrations which result from the lack of hydrologic exchange between channel and off-channel areas.
Nitrification
Nitrification, the oxidation of NH4 + to NO3 −, is a two-step chemoautotrophic process in which NH4 + is oxidized to NO2 −, and NO2 − is then oxidized to NO3 −. This aerobic process requires adequate quantities of labile organic carbon and NH4 + (usually derived from mineralization of organic matter; Strauss et al., 2004). NH4 + oxidation is generally the rate limiting step; hence NO2 − rarely accumulates in the environment. Despite the prevalence of anoxia in backwater sediments, high rates of nitrification can occur (2 μg cm−2 h−1) because of the proximity of NH4 +-rich organic sediments to oxygenated surficial sediments (Strauss et al., 2004). Average summer rates of nitrification across all habitats are about twice that of winter (Table 1). Periodic high rates of winter nitrification occur in backwaters when snow-free ice conditions promote algal photosynthesis, oxic conditions, and nitrification resulting in an adequate NO3 − supply for elevated rates of ambient denitrification (~0.5 μg cm−2 h−1; Strauss et al., 2006). Nitrification is an important step in N loss from backwaters because it converts the abundant sediment NH4 + into NO3 − which is subsequently subject to denitrification (Strauss et al., 2004, 2006). However, James et al. (2008a) found NO3 − production via nitrification was coincident with a small export of NH4 + from a UMR backwater lake—indicating rates of organic N mineralization were faster than nitrification rates.
Nitrification rates in impounded zones are similar to those in backwaters with favorable conditions including generally higher sediment oxygen concentrations and adequate sediment organic matter. In addition, water velocities are generally slow enough to allow accumulation of sediment porewater NH4 + in backwaters and impounded areas. Nitrification rates tend to be extremely low (~0.5 μg cm−2 h−1) in channels, reflecting the low organic matter and organic N content of the sediments and rapid washout of sediment NH4 + by relatively high water velocity.
N assimilation
Temporary removal of NO3 − and NH4 + from the water column can occur via autotrophic assimilation and burial of N-rich organic particles. Although there are no published direct measurements of assimilation of N by plants in the UMR, James et al. (2008a), using mass balance, estimated assimilation by macrophytes, epiphytes, and biofilm to be equal to about 70% of the total NO3 − load in a backwater lake.
Nitrogen budget
In 2001 (January–December), 80,000 metric tons NO3-N moved through Navigation Pool 8 (La Crosse, Wisconsin; Richardson et al., 2004). In that year there was an estimated net removal of NO3 − (removal = NO3 − input − NO3 − output) of about 20% of the NO3 − load to Navigation Pool 8. Seven percent of the NO3 − load to Navigation Pool 8 was lost in backwaters and impounded areas through denitrification. Nitrification was a significant source of NO3 −, producing a similar quantity of NO3 − to that removed by denitrification. Most of the NO3 − input attributed to nitrification originated in impounded zones and channels because NO3 − generated in backwaters was rapidly denitrified. The fate of about 13% of the NO3 − load was unaccounted for and was attributed to losses via assimilation or burial. This apparent conflict with results of James et al. (2008a, b) highlights the current lack of full understanding of controls of nitrogen cycling in the UMR and need for further research on assimilation and burial nitrogen-rich particles.
Phosphorus cycling
Research on cycling of P within UMR has been limited and focused on Lake Pepin, a natural impoundment of the UMR, where particulate P sedimentation and SRP release from the sediments are important components of the P cycle (e.g., James et al., 1995, 2000, 2004). James & Barko (2004) found that during summer Lake Pepin was a sink for suspended particles (80% retained) and total P (13% retained), but a source of SRP, exporting 30% more SRP than it received. Diffusive flux of SRP from sediments was greatest in summer (10–15 mg m−2 day−1 from June to September) and smallest in winter (generally below detection from November to April; James et al., 1995).
An important aspect of P cycling in rivers is the bioavailability of phosphorus. Generally, bioavailable phosphorus is composed of SRP, some fraction of the dissolved organic fraction, and some fraction of the P adsorbed to particles (particulate P). The interaction between dissolved P and suspended particles is an important determinant of the quantity of bioavailable phosphorus in large turbid rivers and is largely regulated by the phosphate buffer mechanism (Froelich, 1988). The phosphate buffer mechanism functions such that when dissolved P (i.e., phosphate) is above the equilibrium phosphate concentration (EPC), phosphate is adsorbed (and subsequently may be absorbed) by suspended particles, and when dissolved P is below EPC, dissolved P is released from particles to the water column. EPC varies in time and space as a function of the characteristics of the suspended material (Froelich, 1988).
Although there are abundant SRP and TP data for the UMR, there has been little work on the more detailed P fractionation needed to understand the dynamics of bioavailable phosphorus and the factors regulating the exchange of phosphorus between dissolved and particulate fractions. However, phosphorus cycling within the Minnesota River (a major tributary of the UMR), which supplies nearly half of the total P loading to the UMR above Lake Pepin (James & Barko, 2004), has been investigated in detail and provides useful information on the relative abundance of various forms of P in the upper reaches of the UMR and transformations among these forms. In the Minnesota River, soluble P, bioavailable particulate P, and refractory particulate P each comprise approximately one-third of the total phosphorus pool (James & Larson, 2008). During low discharge conditions, concentrations of SRP were inversely related to chlorophyll concentrations suggesting an important biotic component to the transformations between dissolved and particulate P. During periods of higher discharge, the balance of P between dissolved and particulate forms appeared to be regulated by the abiotic process of P equilibrium between soluble and particulate P. Similar patterns of biotic control at low discharge and abiotic control at high discharge have been observed in other large river systems (e.g., River Swale; House & Warwick, 1998).
There is evidence that the riverine macroinvertebrates can affect P cycling. James et al. (2000) found that non-native zebra mussel, D. polymorpha, filtered P-rich particles from the water column and excreted a large quantity of dissolved nutrients. Observed rates of SRP excretion were ~3 mg SRP m−2 day−1, which were similar to the internal P loading from anoxic sediments in other eutrophic freshwater systems. Observed rates of ammonia excretion were ~22 mg NH3–N m−2 day−1, and the N:P ratio of Dreissena excretion was about 3:1 (by atoms)—a ratio favorable for cyanobacteria blooms.
Temporal and spatial patterns in nutrient concentrations in the UMR
Because of differences in sources and the biological and physical processes that affect their concentration, N and P often exhibit contrasting temporal and spatial patterns in the UMR. In the main channel for example, maximum N concentrations generally occur in early summer whereas maximum P concentrations generally occur in late summer (Fig. 3). This is likely due to the fact that N sources are almost entirely diffuse, whereas both diffuse and point sources (e.g., wastewater treatment outflows) and sediment P release can be significant sources of P inputs. In most rivers, point-source inputs have been observed to decrease in relative importance as river discharge increases (because of increased dilution) and the contribution of diffuse source inputs usually increase with river discharge (because of increased runoff; e.g., Bowes et al., 2008); this is consistent with the temporal patterns in nutrient concentrations observed in the UMR.
