Abstract
The landscapes colonized by invasive earthworms in the eastern U.S. are often patchworks of forest stands in various stages of successional development. We established six field sites in tulip poplar dominated forests in the Smithsonian Environmental Research Center in Edgewater, MD, that span mid (50–70 years-three plots) and late (120–150 years-three plots) successional stages where younger sites had greater earthworm density and biomass than older sites and were dominated by non-native lumbricid species. In particular Lumbricus rubellus, a litter-feeding species, was abundant in mid successional forests. Here, we separated particulate organic matter (POM) from the bulk soil by a combination of size and density fractionation and found that patterns in soil POM chemistry were similar to those found previously during litter decay: in younger forests with high abundance of earthworms, organic carbon normalized cutin- and suberin-derived substituted fatty acid (SFA) concentration was lower and lignin-derived phenols greater than in older forests where earthworms were less abundant. The chemistry of the dominant litter from mid versus late successional tree species did not fully explain the differences in POM chemistry between age classes. Instead, the differences in leaf body versus petiole and leaf versus root chemistry were the dominant drivers of POM chemistry in mid versus late successional stands, although aspects of stand age and tree species also impacted POM chemistry. Our results indicate that preferential ingestion of leaf body tissue by earthworms and the subsequent shifts in sources of plant biopolymers in soil influenced POM chemistry in mid successional forests. These results indicate that invasive earthworm activity in North American forests contributes to a shift in the aromatic and aliphatic composition of POM and thus potentially influences carbon stabilization in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The introduction of non-native earthworms into a forested area can cause a strong initial disturbance in the detritusphere (Beare et al. 1995), the region where the organic litter layer interacts with and impacts the mineral soil, and alter ecosystem dynamics. A balance exists between carbon (C) losses and retention in soil (Lajtha et al. 2005; Trumbore 2006) during the transformation of plant litter to soil organic matter (SOM). Invasive earthworm species often heavily reduce the amount of organic litter layer in forests (Bohlen et al. 2004a) and promote rapid mixing of organic debris into the mineral soil horizons (Bohlen et al. 2004b), actions which may ultimately impact SOM dynamics (Bohlen et al. 2004c).
Much research on the impact of earthworms on forest biogeochemistry has been conducted in northern U.S. forests where native populations were eliminated by the geographical maximum extent of the Laurentide ice sheet (~18,000 years ago) (e.g., Reynolds 1994; Hale et al. 2005; Frelich et al. 2006; Eisenhauer et al. 2007). However, more southern regions in the U.S., where native earthworm populations have continuously existed, also are experiencing the effects of the introduction and spread of non-native species. These regions, particularly in the eastern U.S., are often vegetated with forest stands in various stages of successional development since logging or abandoned agriculture and have received less attention than their northern counterparts. As a result, how the complex temporal interactions of earthworm species and forest successional stage affect ecosystem processes is largely unknown (Szlavecz and Csuzdi 2007).
The research forest at the Smithsonian Environmental Research Center (SERC, Edgewater, MD is typical of deciduous forests that cover a large area of the mid-Atlantic and southeastern U.S., with stands in various stages of post-agriculture or logging recovery. Twelve earthworm species have been identified in the SERC forest, three of which are native and nine non-native (Szlavecz and Csuzdi 2007) and the community composition, dominant species, and total biomass differed between early and later successional forest sites (Filley et al. 2008a). In younger forests, earthworm biomass and density were 4–5 times greater than in more mature sites, driven largely by two non-native species, Lumbricus rubellus and Octolasion lacteum (Filley et al. 2008a).
One of the most widespread invasive earthworm species in North America is L. rubellus. This species is epi-endogeic, ingesting both surface litter and soil and promoting rapid mixing of organic debris and their casts into the mineral soil by burrowing (Bohlen et al. 2004a). In particular, the epi-endogeic behavior of L. rubellus could impact plant litter on the forest floor and facilitate mixing of litter residue into the mineral horizon. O. lacteum, is a soil dweller (endogeic) and feeds on both organic debris and mineral soil. Both species could potentially further impact soil chemistry directly by ingesting soil.
Aromatic plant biopolymers (such as lignin) and aliphatic biopolyesters (such as contained within cutin and suberin) are thought to compose a large portion of refractory organic matter in soil (Nierop 1998; Nierop et al. 2003; Mikutta et al. 2006). However, recent work has shown that lignin derived phenols are not always present in stabilized organic matter pools (Dignac and Rumpel 2006) and that aliphatic compounds can be preferentially preserved (Lorenz et al. 2007).