Seasonal patterns in A TN and B TP at three main channel monitoring sites on the UMR: River mile 796.9 (Navigation Pool 4, near Lake City, MN), River mile 701.1 (Navigation Pool 8, near La Crosse, WI), and River mile 556.4 (Navigation Pool 13, near Bellevue, IA). Data are monthly means from 1994 to 2007. Detailed methods are presented in Soballe & Fischer (2004)
The maximum in N concentrations in early summer (June) is most likely caused by high spring runoff and discharge, spring applications of N fertilizer, and low water temperature inhibiting denitrification (Richardson et al., 2004). This early summer peak in N concentration is a modern phenomena, suggesting that a key driver is modern rates and timing of N fertilizer application (Turner & Rabalais, 1991). A distinct minimum in N concentrations is observed in late summer and fall. At this time, N inputs are low because rates of precipitation and runoff are low, and rates of N removal via denitrification are relatively high because of warm water and sediment temperatures (Strauss et al., 2006).
Maximum P concentrations generally occur during late summer and early fall, coincident with minimum N concentration (Fig. 3; Houser, 2005). At this time, high sediment and water temperatures and frequent low dissolved oxygen in the backwaters and lakes of the UMR facilitate P release from the sediments (e.g., James et al., 1995). Low discharge during this time of year minimizes the dilution of P released from the sediments and contributed by wastewater treatment plant inputs (e.g., from Minneapolis/St. Paul; USEPA, 2000). An additional peak in average TP concentration occurs in early spring (March), corresponding to the rising limb of the spring flood, and likely due to runoff of eroded particulate P.
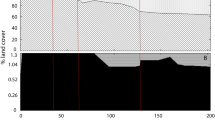
A high degree of spatial heterogeneity is a characteristic of large floodplain rivers (e.g., Junk et al., 1989; Ward et al., 1999) and there are distinct spatial patterns of nutrients in the UMR. For example, there are contrasting lateral patterns in nutrient concentrations for N and P in the UMR. In late summer, TP is generally higher in off-channel areas than the main channel, and this is likely due to P release from the highly organic, anoxic sediment in those areas. NO3 − concentrations in the UMR tend to be higher and less variable in the main channel than in backwater lakes (Richardson et al., 2004; Strauss et al., 2004, 2006). Flow through side channels and backwaters of the UMR have been substantially reduced to support navigable flows in the main channel. As a result of the limited hydrologic exchange with the main channel and high rates of denitrification and assimilation in backwaters, NO3 − concentrations vary inversely with distance from main channel during low flow conditions (Fig. 4A; Richardson et al., 2004). During high flow conditions, when exchange among aquatic areas is increased, NO3 − concentrations are more homogenous across this gradient (Fig. 4B). Similar patterns of increasing homogeneity of nitrate concentrations across the floodplain during high flow conditions and decreases in nitrate concentration as distance to the main channel increases have been observed in other large lowland river systems such as the Danube floodplain (Heiler et al., 1995; Tockner et al., 2000).
There are also large-scale longitudinal patterns in TP in the UMR as a result of the cumulative effects of tributary inputs and downstream increases in catchment area, discharge and suspended solids (Houser et al., in review; Houser, 2005). TP increased in an approximately log–linear relationship with catchment area from below Lake Pepin (near Minneapolis, MN) to Cairo, IL. The downriver increase in TP contrasts with patterns observed in the River Elbe (Czech Republic) where TP was found to be constant or increase only slightly longitudinally (Guhr et al., 2003); but is similar to the strong downstream increase in TP observed in the Parana River system (Bonetto et al., 1994) and the River Swale (Bowes et al., 2003). A similar log-linear increase in TN with increasing catchment area was not observed; only an increase in N concentration below the confluence of the Illinois in Navigation Pool 26 was observed. The increase in TN in the lower reaches likely reflects multiple factors including high nutrient inputs from the Illinois River, more abundant agricultural land use in the catchment, and little in-river N removal. The effects of these differences may be exacerbated by the reduced abundance of contiguous backwaters in the lower reaches of the UMR. In contrast to the UMR, other large rivers often exhibit downriver declines in nitrogen concentration (e.g., Bonetto et al., 1994, Guhr et al., 2003, Neal et al., 2008). For example, in the River Elbe, downriver decline in inorganic N concentrations was attributed to biological uptake and microbial denitrification (Guhr et al., 2003).
Effects of elevated nutrient concentrations on the UMR
The effects of nutrient export from large rivers on estuaries and coastal oceans have been studied extensively (e.g., Turner & Rabalais, 1994; Humborg et al., 1997; Dagg & Breed, 2003), but the ecological effects of elevated nutrient concentrations on large river ecosystems are poorly understood and have recently received increased attention (Mainstone & Parr, 2002; Hilton et al., 2006). River eutrophication can lead to the following problems: (1) excessive growth of planktonic, benthic, and filamentous algae, and aquatic macrophytes; (2) reduced diversity of macrophytes; (3) changes from macrophyte to benthic, filamentous or planktonic algal dominance; (4) low dissolved oxygen concentrations; (5) large pH changes; and (6) cyanobacteria blooms (Hilton & Irons, 1998 as cited in Hilton et al., 2006). Assessing the extent to which the above-listed problems occur in the UMR, and result from increased nutrient input, is difficult because there is little data on ecological conditions of the river (e.g., DO, pH, abundance of cyanobacteria) before elevated nutrient input began. However, eutrophication may be affecting the UMR in important ways including excessive growth of metaphyton, localized occurrence of high pH values, low DO concentrations in off-channel areas, extensive cyanobacteria blooms, and changes in phytoplankton community composition. For example, paleolimnological evidence indicates that in the last 200 years, the diatom community in riverine Lake Pepin has shifted from primarily benthic forms indicative of clear water to planktonic assemblages typically of eutrophic conditions (Engstrom et al., 2009).
In other rivers excessive periphyton growth is detrimental to macrophyte abundance (e.g., Jones et al., 2002; Wade et al., 2002), and in the UMR high metaphyton biomass (filamentous algae and duckweeds) has been found associated with high TN and TP concentrations (Fig. 5A). The establishment of a heavy canopy of surface metaphyton is associated with low DO concentrations probably because of reduced re-aeration and lower photosynthetic rates by aquatic macrophytes, periphyton, and sestonic algae due to shading (Fig. 5B).
Nutrient and dissolved oxygen concentrations versus metaphyton biomass. A Mean total nitrogen (TN) and total phosphorus (TP) versus metaphyton biomass; B dissolved oxygen (DO) concentration versus metaphyton biomass. Biomass classes were defined as follows: A = 0, B = 1–10, C = 11–30, D = 31–49, E = >50 g DW m−2. Error bars are standard error. Figure modified from Sullivan (2008)
Low DO concentrations (<5 mg l−1) are regularly observed in off-channel areas within the UMR during summer and winter (Houser, 2005; Table 2). Backwater areas often are shallow and have fine, highly organic sediments (Strauss et al., 2004)—conditions that would be expected to produce low dissolved oxygen concentrations. Because there are no data on the historical frequency and extent of low DO prior to the eutrophication of the river and modifications for navigation, it is difficult to assess the extent to which elevated nutrient concentrations in the river have increased the frequency and extent of low DO relative to historical conditions.
Cyanobacteria can be abundant in the UMR forming noticeable green films on the water surface in late summer (Huff, 1986). Nuisance cyanobacteria blooms and associated fish kills have occurred in Lake Pepin (Lake City, MN; Lung & Larson, 1995). Cyanobacteria blooms generally coincide with low river discharge, low backwater NO3 − concentrations, relatively high soluble P concentrations, and elevated pH, all of which are known to favor cyanobacterial growth (Scheffer et al., 1997; Downing et al., 2001). There is evidence indicating abrupt increases in cyanobacterial pigments in Lake St. Croix (an impoundment of the St. Croix River just upstream of its confluence with the UMR) coincident with increases in nutrient inputs occurring in the 1960s (Edlund et al., 2009).
High rates of N input to the UMR have raised concerns about toxic effects of high sediment ammonia concentrations on benthic invertebrates. Ammonia occurs in pH-dependant equilibrium as ionized ammonium (NH4 +) or unionized ammonia (NH3) and is a natural product of decomposition and mineralization of organic N (Wetzel, 2001). NH3 becomes the dominant form at pH >8.5 and has been shown to be toxic to many organisms (e.g., Ankley et al., 1995; Schubauer-Berigan et al., 1995). Of major concern are potential effects on declining populations of unionid mussels. For example, juvenile Lampsilis mussels exhibit reduced growth with concentrations of NH3 of 30 μg NH3–N l−1 (EC50) and death at 124 μg NH3–N l−1 (LC50) in laboratory sediment tests (Newton & Bartsch, 2007).
In unenriched waters, concentrations of NH3 or NH4 + are rarely above detection (Wetzel, 2001). However, sediment porewaters in the UMR can contain abundant NH4 +, particularly in carbon-rich, anoxic sediments (Strauss et al., 2004, 2006; James et al., 2008a). Yet, in a spatially and temporally extensive study of sediment porewater total ammonia N (TAN) and NH3, median NH3 in porewaters of the top 5 cm of saturated sediment was 22 μg l−1 (Fig. 6), a concentration below that likely to result in impairment for benthic invertebrates. TAN concentrations were often substantial, but pH levels rarely exceeded 7.5–8.0, not high enough to cause an equilibrial shift to NH4 +. Frazier et al. (1996) also found a wide range of concentrations (TAN: 0.7–4.0 mg l−1) in sediments of the lower portion of Navigation Pool 8. While there are concerns that ammonia toxicity contributes to large-scale declines in freshwater invertebrates (e.g., Sparks & Sandusky, 1981; Wilson et al., 1995), we found no published evidence that natural conditions in the UMR lead to toxic levels of unionized ammonia (NH3). The only in situ study we are aware of that evaluated ammonia toxicity on a highly susceptible organism (juvenile unionid mussels) found no evidence of elevated mortality or decreased growth in a wide range (range: 7.9–187 μg l−1 NH3–N) of porewater ammonia concentrations (Bartsch et al., 2003). In fact, the highest proportion of live juveniles was found at the highest NH3 concentrations, and growth rates were positively related to NH3 concentrations (Bartsch et al., 2003, Fig. 3). Further, it should not be surprising to find a co-occurrence of unionid mussels and elevated sediment ammonia—mussels excrete ammonia as a result of normal metabolism (Baker & Hornbach, 1997, Vaughn et al., 2004). Mussel mortality from elevated sediment ammonia may be possible in river sediment, but existing in situ studies do not indicate that unionid mussels are generally susceptible to ammonia toxicity—except under extreme situations (e.g., wastewater treatment plant outfalls).
The limited studies on the effects of nutrients on algal biomass and primary production in the UMR suggest that nutrients are generally not limiting in the channel areas of the river (Huff, 1986). Similarly, phytoplankton production in Lake Onalaska, a large backwater lake near La Crosse, Wisconsin, was found to be generally light limited (Owens & Crumpton, 1995). The relatively low N concentrations and relative high P concentrations that occur in more isolated backwaters, and the abundant cyanobacteria that can occur there, suggest that N limitation may be a factor in these areas at certain times. The occurrence of midsummer N limitation in isolated backwaters is also supported by nutrient content analysis of filamentous algae and duckweed (e.g., Lemna spp. and Wolfia spp.) tissue (Sullivan, 2008).
Management applications: actions to reduce river nutrient concentrations
Scavia and Donnelly (2007) estimated that a 37–45% reduction in N load to the Gulf of Mexico may be needed to meet the goal of reducing the 5-year running mean area of hypoxia to <5000 km2 by 2015 (Mississippi River/Gulf of Mexico Watershed Nutrient Task Force 2001). Other studies have indicated that, for some climate change scenarios, a 50–60% reduction in N load may be needed (Justić et al., 2003; Donner & Scavia, 2007). Evidence linking P inputs to Gulf hypoxia has emerged only more recently (e.g., Sylvan et al., 2006), and thus goals for P reduction have not been well established. Ongoing storage of N in the UMR basin soils makes it increasingly difficult to reduce N input to the Gulf in the short term (Donner & Kucharik, 2003), and recent increases in corn cultivation in response to the increased demand for ethanol may increase dissolved N export by 10–34% (Donner & Kucharik, 2008). Despite these challenges, there are ongoing efforts to develop methods to reduce N, and to a lesser extent, P export by the Mississippi River, which include constructing wetlands and riparian buffer zones (especially in areas of extensive agriculture), improving agricultural practices, diverting water through backwater areas (James et al., 2008b) and coastal wetlands, increasing control of point sources, and implementing water level drawdowns (Mitsch et al., 2001; Cavanaugh et al., 2006).
Constructing wetlands and riparian buffers to intercept agricultural runoff reduces N input to the river through denitrification and N assimilation by plants, microbes, and macrophytes (Mitsch et al., 2005). However, the area of new wetland required to significantly reduce NO3 − export is enormous. Mitsch et al. (2005) estimated that 22,000 km2 of wetland needs to be created to reduce NO3 − export by 40%. They noted that this is a very small fraction of the MRB catchment (<1%), but is 65 times the net gain of wetlands in the USA over the previous 10 years because of activities related to the Clean Water Act, and four times the cumulative total of the USDA Wetland Reserve Program for the entire USA at that time. There also remain some concerns about the effect of extensive wetland creation on N2O (a greenhouse gas and byproduct of denitrification) emissions. However it appears that N2O production is a very small fraction of NO3 −–N removed by denitrification (<0.5%; Hernandez & Mitsch, 2006), and the overall benefits of wetland protection, creation, and restoration likely outweigh any slight increase in N2O that might result.