During decomposition of leaf litter from tulip poplar (Liriodendron tulipifera L.), the dominant tree species at SERC, earthworm activity fundamentally altered the chemical trajectory of litter residue (Filley et al. 2008a). High earthworm abundance was associated with tulip poplar litter residue that was strongly depleted in cinnamyl-based lignin and aliphatic compounds measured as alkaline cupric-oxide extractable hydroxyl-substituted fatty acids (e.g., cutin) and enriched in non-cinnamyl lignin compared to litter residue from low earthworm abundance sites. These patterns were likely a result of feeding habits of the earthworms, which selectively ingest the leaf body but not the petioles (Suárez et al. 2006), thereby leaving the cinnamyl lignin- and aliphatic-poor but non-cinnamyl lignin-rich petiole behind on the soil surface. Slight differences among sites were also seen in leaves within earthworm-excluding litter bags as well as in soil enzyme assays, which suggest microbiological shifts may also play a role (Filley et al. 2008a).
Over time, this chemical trajectory of litter decay should be transferred belowground to SOM (Kögel-Knabner 2002). Earthworms digest carbohydrates and proteins in leaf litter (Brown et al. 2004) and have been shown to have esterase enzymes with the potential to degrade aliphatic polyesters such as cutin and suberin (Nakajima et al. 2005). This, combined with the affinity for eating aliphatic-rich leaf body material leaving aliphatic-poor litter residue behind, implies that the dominance of soil mixing species such as L. rubellus could potentially impact the composition and flux of stabilized soil C. However, whether the earthworm-induced chemical trajectory observed for tulip poplar leaf litter persists belowground at SERC has not been determined.
In this study we sought to answer two questions: (1) Will the chemical trajectory promoted by invasive earthworms previously seen for tulip poplar leaf decomposition be transferred to soil organic matter? (2) In a complex ecosystem, representative of much of eastern US forested land, can we distinguish the impact on soil chemistry among factors associated with forest succession, i.e., stand age, tree species and invasive earthworm populations?
Based on the chemical trajectory of leaf decomposition promoted by earthworms and their ability to mix surface residues into the soil horizons, we hypothesized that non-mineral associated soil organic matter, representing the closest physical and temporal link to the surface litter, in the high earthworm abundance-younger sites would be depleted in aliphatic plant biopolymers and enriched in lignin-derived phenols compared to the low earthworm abundance-older sites. If earthworm preferential feeding on leaf body tissue was the dominant driver of organic matter chemistry in POM, then we hypothesized that differences in plant biopolymer chemistry between leaf bodies and petioles would influence POM plant biopolymer chemistry to a greater extent than existing differences between age classes in plant litter input chemistry.
Methods
Site description
The Smithsonian Environmental Research Center (SERC) lies along the western shore of Maryland on the Rhode River estuary (www.serc.si.edu). Within the 2,886 ha SERC research forest (38°53′ N, 76°33′ W), long-term experimental sites were established in 2003 within two groups of three stands that differed in forest successional stage since abandonment of agriculture or logging: one group of three mid successional stands (50–70 years) and another of three late successional stands (120–150 years). The experimental sites are located in a 1,500 ha region within the SERC forest and the successional stands are interspersed within that area. Leaf input rates at SERC typically are 330–450 g/m2/year with high and low rates ranging from 272 to 525 g/m2/year (G. Parker, unpublished data). Tree biomass is 375 Mg/ha in mid successional stands and 555 Mg/ha in late successional stands at SERC (G. Parker, unpublished data). Tulip poplar is the most abundant tree species in both age classes. In mid successional stands, the dominant species are tulip poplar (150 Mg/ha) and sweet gum (Liquidambar styraciflua L.) (145 Mg/ha). Red maple (Acer rubrum L.) (40 Mg/ha), white oak (Quercus alba) (20 Mg/ha), American beech (Fagus grandifolia Ehrh) (15 Mg/ha), and red/black oaks (Q. falcata, Q. coccinea, Q. velutina, and Q. rubra) (5 Mg/ha) are the secondary species (G. Parker, unpublished data). In late successional stands, tulip poplar alone is the dominant species (250 Mg/ha). White oak (100 Mg/ha), red/black oaks (75 Mg/ha), American beech (70 Mg/ha), and hickories (Carya spp.) (40 Mg/ha) are the secondary tree species (G. Parker, unpublished data).
The soils at all the sites are considered to have fine sandy loam in the Collington-Monmouth series and are classified as associations of the Collington sandy loam (fine-loamy mixed, active, mesic Typic Hapludult) and the Monmouth fine sandy loam (fine, mixed, active, mesic Typic Hapludult) (Soil Survey Staff http://soils.usda.gov/technical/classification/osd/index.html). The late succession sites are Collington series, two of the mid succession sites are Monmouth series, and one of the mid succession sites is Donlonton series. The primary difference between these series is greater fine sand (particle diameter 0.2–0.02 μm) content in the Monmouth series than in the Collington series. Differences in land use history, e.g., agriculture and logging, resulted in only minor differences in mineralogy and soil chemistry within the SERC forest (Pierce 1974). The average pH was 5.4 in late successional stands and 5.7 in mid successional stands at SERC (Szlavecz and Csuzdi 2007) and volumetric soil moisture measured in spring-early summer was 33.0 ± 1.5% in our mid succession sites and 25.0 ± 1.9% in our late succession sites (unpublished data). There is little persistent forest floor in any of the sites.