Studies reviewed by Randall & Mulla (2001) found that improved soil nutrient testing, proper credit for previous legume crops and animal manure applications, and application of N fertilizer at the proper rate and optimal time of year could substantially reduce the N export into the MRB. Reducing the acreage of drained fields and increasing reliance on perennial crops such as alfalfa would likely have a stronger effect than the above practices, but economic considerations suggest that changes of this magnitude in agricultural practices are unlikely to occur in the near future. Envisioning more dramatic changes in agricultural (and cultural) practices, Donner (2007) estimated that shifting agricultural emphasis from feed for meat production to corn, soybean, wheat, and dairy for human consumption could reduce land and fertilizer demands by >50% without changing the total human food protein produced. Such a change could reduce NO3 − export to the Gulf of Mexico by 40–50%. Modeling work in a small catchment within the UMR drainage indicates that increasing conservation tillage, reducing application rates of P fertilizer, and converting crop land to pasture could reduce P loads by approximately 20%, but meeting local TMDL requirements would require additional controls of point sources (Dalzell et al., 2004).
Demonstrated rapid uptake of NO3 − in backwaters suggests the potential to reduce TN transport to the Gulf of Mexico by routing NO3 −-rich water into backwater areas. Several studies have documented the effect of diverting NO3 −-rich water through backwater lakes (James et al., 2008a, b). In general, lake-wide NO3 − uptake length (linear distance needed for total mass of NO3 − to be removed) increased linearly with increasing inflow concentration—up to a concentration of 2 mg l−1. Above 2 mg l−1, N saturation occurred and NO3 − uptake efficiency was limited to 40%. Diversions of N-rich water through backwaters may be an effective management tool to remove small amounts of NO3 −. James et al. (2008b) estimated it would require connecting ~10,000 ha of backwaters (~13% of the backwater habitat from Navigation Pool 1 to the confluence with the Ohio River) to the main channel to remove ~40% of the summer NO3 − load. However, there are serious potential problems with using large-scale water diversions as a tool for managing NO3 − retention in the UMR such as reductions in backwater volume and surface area because of increased sediment delivery (e.g., McHenry et al., 1984) and nutrient-induced changes in macrophyte communities (Sullivan, 2008) that may result from increased flow through backwater lakes.
Similar research on the LMR has investigated the effectiveness of diverting LMR flow through coastal wetlands and spillways for reducing delivery of nutrients to the Gulf of Mexico (Lane et al., 2001, 2004; Mitsch et al., 2001). Diverting LMR water through a coastal wetland reduced TN and TP by 44 and 62%, respectively, and increased the DSi:DIN ratio from 0.9 to 2.6 (Lane et al., 2004). Similar results were observed during an experimental diversion of the LMR through the Bonnet Carre Spillway into Lake Pontchartrain (Lane et al., 2001). However, this study found diversions have been associated with cyanobacteria blooms and fish kills in the receiving water bodies and recommended that diversions of LMR water into coastal wetlands include wetland overland flow to partially remove sediments and nutrients before the diverted water reaches larger bays where algal blooms and anoxia may occur (Lane et al., 2001). Furthermore, the overall potential NO3 − reduction has been estimated to probably be <10 to 15% of total river N flux, even with complete diversion through delta (Mitsch et al., 2001).
Water level manipulations or ‘drawdowns’ are an increasingly common large-scale management technique employed on the UMR and other large rivers for restoration of rooted aquatic vegetation and sediment stabilization (WLMTF, 2007). Laboratory sediment desiccation experiments by James et al. (2004) suggested that sediment drying resulting from drawdowns could promote N loss through volatilization of sediment-bound NH4 +. Cavanaugh et al. (2006) evaluated the effects of a large-scale water level drawdown on N cycling processes and NO3 − retention in the UMR. They found that although sediment NH4 + was significantly decreased in desiccated sediments, nitrification and denitrification were generally reduced or showed no significant trend. Reductions in sediment NH4 + from field samples co-occurred with increased growth of rooted macrophytes suggesting macrophyte assimilation, and not nitrification or volatilization caused the reduction in sediment NH4 +. Cavanaugh et al. (2006) concluded that drawdown effects on N-cycling processes are of insufficient magnitude to create significant change in the enormous pool of NH4 + contained in the carbon-rich sediments underlying macrophyte beds in backwater lakes. Furthermore, rates of denitrification are reduced by drawdowns because reductions in inundation of sediments by nitrate-rich water and sediment desiccation tend to oxygenate previously anoxic sediments.
Most of the nutrient mitigation methods reviewed here can contribute to reducing nutrient concentration and flux in the UMR, but none constitutes a singular and rapid solution to the eutrophication of the UMR and the hypoxia in the Gulf of Mexico (Mitsch et al., 2001). In fact, given the storage of nutrients in the catchment and river sediments, nutrient mitigation efforts will need to be sustained and their effects will likely be only slowly realized (Goolsby & Battaglin, 2001). Management actions to reduce nutrient impairment in the Mississippi River have largely (and appropriately) focused on land management practices and wetland and riparian buffer restoration. Increasing the flow of water through backwaters and coastal wetlands may contribute small additional reductions in nutrient flux to the Gulf of Mexico, but the effects of these diversions on the receiving backwaters and wetlands need further consideration, and the emphasis should remain on reducing nutrient flux to the UMR rather than increasing rates of processing in the river.
Linkages, synthesis and research needs
The bulk of the published research on nutrients in the UMR focuses on the effects of increased nutrient inputs to the Gulf of Mexico, the factors that affect those inputs, and how those inputs may be reduced. There are obvious ecological and economic reasons to emphasize these topics. However, our understanding of the effects of eutrophication on the UMR and the basic processes that dominate nutrient cycling and mediate those effects lags behind our understanding of the river and its catchment as a source and conveyor of nutrients. Evidence suggests that the high nutrient concentrations in the river may be contributing to: (1) increased metaphyton (duckweed and filamentous algae) that may be increasing the extent and duration of low DO concentration and may eventually reduce submersed aquatic vegetation abundance and diversity (Moore et al., this volume); (2) cyanobacteria blooms on the river which cause esthetic problems and may reduce food quality for higher trophic levels (see Delong, this volume, for additional discussion of important energy sources for the UMR food web); (3) increased frequency and extent of high pH concentrations (because of high rates of photosynthesis at certain times and places) that affect plants and other organisms (and potentially increasing concentrations of toxic NH3 in sediment porewater); and (5) increased frequency and duration of low DO concentrations. We suggest that the connections among these phenomena, the extent to which they are caused or exacerbated by the current rate of nutrient input, and the extent to which they are affected by hydrology, climate, and morphology are areas worthy of increased research. Because there is little data available describing the ecology of UMR before the onset of high nutrient inputs and modifications to facilitate navigation, paleolimnological approaches in relatively isolated off-channel areas may be needed to increase our understanding of ecosystem changes associated with changes in nutrient inputs.
Important basic components of nutrient cycling within the river remain poorly understood, particularly, the complex linkages between nutrient cycling and other physical and biotic components of the river. In the following sections we suggest a number of research topics that would improve our understanding of nutrient cycling in the UMR and other large rivers.
The role of specific processes in cycling N and P
Important processes for the cycling of N and P in the UMR and large rivers in general remain poorly understood. For example, benthic sediment mediated processes likely dominate P dynamics in backwaters, but little is known about the role of sediment P release in determining backwater P concentrations and the timing of seasonal P concentration minima and maxima. For N, the magnitude and spatial patterns of microbially mediated processes (e.g., nitrification and denitrification) are reasonably understood. However, little is known about the magnitude and fate of N assimilated by aquatic plants which is likely a significant proportion of N loss in backwaters.
Hydrological connectivity to the main channel and nutrient cycling in off-channel areas
Nutrient cycling in large floodplain rivers is strongly affected by river hydrology, connectivity among aquatic areas, and local morphometry (Hamilton & Lewis, 1990; Amoros & Bornette, 2002). Extensive and complex off-channel aquatic areas in the UMR exhibit a broad range of hydrologic connectivity to the main channel, but generally maintain surface water continuity with the main channel throughout the growing season. During high discharge conditions, nutrient-rich water flows through most aquatic areas in the UMR. During low discharge conditions, hydrological connectivity and resulting nutrient transport between off-channel areas and the main channel are highly variable among off-channel areas. This spatial and temporal variability in nutrient input to off-channel areas may have important consequences for biotic productivity in these areas. Existing work on hydrologic connectivity in rivers has generally investigated the effects of the frequency and timing of hydrologic connection to the main channel on floodplain lakes and backwaters that do not maintain surface water connections with the main channel throughout the growing season (e.g., Knowlton & Jones, 1997; Tockner et al., 1999). Therefore, the role of connectivity in determining nutrient cycling and biotic productivity in the UMR may differ from that observed in other rivers.
Effects of cyanobacterial abundance on higher trophic levels and the contribution of N fixation by cyanobacteria to the N budget of the UMR
The ecosystem effects of cyanobacterial blooms on the UMR are not well understood. In addition to causing esthetic problems, cyanobacteria are generally a relatively poor food source (Brett et al., 2006; Ravet & Brett, 2006) and their dominance of the algal community at certain times and places may reduce food quality in a way that propagates throughout the food web. In addition, little is known about contribution of N-fixation by cyanobacteria to the overall N budget of the UMR. Although N fixation within the river is likely a small proportion of the total N budget at the reach scale, it may be locally important in off-channel areas during certain times of the year.
Macroinvertebrate fauna and nutrient cycling
River invertebrates can influence nutrient cycling in the UMR. For example, zebra mussel beds are hot spots for denitrification (Richardson et al., 2004; Bruesewitz et al., 2006). Furthermore, zebra mussels filter P-rich particles from the water column and excrete dissolved N and P in a low N:P ratio (James et al., 2000). However, the role of nutrient loading on invertebrate production is poorly understood for the UMR and other large river systems. There is evidence that addition of phosphorus to oligotrophic rivers can increase biomass and abundance of invertebrates (e.g., Hinterleitner-Anderson, et al., 1992; Rublee & Partusch-Talley, 1995; Slavik et al., 2004) but we know of no such information for the UMR or similar rivers. Although rivers tend to support high rates of invertebrate production (Benke & Wallace, 1997), we know of no published estimates of secondary production for the UMR, and linkages to nutrient loading are speculative.
References
Alexander, R. B., R. A. Smith & G. E. Schwarz, 2000. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403: 758–761.
Alexander, R. B., R. A. Smith & G. E. Schwarz, 2004. Estimates of diffuse phosphorus sources in surface waters of the United States using a spatially referenced watershed model. Water Science and Technology 49: 1–10.
Alexander, R. B., R. A. Smith, G. E. Schwarz, E. W. Boyer, J. V. Nolan & J. W. Brakebill, 2008. Differences in phosphorus and nitrogen delivery to the Gulf of Mexico from the Mississippi River Basin. Environmental Science and Technology 42: 822–830.
Amoros, C. & G. Bornette, 2002. Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biology 47: 761–776.
Ankley, G. T., M. K. Schubauer-Berigan & P. D. Monson, 1995. Influence of pH and hardness on toxicity of ammonia to the amphipod Hyalella azteca. Canadian Journal of Fisheries and Aquatic Sciences 52: 2078–2083.
Baker, S. M. & D. J. Hornbach, 1997. Acute physiological effects of zebra mussels (Dreissena polymorpha) infestation on two unionid mussels, Actinonais ligamentina and Amblema plicata. Canadian Journal of Fisheries and Aquatic Sciences 54: 512–519.
Bartsch, M. R., T. J. Newton, J. W. Allran, T. A. O’Donnell & W. B. Richardson, 2003. Effects of pore-water ammonia on in situ survival and growth of juvenile unionids (Lampsilis cardium) in the St. Croix Riverway, Wisconsin, USA. Environmental Toxicology and Chemistry 22: 2561–2568.
Benke, A. C. & J. B. Wallace, 1997. Trophic basis of production among riverine caddisflies: implications for food web analysis. Ecology 78: 1132–1145.
Bonetto, C., L. Decabo, N. Gabellone, A. Vinocur, J. Donadelli & F. Unrein, 1994. Nutrient dynamics in the deltaic floodplain of the Lower Parana River. Archiv Fur Hydrobiologie 131: 277–295.
Bowes, M. J., W. A. House & R. A. Hodgkinson, 2003. Phosphorus dynamics along a river continuum. Science of the Total Environment 313: 199–212.
Bowes, M. J., J. T. Smith, H. P. Jarvie & C. Neal, 2008. Modelling of phosphorus inputs to rivers from diffuse and point sources. Science of the Total Environment 395: 125–138.
Brett, M. T., D. C. Muller-Navarra, A. P. Ballantyne, J. L. Ravet & C. R. Goldman, 2006. Daphnia fatty acid composition reflects that of their diet. Limnology and Oceanography 51: 2428–2437.
Bruesewitz, D. A., J. L. Tank, M. J. Bernot, W. B. Richardson & E. A. Strauss, 2006. Seasonal effects of the zebra mussel (Dreissena polymorpha) on sediment denitrification rates in Pool 8 of the Upper Mississippi River. Canadian Journal of Fisheries and Aquatic Sciences 63: 957–969.
Carey, A. E., W. B. Lyons, J. C. Bonzongo & J. C. Lehrter, 2001. Nitrogen budget in the Upper Mississippi River watershed. Environmental & Engineering Geoscience 7: 251–265.
Cavanaugh, J. C., W. B. Richardson, E. A. Strauss & L. A. Bartsch, 2006. Nitrogen dynamics in sediment during water level manipulation on the Upper Mississippi River. River Research and Applications 22: 1–17.