At our experimental sites, surveys conducted over several years showed that the dominant earthworm species were L. rubellus, O. lacteum, and Eisenoides loennbergi, all of the Lumbricidae family (Szlavecz and Csuzdi 2007). Lumbricus rubellus and O. lacteum are non-native species and E. loennbergi is a native, endogeic species. Earthworm biomass (g/m2) and density (individuals/m2) were significantly greater in the mid successional stands than the late successional stands (Filley et al. 2008a). In 2004, earthworm biomass was four times greater at the younger sites than at the older sites. Density was 130 individuals/m2 at the younger sites and 18 individuals/m2 at the older sites. About 65% of individuals at the younger site were L. rubellus and Lumbricus juveniles compared to 35% at the older sites. Lumbricus rubellus is the only earthworm species at SERC that dwells in the litter layer (epi-endogeic), all other species are soil dwelling (anecic or endogeic) (Filley et al. 2008a). Although anecic species also ingest leaf litter and transport litter into their burrows, less than 5% of individuals in either age class were anecic. Long term sampling of the SERC forests indicates this distinction is consistent over multiyear periods (Szlavecz and Csuzdi 2007), although earthworm abundance may fluctuate due to changes in regional precipitation.
Collection of soil and litter
In each of the six sites a 3 × 3 m plot was delineated and then divided into a 0.5 m grid for a total of 36 subplots as part of a larger long-term litter amendment experiment. In November 2005, six cores were taken from each site of the 0–5 cm layer of soil A horizon from the control treatment grids. The moist soil was bagged and kept refrigerated during transit from SERC to Purdue University. Within a week of collection the field-moist soils were passed through a 2 mm sieve to remove rocks, large roots, and other debris, and then dried at 50°C to a constant weight. A composite was made randomly of three of the six samples collected (the remaining three were used for analyses not included in this manuscript), mixed well inside a plastic bag, and stored for use in our analyses. Of the >2 mm material, roots were hand picked and re-rinsed with distilled and deionized water to remove as much soil as possible.
Leaf litter from the dominant tree species was collected for archive at the SERC forest in the fall of 1999, 2003, and 2006. Leaves were collected from leaf litter traps during the period of leaf senescence and were subjected to little, if any, decomposition and no earthworm processing while in the traps. A subset of each year’s collection was combined for chemical and elemental analysis. For the tulip poplar, oak spp., hickory spp., and sweet gum leaves, which had distinct petioles/mid-veins (henceforth referred to as “petiole tissue”), the leaf body was separated from the petiole, prominent mid-vein, and large secondary veins with a razor blade and analyzed individually. Red maple and beech leaves did not have prominent mid-veins and secondary veins that were easily distinguished and separated from the leaf body, thus they were analyzed as whole leaves.
Isolation of soil particulate organic matter (POM)
A combination of size and density fractionation was used to isolate the partially decomposed, highly organic debris present in the 0–5 cm layer of the sieved mineral soil (Cambardella and Elliott 1992, 1993). To remove potential differences between individuals collecting soil samples (in particular contamination of mineral soil by surface litter or organic horizon), free-floating particulate organic matter was removed. To do this, 100 ml of distilled, deionized water was added to a 30 g subsample and gently shaken on a benchtop shaker for one hour. After settling for 24 h, material floating in the water was aspirated and removed. Shaking and aspiration was repeated until no material floated (typically 2 cycles). The maximum amount removed in this manner was 20 mg, the equivalent of 0.3% of total bulk soil C. The volume in the bottle with remaining sample was raised to 200 ml and sodium hexametaphosphate (HMP) was added to make a 0.5% solution of HMP. Bottles were shaken overnight (~15 h) to fully disrupt the soil aggregate structure. The soil-HMP slurry was passed through a micrometer sieve to remove silt and clay sized particles and mineral associated C. The >53 μm material (consisting of organic debris and sand) was rinsed with distilled, deionized water, collected, dried at 50°C overnight, and weighed.
To facilitate separation of organic debris from sand, 50 ml of a 1.4 g ml−1 solution of sodium polytungstate (SPT, Geoliquids, Chicago, IL, USA) was added to the >53 μm material and vigorously mixed in a beaker with a stirring stick for 30 s and was allowed to settle overnight. The heavy fraction (sand) sank and the POM (remaining organic debris) floated and was aspirated from the surface. The mixing and aspiration cycle was repeated until no material remained floating after mixing (typically 2–3 cycles). The POM from each sample was collected on a 20 μm nylon filter, rinsed thoroughly with distilled, deionized water to remove excess SPT, dried at 50°C overnight, and weighed. At 1.85 g ml−1, the density typically used during this step (Cambardella and Elliott 1992, 1993), all >53 μm material floated, thus a lower density of 1.4 g ml−1 was chosen based on a trial conducted with a range of solution densities from 1.4–1.7 g ml−1 to determine the most appropriate density at which to achieve a clear separation between the organic debris and sand.
To ensure that we had homogenous samples for analysis, a sub-sample of each bulk soil as well as the recovered POM for each sample were individually ground with a mortar and pestle until the entire sample passed through a 250 μm sieve. Litter samples, including petiole, leaf body, whole leaf, and roots, were more fibrous than the soil and were ground with a liquid N2 SPEX CertiPrep (Metuchen, NJ, USA) freezer mill to a powdered consistency.
Elemental analysis
The organic carbon (OC) concentration of bulk soil and POM were determined by dry micro-Dumas combustion (CHN EA1108 Elemental Analyzer, Carlo Erba Instruments, Milan, Italy). Duplicate analyses were run for every sample; in some cases triplicate analyses were necessary to reduce variability.
Lignin and substituted fatty acid quantification
Lignin-derived phenols and cutin- and suberin-derived substituted hydroxy fatty acids (SFA) were quantified using alkaline cupric-oxide (CuO) oxidation (Hedges and Mann 1979; Goñi and Hedges 1990a, b, c) with modifications based upon Dalzell et al. (2005) for plant litter and soil POM. The reactions utilized Monel reaction vessels (Prime Focus, Inc. Seattle, WA, USA). Internal recovery standards for lignin and SFA biopolymers (ethyl vanillin and DL-12 hydroxystearic acid) were added to each vessel following the initial alkaline reaction and before the solvent extraction phase. Lignin-derived phenols were quantified by the analysis of trimethylsilane derivatives of three classes of lignin monomers: vanillyl (V), syringyl (S), and cinnamyl (Ci) (Table 1) relative to the internal standard ethyl vanillin. The abbreviation Ci for cinnamyl is typically referred to as C in the literature (Hedges and Mann 1979) but to avoid confusion with references to carbon, Ci is used throughout this manuscript. Lignin content was expressed as both the sum of S + V + Ci (SVCi-Lignin) and as S + V (SV-Lignin) (Hedges and Ertel 1982). Ratios of the classes of lignin monomers in the POM can be used to identify dominant sources of lignin (Hedges and Mann 1979) and ratios of acid and aldehyde monomers within the S and V classes (Ac/AlS,V) can be used as an index for the degree of lignin oxidation where higher values suggest increased microbial oxidation (Kögel 1986). The derivatives of eight SFA were assessed by extracted ions based on similar proxy standard calibration curves relative to the IRS DL-12, hydroxystearic acid (Table1). Within an ecosystem, groups of SFA can be identified as either cutin- or suberin- derived in plant litter and soil (Kögel-Knabner 1989; Riederer et al. 1993; Filley et al. 2008b).
A Hewlett-Packard (5971) quadrupole mass spectrometer interfaced to a 5890 series II gas chromatograph was used in the quantification of individual compounds by extracted ion calibration curves. Derivitization of samples and GC-MS performance were verified by the addition of a methyl-3,4-dimethoxybenzoate recovery standard immediately prior to derivatization. Duplicate CuO analyses were performed for every plant litter sample to assure consistency. Beyond the calibration curves and internal standards, a standard peach leaf (NIST 1547) was used as a lab CuO reference material in each batch of analyses and used to monitor the consistency of the reaction and GC performance on a biological substrate similar to our samples. Mean standard reproducibility for the analytical method was 2–5% for lignin phenols and 2–9% for SFA. The SFA were assigned abbreviated names that are used throughout the text (Table 1).
Calculation of reconstructed leaves and litter
To gain the most detailed information about plant chemistry in light of the known impact of selective plant tissue detritivorous feeding, CuO analysis was done on leaf body and petiole tissue separately when leaf morphology allowed the physical separation of the two tissues (Filley et al. 2008a). The chemistry of the whole leaf was then reconstructed using a weighted mean of the leaf body and petiole tissues for each species (WLR). To determine the amount of weight given to each tissue in this calculation, five leaves of each species were carefully dissected into leaf body and petiole (including a prominent mid-vein and any large secondary veins) tissues. Leaf bodies and petioles were dried at 70°C for 10 h and weighed. The proportion of leaf body and petiole mass was then calculated for each leaf and averaged for each species. The mean proportion of dry weight ± one standard error within the leaf body was 0.77 ± 0.02 for sweet gum, 0.81 ± 0.02 for tulip poplar, 0.79 ± 0.02 for red/black oak spp., 0.83 ± 0.02 for white oak, and 0.76 ± 0.02 for hickory. Since the final CuO data are normalized to C, the mass balance and %C values for each species were used to calculate the proportion of leaf C within leaf body and petiole tissue for each species with the following equation:
where LBC is the proportion of total C within the leaf body, LBmass is the proportion of total dry weight with the leaf body, LB%C is the %C of leaf body tissue, P mass is the proportion of total dry weight with the petiole, and P %C is the %C of petiole tissue. The leaf C balance between leaf body and petiole tissue was then used to calculate the weighted means for the whole leaf chemical parameters:
where WLR is the reconstructed whole leaf, LBC is the proportion of total C within the leaf body, LBx is the parameter value measured for leaf body tissue, P C is the proportion of total C within the petiole, and P x is the parameter value measured for petiole tissue.