Dagg, M. J. & G. A. Breed, 2003. Biological effects of Mississippi River nitrogen on the Northern Gulf of Mexico—a review and synthesis. Journal of Marine Systems 43: 133–152.
Dalzell, B. J., P. H. Gowda & D. J. Mulla, 2004. Modeling sediment and phosphorus losses in an agricultural watershed to meet TMDLs. Journal of the American Water Resources Association 44: W12414.
Delong, M. D., this volume. Food Webs and the Upper Mississippi River: contributions to our understanding of ecosystem function in large rivers. doi:10.1007/s10750-009-0065-6
Dodds, W. K., W. W. Bouska, J. L. Eitzmann, T. J. Pilger, K. L. Pitts, A. J. Riley, J. T. Schloesser & D. J. Thornbrugh, 2009. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environmental Science and Technology 43: 12–19.
Donner, S. D., 2003. The impact of cropland cover on river nutrient levels in the Mississippi River Basin. Global Ecology and Biogeography 12: 341–355.
Donner, S. D., 2007. Surf or turf: a shift from feed to food cultivation could reduce nutrient flux to the Gulf of Mexico. Global Environmental Change 17: 105–113.
Donner, S. D. & C. J. Kucharik, 2003. Evaluating the impacts of land management and climate variability on crop production and nitrate export across the Upper Mississippi Basin. Global Biogeochemical Cycles 17: 1085. doi:10.1029/2001GB1808.
Donner, S. D. & C. J. Kucharik, 2008. Corn-based ethanol production compromises goal of reducing nitrogen export by the Mississippi River. Proceedings of the National Academy of Sciences 105: 4513–4518.
Donner, S. D. & D. Scavia, 2007. How climate controls the flux of nitrogen by the Mississippi River and the development of hypoxia in the Gulf of Mexico. Limnology and Oceanography 52: 856–861.
Donner, S. D., M. T. Coe, J. D. Lenters, T. E. Twine & J. A. Foley, 2002. Modeling the impact of hydrological changes on nitrate transport in the Mississippi River Basin From 1955 to 1994. Global Biogeochemical Cycles 16: 1043. doi:10.1029/2001GB00396.
Donner, S. D., C. J. Kucharik & J. A. Foley, 2004. Impact of changing land use practices on nitrate export by the Mississippi River. Global Biogeochemical Cycles 18: GB1028. doi:10.1029/2003GB002093.
Downing, J. A., D. B. Watson & E. McCauley, 2001. Predicting cyanobacteria dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences 58: 1905–1908.
Duff, J. H. & F. J. Triska, 2000. Nitrogen biogeochemistry and surface—subsurface exchange in streams. In Jones, J. B. & P. J. Mulholland (eds), Streams and Ground Waters. Academic Press, San Diego, CA: 197–217.
Edlund, M. B., D. R. Engstrom, L. D. Triplett, B. M. Lafrancois & P. R. Leavitt, 2009. Twentieth century eutrophication of the St. Croix River (Minnesota-Wisconsin, USA) reconstructed from the sediments of its natural impoundment. Journal of Paleolimnology 41: 641–657.
Engstrom, D. R., J. E. Almendinger & J. A. Wolin, 2009. Historical changes in sediment and phosphorus loading to the Upper Mississippi River: mass-balance reconstructions from the sediments of Lake Pepin. Journal of Paleolimnology 41: 563–588.
Frazier, B. E., T. J. Naimo & M. B. Sandheinrich, 1996. Temporal and vertical distribution of total ammonia nitrogen and un-ionized ammonia nitrogen in sediment pore water from the Upper Mississippi River. Environmental Toxicology and Chemistry 15: 92–99.
Fremling, C. R., 2005. Immortal River: The Upper Mississippi in Ancient and Modern Times. University of Wisconsin Press, Madison, WI.
Froelich, P. N., 1988. Kinetic control of dissolved phosphate in natural rivers and estuaries: a primer on the phosphate buffer mechanism. Limnology and Oceanography 33: 649–668.
Goolsby, D. A. & W. A. Battaglin, 2001. Long-term changes in concentrations and flux of nitrogen in the Mississippi River Basin, USA. Hydrological Processes 15: 1209–1226.
Guhr, H., D. Spott, G. Bormiki, M. Baborowski & B. Karrasch, 2003. The effects of nutrient concentration in the River Elbe. Acta Hydrochimica et Hydrobiologica 31: 282–296.
Hamilton, S. K. & W. M. Lewis, 1990. Basin morphology in relation to chemical and ecological characteristics of lakes on the Orinoco River Floodplain, Venezuela. Archiv Fur Hydrobiologie 119: 393–425.
Heiler, G., T. Hein & F. Schiemer, 1995. Hydrological connectivity and flood pulses as the central aspects for the integrity of a river–floodplain system. Regulated Rivers: Research and Management 11: 351–361.
Hernandez, M. E. & W. J. Mitsch, 2006. Influence of hydrologic pulses, flooding frequency, and vegetation on nitrous oxide emissions from created riparian marshes. Wetlands 26: 862–877.
Hilton, J., M. O’Hare, M. J. Bowes & J. I. Jones, 2006. How green is my river? A new paradigm of eutrophication in rivers. Science of the Total Environment 365: 66–83.
Hinterleitner-Anderson, D., A. E. Hershey & J. A. Schuldt, 1992. The effects of river fertilization on mayfly (Baetis sp.) drift patterns and population density in an arctic river. Hydrobiologia 240: 247–258.
House, W. A. & M. S. Warwick, 1998. A mass-balance approach to quantifying the importance of in-stream processes during nutrient transport in a large river catchment. The Science of the Total Environment 210(211): 139–152.
Houser, J. N. (ed.), 2005. Multiyear synthesis of limnological data from 1993 to 2001 for the Long Term Resource Monitoring Program. U.S. Geological Survey, Upper Midwest Environment Sciences Center, La Crosse, Wisconsin, March 2005. LTRMP Technical Report 2005-T003: 59 pp (NTIS PB2005-105228).
Houser, J. N., D. W. Bierman, R. M. Burdis & L. A. Soeken-Gittinger. Large-scale longitudinal and seasonal patterns in nutrients, chlorophyll, and suspended solids in a large floodplain river: the Upper Mississippi River from Lake City, MN to Cairo, IL. In review.
Huff, D. R., 1986. Phytoplankton communities in Navigation Pool No. 7 of the Upper Mississippi River. Hydrobiologia 136: 47–56.
Humborg, C., V. Ittekkot, A. Cociasu & B. Vonbodungen, 1997. Effect of Danube River Dam on Black Sea biogeochemistry and ecosystem structure. Nature 386: 385–388.
James, W. F. & J. W. Barko, 2004. Diffusive fluxes and equilibrium processes in relation to phosphorus dynamics in the Upper Mississippi River. River Research and Applications 20: 473–484.