Similarly, we reconstructed what the average chemistry of leaf litter inputs would have been in the mid and late successional forests. Species-specific litterfall rates were not available for our study sites at SERC, therefore the proportion of total biomass (Mg/ha) each tree species comprised for each age class was used as a first estimate to calculate a weighted mean for whole leaf (or WLR when appropriate), leaf body, and petiole chemical parameters for the mid and late successional stands:
where RL is the reconstructed litter, SGp is the proportion of total tree biomass that is sweet gum, SGx is the parameter value measured for sweet gum WLR, TPp is the proportion of total tree biomass that is tulip poplar, TPx is the parameter value measured for tulip poplar WLR, RMp is the proportion of total tree biomass that is red maple, RMx is the parameter value measured for red maple whole leaf, ROp is the proportion of total tree biomass that is red/black oak spp., ROx is the parameter value measured for red/black oak spp. WLR, WOp is the proportion of total tree biomass that is white oak, WOx is the parameter value measured for white oak WLR, ABp is the proportion of total tree biomass that is American beech, ABx is the parameter value measured for American beech whole leaf, H p is the proportion of total tree biomass that are hickory spp., and H x is the parameter value measured for hickory spp. This method provided approximate values for reconstructed litter chemistry representative of the age classes relevant to our study sites at SERC.
Statistical analyses
Means for POM from the two age classes were compared using a two-tailed t-test (PROC TTEST) in SAS v.9.1 (SAS Inc., Cary, NC). Due to the influence of the low number of replicates (n = 3) and high natural heterogeneity common in forest soils on the interpretation of statistical analyses, differences between means with a significance level of P < 0.100 are discussed.
The measured values of SFA and lignin-derived phenols for all 16 compounds identified were used to examine compositional similarities and differences between plant inputs and soil POM. Concentrations were converted to relative abundance by dividing each concentration by the sum total concentration for all the plant biopolymers measured. This relativization is representative of biopolymer composition and is more meaningful than concentration for identifying compositional similarities and differences among plant inputs and POM. The chemical composition was examined by non-metric principal components analysis (PCA) using the PC-ORD software package (McCune and Mefford 1999). A correlation cross-products matrix, which produces correlation coefficients among the compounds, was used in the PCA. This method provides a broken stick eigenvalue; the broken-stick eigenvalue was less than the actual eigenvalue for the first and second axes; therefore these were used for interpretation (McCune and Mefford 1999). Pearson and Kendall correlations with the main matrix were calculated in PC-ORD. Suberin-derived SFA (Σ Suberin acids) and cutin-derived SFA (Σ Cutin acids) were identified by superimposing relativized abundances of each SFA onto the ordination produced with all of the chemical compounds. Using this method the SFA dominant in roots versus foliar tissue were apparent.
Results
Litter chemistry
Large differences existed in the CuO compositional data, ratios of lignin classes, and plant biopolymer (lignin-, cutin, and suberin-derived compounds) parameters among the plant litter types analyzed (Table 2, see also Appendix Table 7 for concentrations of individual compounds). The total concentration of lignin-derived phenols (SVCi-Lignin) was 2.7–11.7/100 mg OC for all litter. American beech leaves and tulip poplar petioles had the greatest concentrations of SVCi-Lignin. Petiole tissue had greater concentrations of V and S lignin-derived phenols than the leaf body tissue for every species. The total concentration of extractable substituted fatty acids (Σ SFA) was 1.2–8.1/100 mg OC for all litter. Red/black oak spp. and tulip poplar leaf bodies had the greatest Σ SFA and, within each species, Σ SFA was always greater in leaf bodies than in petioles (Table 2).
The ratio of S-to-V lignin classes (S/V) was greater in petioles than in leaf bodies for every species. Tulip poplar petioles had the greatest S/V. The ratio of Ci-to-V lignin classes (Ci/V) was greater in leaf bodies than petioles for every species. Sweet gum leaf body had the greatest Ci/V of all the species as a result of lower V concentration. The ratio of acid-to-aldehyde moieties within the V [Ac/Al(V)] and S [Ac/Al(S)] lignin classes, was less than 0.25 for all of the plant inputs, which is typical of fresh litter (Hedges et al. 1988; Filley et al. 2008b).
Mid and late successional species differed in Ci/V and the ratio of SFA-to-SV-Lignin (Σ SFA/SV-Lignin) (Fig. 1). Sweet gum and tulip poplar are dominant early in the development of SERC forests following agricultural abandonment or logging. These early and mid successional species had the highest Ci/V and Σ SFA/SV-Lignin in leaf bodies and the greatest difference between leaf bodies and petioles among all of the dominant tree species at SERC. Oak spp., hickory spp., and American beech become more abundant later in forest succession and generally had lesser Ci/V and Σ SFA/SV-Lignin values and smaller difference in chemistry between leaf body and petiole than the mid succession species. Overall, roots had lower Ci/V and Σ SFA/SV-Lignin than the foliar tissue (Table 2). There were no similar generalized patterns along the successional gradient in S/V in leaf tissues (Table 2).
Comparison of the ratio of cinnamyl-to-vanillyl based lignin phenols (Ci/V, upper panel) and extractable substituted fatty acids-to-SV-Lignin-derived phenols (Σ SFA/SV-Lignin, lower panel) in leaf tissue among various plant species along a successional gradient. Whole leaf values for tree species indicated with an asterisk (*) are calculated using a weighted mean of the measured petiole and leaf body values
Among the plant litter analyzed from SERC, different groups of SFA were found to be more concentrated in foliar tissues or roots. In foliar tissues, 7&8,ω-C16:1DA (particularly in sweet gum and tulip poplar) and 9,10,ω-C18 (particularly in oak spp.) were concentrated. As found previously for tulip poplar (Filley et al. 2008a), 9&10,ω-C16 was characteristically high in leaf bodies and low in petioles of all species analyzed (Appendix Table 7). The sum of these compounds (Σ Cutin acids) was high in foliar tissues and low in roots comprising on average 91% of the total SFA extracted from foliar tissues (Table 3). In roots, ω-C16, C16DA, ω-C18:1, and C18:1DA were concentrated compared to the other plant inputs (Appendix Table 7). The sum of these compounds (Σ Suberin acids) was high in root tissue and low in foliar tissue comprising on average 89% of the total SFA extracted from roots (Table 3).
Among the various plant biopolymer concentrations and parameters measured, there were no differences between roots from mid versus late successional stands that fell outside the overlap of variances (Tables 2, 3).
POM recovery and chemistry
Bulk soil %C was greater and more POM was recovered by mass in the late successional stands than in the mid successional stands. There was no difference in POM %C between age classes. As a result, a significantly larger proportion of bulk soil C was recovered within the POM from older sites than from the younger sites (Table 4).
Overall, POM from the mid succession sites was depleted in SFA and enriched in SV-Lignin compared to the late succession sites (Table 4). In particular, the S concentration and S/V was significantly greater in POM from the younger sites compared to the older sites. The SFA concentration in POM from the older sites was more than twice that from the younger sites; however, high variability within the older sites kept the difference in POM Σ SFA between age classes from being statistically significant. Particulate OM Σ SFA/SV-Lignin, Ac/Al(V,S) and Ci/V were significantly greater from the older sites compared to the younger sites. The concentration of Cutin acids and Σ Cutin acids/Σ SFA was greater in POM from the older sites than from the younger sites. There were no differences between age classes in the concentration of Suberin acids but in the younger sites, Σ Suberin acids/Σ SFA was significantly greater than in the older sites.
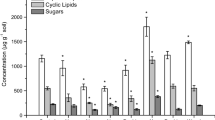
The patterns previously documented by Filley et al. (2008a) during tulip poplar litter decay, i.e., a depletion in Ci and SFA and enrichment in SV-Lignin when invasive earthworms are present, persisted in POM and were not explained by differences in initial litter chemistry alone (Fig. 2a–d). Particulate OM Ci/V was greater in the late succession sites compared to the mid succession sites, yet the reconstructed litter values showed the opposite pattern. At the younger sites, POM Ci/V was approximately equal to that of the reconstructed petioles (Fig. 2a). Although not significant, Σ SFA was substantially greater in the POM from the older sites than the younger, but Σ SFA in reconstructed litter was similar between the age classes (Fig. 2b). SV-Lignin was significantly greater in POM from the younger sites compared to the older sites. In contrast, SV-Lignin in reconstructed litter was greater in the older sites than the younger sites. At the younger sites SV-Lignin was more concentrated in POM than in reconstructed litter whereas SV-Lignin was less concentrated in POM than in reconstructed litter at the older sites (Fig. 2c). Particulate OM Σ SFA/SV-Lignin was significantly greater at the older sites compared to the younger sites (Fig. 2d). At the older sites, Σ SFA/SV-Lignin was approximately equal between the POM and reconstructed litter. In contrast, POM was enriched in lignin and depleted in SFA compared to the reconstructed litter at the younger sites. At the younger sites, ΣSFA/SV-Lignin of POM was approximately equal to that of the reconstructed petioles.
Patterns in the chemical trajectory of plant biopolymers during the transformation of mixed plant litter (bars) to particulate organic matter (POM, ●) at mid (50−70 years) and late (120–150 years) successional forest sites at SERC. The values shown for leaf body, whole leaf, and petiole are weighted means of the measured tissues from all of the plant species present at each site. Weight was given to each tree species based on the relative biomass of each species in young and old forests at SERC. Thus, the bars are representative of the average aboveground plant litter expected in each age class. The values for POM are means ± one standard error, significant differences between the age classes are indicated by asterisks (*), see Table 3 for P-values. Arrows indicate the direction of the chemical trajectory along the successional gradient when comparisons were significant
PCA, chemical composition
The first two axes of the PCA accounted for 62.6% of total variability present within the relative abundance compositional data (see Table 5 for all correlation coefficients). Axis 1 (40.7%) segregated roots (more negative scores) from foliar tissues (more positive scores) with POM intermediate (Fig. 3). Along the first axis, the C16DA, ferulic acid, and C18:1DA (high negative correlation) were separated from 9&10 ω-C16, p-hydroxycinnamic acid, and 9,10,ω-C18:1 (high positive correlation) (Fig. 3). Axis 2 (21.9%) segregated petioles and POM from mid succession sites (more negative scores) from leaf bodies and POM from late succession sites (more positive scores). Along the second axis, acetosyringone, syringaldehyde, and syringic acid (high negative correlation) separated from ω-C16, p-hydroxycinnamic acid, and ω-C18:1 (high positive correlation).