James, W. F. & C. E. Larson, 2008. Phosphorus dynamics and loading in the turbid Minnesota River (USA): controls and recycling potential. Biogeochemistry 90: 75–92.
James, W. F., J. W. Barko & H. L. Eakin, 1995. Internal phosphorus loading in Lake Pepin, Upper Mississippi River. Journal of Freshwater Ecology 10: 269–276.
James, W. F., J. W. Barko, M. Davis, H. L. Eakin, J. T. Rogala & A. C. Miller, 2000. Filtration and excretion by zebra mussels: implications for water quality impacts in Lake Pepin, Upper Mississippi River. Journal of Freshwater Ecology 15: 429–437.
James, W. F., J. W. Barko & H. L. Eakin, 2004. Impacts of sediment dewatering and rehydration on sediment nitrogen concentration and macrophyte growth. Canadian Journal of Fisheries and Aquatic Sciences 61: 538–546.
James, W. F., W. B. Richardson & D. M. Soballe, 2008a. Contribution of sediment fluxes and transformations to the summer nitrogen budget of an Upper Mississippi River backwater system. Hydrobiologia 598: 95–107.
James, W. F., W. B. Richardson & D. M. Soballe, 2008b. Effects of residence time on summer nitrate uptake in Mississippi River flow-regulated backwaters. River Research and Applications. doi:10.1002/rra.1150.
Jones, J. I., J. O. Young, J. W. Eaton & B. Moss, 2002. The influence of nutrient loading, dissolved inorganic carbon and higher trophic levels on the interaction between submerged plants and periphyton. Journal of Ecology 90: 12–24.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The Flood Pulse Concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Justić, D., N. N. Rabalais & R. E. Turner, 2003. Simulated responses of the Gulf of Mexico hypoxia to variations in climate and anthropogenic nutrient loading. Journal of Marine Systems 42: 115–126.
Kloiber, S. M., 2006. Estimating nonpoint source pollution for the Twin Cities metropolitan area using landscape variables. Water, Air and Soil Pollution 172: 313–335.
Knowlton, M. F. & J. R. Jones, 1997. Trophic status of Missouri River floodplain lakes in relation to basin type and connectivity. Wetlands 17: 468–475.
Lafrancois, B. M., S. Magdalene & D. K. Johnson, 2009. Recent water quality trends and a comparison to sediment-core records for two riverine lakes of the Upper Mississippi River basin: Lake St. Croix and Lake Pepin. Journal of Paleolimnology 41: 603–622.
Lane, R. R., J. W. Day, D. Justić, E. Reyes, B. Marx, J. N. Day & E. Hyfield, 2004. Changes in stoichiometric Si, N and P ratios of Mississippi River water diverted through coastal wetlands to the Gulf of Mexico. Estuarine Coastal and Shelf Science 60: 1–10.
Lane, R. R., J. W. Day, G. P. Kemp & D. K. Demcheck, 2001. The 1994 Experimental opening of the Bonnet Carre spillway to divert Mississippi River water into Lake Pontchartrain, Louisiana. Ecological Engineering 17: 411–422.
Lung, W. S. & C. E. Larson, 1995. Water quality modeling of Upper Mississippi River and Lake Pepin. Journal of Environmental Engineering – ASCE 121: 691–699.
Mainstone, C. P. & W. Parr, 2002. Phosphorus in rivers—ecology and management. Science of the Total Environment 282: 25–47.
McHenry, J. R., J. C. Ritchie, C. M. Cooper & J. Verdon, 1984. Recent rates of sedimentation in the Mississippi River. In Wiener, J. G., R. V. Anderson & D. R. McConville (eds), Contaminants in the Upper Mississippi River: Proceedings of the 15th Annual Meeting of the Mississippi River Research Consortium. Butterworth Publishers, Stoneham, MA: 99–117.
McIsaac, G. F. & X. T. Hu, 2004. Net N input and riverine N export from Illinois agricultural watersheds with and without extensive tile drainage. Biogeochemistry 70: 251–271.
McIsaac, G. F., M. B. David, G. Z. Gertner & D. A. Goolsby, 2001. Nitrate flux in the Mississippi River. Nature 414: 166–167.
Mississippi River/Gulf of Mexico Watershed Nutrient Task Force, 2001. Action Plan for Reducing, Mitigating, and Controlling Hypoxia in the Northern Gulf of Mexico. Mississippi River/Gulf of Mexico Watershed Nutrient Task Force. Available from http://oceanservice.noaa.gov/products/hypox_final.pdf.
Mitsch, W. J., J. W. Day, J. W. Gilliam, P. M. Groffman, D. L. Hey, G. W. Randall & N. M. Wang, 2001. Reducing nitrogen loading to the Gulf of Mexico from the Mississippi River Basin: strategies to counter a persistent ecological problem. Bioscience 51: 373–388.
Mitsch, W. J., J. W. Day, L. Zhang & R. R. Lane, 2005. Nitrate-nitrogen retention in wetlands in the Mississippi River Basin. Ecological Engineering 24: 267–278.
Moore, M., S. P. Romano & T. Cook, this volume. Synthesis of Upper Mississippi River System submersed and emergent aquatic vegetation: past, present, and future. doi:10.1007/s10750-009-0062-9
Newton, T. J. & M. R. Bartsch, 2007. Assessing contaminant sensitivity of early life stages of freshwater mussels (Unionidae): lethal and sublethal effects of ammonia to juvenile Lampsilis mussels in sediment and water-only exposures. Environmental Toxicology and Chemistry 26: 2057–2065.
Neal, C., H. P. Jarvie, A. Love, M. Neal, H. Wickham & S. Harman, 2008. Water quality along a river continuum subject to point and diffuse sources. Journal of Hydrology 350: 154–165.
Owens, J. L. & W. G. Crumpton, 1995. Primary production and light dynamics in an upper Mississippi River backwater. Regulated Rivers: Research and Management 11: 185–192.
Panno, S. V., K. C. Hackley, W. R. Kelly & H. H. Hwang, 2006. Isotopic evidence of nitrate sources and denitrification in the Mississippi River, Illinois. Journal of Environmental Quality 35: 495–504.
Rabalais, N. N., W. J. Wiseman, R. E. Turner, B. K. Sengupta & Q. Dortch, 1996. Nutrient changes in the Mississippi River and system responses on the adjacent continental shelf. Estuaries 19: 386–407.
Randall, G. W. & D. A. Mulla, 2001. Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural practices. Journal of Environmental Quality 30: 337–344.
Ravet, J. L. & M. T. Brett, 2006. Phytoplankton essential fatty acid and phosphorus content constraints on Daphnia somatic growth and reproduction. Limnology and Oceanography 51: 2438–2452.
Richardson, W. B., E. A. Strauss, L. A. Bartsch, E. M. Monroe, J. C. Cavanaugh, L. Vingum & D. Soballe, 2004. Denitrification in the Upper Mississippi River: rates, controls, and contribution to nitrate flux. Canadian Journal of Fisheries and Aquatic Sciences 61: 1102–1112.