Principal components analysis with all of the alkaline CuO-extractable substituted fatty acids and lignin-derived phenols quantified for leaf bodies (grey circles), petioles (black circles), whole leaves (white circles), roots from mid (50–70 years, black upside down triangles) and late (120–150 years, white upside down triangles) successional stands, particulate organic matter (POM) from young (black squares) and old (white squares) forest sites. Major groups with distinct chemistries are circled and the three most correlated chemical species are listed for both directions of each axis, the r-value of each is in parentheses
The overall plant biopolymer composition among the major groups of plant inputs and POM was distinct (Fig. 3). Within the cluster of root samples, there was no distinction between the age classes. In contrast, within the cluster of POM samples the older sites had more positive scores along axis 2 whereas younger sites were more negative. Among the cluster of foliar tissues, there was no clear distinction between mid- and late successional tree species, although the mid-successional species (tulip poplar, sweet gum, and red maple) tended to have more positive scores along both axes than the late successional species (tulip poplar, oak spp., beech, and hickory spp.). However, there was a clear distinction between leaf body and petiole composition. Leaf bodies had more positive scores along both axes while petioles were more negative.
Relative importance of individual compounds
Superposition of the relativized abundances of each SFA onto the ordination revealed characteristic groups of compounds were present in high relative abundance within plant tissues and distinguished a given input type from the others. In addition to the suberin-derived SFA identified previously, the lignin monomer ferulic acid was also present in high abundance in roots relative to other plant inputs (Table 6). In addition to the cutin-derived SFA discussed previously, syringaldehyde, acetosyringone, and syringic acid were present in characteristically high relative abundance in petioles compared to the other plant inputs (Table 6).
We found that some compounds important in plant inputs were not conserved in POM. The relative abundances of ω-C18:1, and C18:1DA were high in roots and 9,10,ω-C18:1 was high in leaves compared to other inputs but were relatively depleted in POM (Table 6). The relative decay rates of the cutin and suberin monomers appears to be environment specific but a number of studies that have shown hydroxy acids with double bonds and ones with more than one hydroxyl group can be preferentially degraded compared to compounds without these features (Goñi and Hedges 1990c; Otto and Simpson 2006; Feng and Simpson 2007). The relative abundance of p-hydroxycinnamic acid was high in sweet gum leaf body tissue but did not remain high in POM. Conversely, acetovanillone, vanillin, vanillic acid, and syringic acid were present in relatively high abundance in POM compared to the original plant inputs (Table 6).
The chemical composition of POM differed between age classes in several aspects. The relative abundance of syringaldehyde, acetosyringone, and syringic acid was higher for POM from the younger sites compared to the older sites (P = 0.005) whereas ferulic acid, ω-C16, C16DA, 9,10, ω-C18, and 9&10 ω-C16 were present in higher abundance in POM from the older sites compared to the younger sites (P = 0.022) (Fig. 4).
The sum relative abundances of suites of compounds that distinguished the composition of POM between age classes. Values are means ± one standard error for the concentration of each suite relative to the total amount of lignin-derived phenols and SFA extracted. ** Differences between age classes were significant at the P < 0.05 level, see text for P-values. Ci2, ferulic acid; S1, syringaldehyde; S2, acetosyringone; S3, syringic acid
Discussion
Leaf chemistry and food source potential
Tulip poplar is the dominant tree species at both our mid and late succession sites at SERC, but many other species also are present and the relative abundance of these other species change over time. Tulip poplar leaves shared many basic chemical characteristics with leaves from all of the trees measured at SERC (Fig. 1). For all species measured, the leaf body and petiole were distinguishable by their plant biopolymer signature, e.g., the leaf body SFA concentration was greater than the petiole and petiole S- and V-lignin concentrations were greater than leaf body (Table 2). Lumbricus rubellus is the primary litter feeding species present at SERC, but L. friendi, an anecic species comprising less than 5% of adult individuals in both age classes, also feeds on leaf litter. In addition, even endogeic species ingest leaf litter, although perhaps less than L. rubellus. Filley et al. (2008a) found that high earthworm activity heavily impacted the chemistry of tulip poplar litter residue during decomposition primarily through selective ingestion of the leaf body causing residue to selectively accrue S- and V-lignin phenols.