Royer, T. V., M. B. David & L. E. Gentry, 2006. Timing of riverine export of nitrate and phosphorus from agricultural watersheds in Illinois: implications for reducing nutrient loading to the Mississippi River. Environmental Science and Technology 40: 4126–4131.
Rublee, P. A. & A. Partusch-Talley, 1995. Microfaunal responses to fertilization of an arctic tundra stream. Freshwater Biology 34: 81–90.
Scavia, D. & K. A. Donnelly, 2007. Reassessing hypoxia forecasts for the Gulf of Mexico. Environmental Science and Technology 41: 8111–8117.
Scheffer, M., S. Rinaldi, A. Gragnani, L. R. Mur & E. H. van Nes, 1997. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 78: 272–282.
Schubauer-Berigan, M. K., P. D. Monson, C. W. West & G. T. Ankley, 1995. Influence of pH on the toxicity of ammonia to Chironomus tentans and Lumbriculus variegatus. Environmental Toxicology and Chemistry 14: 713–717.
Slavik, K., B. J. Peterson, L. A. Deegan, W. B. Bowden, A. E. Hershey & J. E. Hobbie, 2004. Long-term responses of the Kuparuk River ecosystem to phosphorus fertilization. Ecology 85: 939–954.
Soballe, D. M. & J. R. Fischer, 2004. Long Term Resource Monitoring Program Procedures: Water quality monitoring. U.S. Geological Survey, Upper Midwest Environmental Sciences Center, La Crosse, WI. Technical Report LTRMP 2004-T002-1 (Ref. 95-P002-5). 73 pp + Appendixes A–J. Available from http://www.umesc.usgs.gov/documents/reports/2004/04t00201.pdf.
Sparks, R. E. & M. J. Sandusky, 1981. Identification of factors responsible for decreased production of fish food organisms in the Illinois and Mississippi Rivers. Final Report for Project No. 3-291-R, Illinois Natural History Survey, River Research Laboratory, Havana, IL.
Strauss, E. A., W. B. Richardson, L. A. Bartsch, J. C. Cavanaugh, D. A. Buresewitz, H. Imker, H. A. Heinz & D. M. Soballe, 2004. Nitrification in the Upper Mississippi River: patterns, controls, and contribution to NO3 budget. Journal of the North American Benthological Society 23: 1–14.
Strauss, E. A., W. B. Richardson, J. C. Cavanaugh, L. A. Bartsch, R. M. Kreiling & A. J. Standorf, 2006. Variability and regulation of denitrification in an Upper Mississippi River backwater. Journal of the North American Benthological Society 25: 596–606.
Sullivan, J. F., 2008. The use of metaphyton to evaluate nutrient impairment and proposed nutrient criteria for wetlands and backwaters in the Upper Mississippi River. Wisconsin Department of Natural Resources Report. La Crosse, WI.
Sylvan, J. B., Q. Dortch, D. M. Nelson, A. F. M. Brown, W. Morrison & J. W. Ammerman, 2006. Phosphorus limits phytoplankton growth on the Louisiana shelf during the period of hypoxia formation. Environmental Science & Technology 40: 7548–7553.
Theiling, C. H. & J. M. Nestler, this volume. River stage response to alteration of Upper Mississippi River channels, floodplains, and watersheds. doi:10.1007/s10750-009-0066-5
Tockner, K., D. Pennetzdorfer, N. Reiner, F. Schiemer & J. V. Ward, 1999. Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river-floodplain system (Danube, Austria). Freshwater Biology 41: 521–535.
Tockner, K., F. Malard & J. V. Ward, 2000. An extension of the flood pulse concept. Hydrological Processes 14: 2861–2883.
Turner, R. E. & N. N. Rabalais, 1991. Changes in Mississippi River water quality this century. BioScience 41: 140–147.
Turner, R. E. & N. N. Rabalais, 1994. Coastal eutrophication near the Mississippi River Delta. Nature 368: 619–621.
Turner, R. E. & N. N. Rabalais, 2004. Suspended sediment, C, N, P, and Si yields from the Mississippi River Basin. Hydrobiologia 511: 79–89.
USEPA, 2000. Upper Mississippi River Case Study. In Progress in Water Quality: An Evaluation of the National Investment in Municipal Wastewater Treatment. U.S. Environmental Protection Agency. Office of Water, Washington, D.C. EPA-832-R-00-008. Available from http://www.epa.gov/OWM/wquality/benefits.htm.
Vaughn, C. C., K. B. Gido & D. E. Spooner, 2004. Ecosystem processes performed by unionid mussels in stream mesocosms: species roles and effects of abundance. Hydrobiologia 527: 35–47.
Wade, A. J., P. G. Whitehead, G. M. Hornberger & D. J. Snook, 2002. On modelling the flow controls on macrophyte and epiphyte dynamics in a lowland permeable catchment: the River Kennet, Southern England. Science of the Total Environment 282: 375–393.
Ward, J. V., K. Tockner & F. Schiemer, 1999. Biodiversity of floodplain river ecosystems: ecotones and connectivity. Regulated Rivers: Research and Management 15: 125–139.
Wasley, D., 2000. Concentration and movement of nitrogen and other materials in selected reaches and tributaries of the Upper Mississippi River System. MS Thesis, University of Wisconsin-La Crosse.
Water Level Management Task Force (WLMTF), 2007. Summary of results of the Pool 5 and Pool 8 draw downs on the Upper Mississippi River. River Resources Forum, US Fish and Wildlife Service.
Wetzel, R. G., 2001. Limnology: Lake and River Ecosystems. Elsevier Academic Press, San Diego, CA.
Wilcox, D. B, 1993. An aquatic habitat classification system for the Upper Mississippi River System. U.S. Fish and Wildlife Service. EMTC 93-T003. 9 pp + Appendix A (NTIS PB93-208981). Available from http://www.umesc.usgs.gov/documents/reports/1993/93t003.pdf [accessed 2 June 2009].
Wilson, D. M., T. J. Naimo, J. G. Wiener, R. V. Anderson, M. B. Sandheinrich & R. E. Sparks, 1995. Declining populations of the fingernail clam Musculium transversum in the upper Mississippi River. Hydrobiologia 304: 209–220.
Wollheim, W. M., B. J. Peterson, S. M. Thomas, C. H. Hopkinson & C. J. Vorosmarty, 2008. Dynamics of N removal over annual time periods in a suburban river network. Journal of Geophysical Research-Biogeosciences 113: G03038.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: S. P. Romano & B. Ickes / Upper Mississippi River Research Synthesis: Forty Years of Ecological Research Synthesis: Forty Years of Ecological Research
Rights and permissions
About this article
Cite this article
Houser, J.N., Richardson, W.B. Nitrogen and phosphorus in the Upper Mississippi River: transport, processing, and effects on the river ecosystem. Hydrobiologia 640, 71–88 (2010). https://doi.org/10.1007/s10750-009-0067-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0067-4