Although there is the potential for alteration of the chemical trajectory for litter residue of all tree species, earthworms and other invertebrates prefer highly nutritious leaf litter such as sweet gum, maple, tulip poplar, and ash, to oak and beech, which initially have higher concentrations of polyphenols and lower N and P concentration (Satchel and Lowe 1967; Zicsi 1983; Curry 2004; Curry and Schmidt 2007). Calcium content of leaf litter may also enhance earthworm feeding activity and thus leaf litter transformation (Hobbie et al. 2006). With only beech leaves as a food source for 25 days, L. rubellus lost 50% of body-mass (Hedde et al. 2007). Yet, when preferred food sources are not available, earthworms will consume what is available. Although oak is not a preferred food source, L. rubellus increased litter mineralization of fresh oak leaves (Zimmer et al. 2005). Partial decomposition of leaf material reduces the amount of deterrent chemicals and recalcitrant compounds making it more palatable for soil invertebrates such as isopods and earthworms (Zimmer 2002).
At SERC L. rubellus and Lumbricus juveniles are neither abundant nor dominant within the earthworm community in the late successional forests where oaks and American beech are common, instead large earthworm populations occur primarily in early and mid successional stands at SERC (Szlavecz and Csuzdi 2007; Filley et al. 2008a). In these mid successional forests, tulip poplar and sweet gum comprise ~80% of aboveground biomass and have the greatest ratio of Ci/V and SFA-to-SV-Lignin in leaf body and whole leaf and among the lowest ratio of Ci/V and SFA-to-SV-Lignin in petioles of all the species (Fig. 1). Because of the large difference in chemistry between the leaf body and petiole in tulip poplar and sweet gum and high abundance in young forests where L. rubellus is abundant, among the tree species at SERC, these have the greatest potential for impacting litter residue chemistry through earthworms selectively feeding on the leaf body tissue.
Continuation of the earthworm-driven chemical trajectory of litter
The partially-decayed organic fraction from the soil (i.e., POM) represented a starting point to test our hypothesis that the generalized chemical trajectory observed during tulip poplar litter decay in the presence of earthworms would be transferred belowground to soil organic matter. We found that the plant biopolymer chemistry of POM in mid versus late succession sites was consistent with earlier findings of a distinct chemical trajectory in tulip poplar litter decay associated with earthworm abundance (Fig. 2a–d). These patterns suggest that invasive earthworms may directly impact SOM chemistry and thus potentially SOM dynamics. However, factors other than earthworm abundance, including stand age and tree species, also influence POM chemistry and we sought to distinguish between these three factors as drivers of POM plant biopolymer chemistry.
Stand age as a driver of POM chemistry
Some aspects of POM recovery and chemistry were consistent with expected differences due to stand age. Soil generally accumulates organic carbon during early forest succession (Chapin et al. 2002). The older sites at SERC contained more C within POM than the younger sites (Table 4), perhaps as a result of greater time for accumulation and NPP in the older sites. The lignin-derived phenols in POM from the older sites were more decayed, i.e., higher Ac/Al(S,V), than in POM from the younger sites (Table 4), which is indicative of greater microbial processing either over time or by more active populations of lignin degrading organisms such as fungi.
Roots can contribute a greater relative amount of soil C than shoots (Rasse et al. 2005). Ferulic acid, ω-C16, and C16DA were part of the suite of compounds prevalent in POM from the old sites (Fig. 4). These compounds were all predominant in root tissue and remained prevalent, and in some cases accumulated (i.e. the relative abundance was greater in POM than in the root tissue itself) (Table 5), in POM from the late successional forests.
Aliphatic components, including suberin, are considered to be major contributors to stabilized soil carbon (Nierop 1998; Nierop et al. 2003; Mikutta et al. 2006). Recent work showed that ω-C16, in particular, is stable in soil and remained constant relative to other long chain (C16) substituted fatty acids during incubation, even at elevated temperatures (Feng and Simpson 2007). The relative abundance of ω-C16, and C16DA was similar in the roots collected from both age classes and the differences observed in POM (Fig. 4) were likely due in part to greater root inputs and/or accumulation over time in the older sites (i.e. stand age).
Tree species as a driver of POM chemistry
The difference in POM plant biopolymer concentration and bulk parameters between the age classes was not a direct reflection of the dominant tree species’ chemistry. For example, Ci/V and Σ SFA/SV-Lignin were greater in mid successional species than in late successional species (Fig. 1) but both parameters were greater in POM from the late successional forests than from the mid successional forests (Fig. 2a, d). Additional inputs, such as roots, impact the bulk POM values, e.g., Ci/V and Σ SFA/SV-Lignin in POM is lower than the foliar tissue in part due to the influence of root inputs, which have lower ratio values than aboveground tissue (Table 2).
Preferential decay of specific compounds is likely responsible for some of the differences in POM chemistry between sites (e.g., Riederer et al. 1993; Filley et al. 2008b). A large percentage of Ci class lignin phenols are thought to be bonded to lignin polymers and sugars by ester linkages that are readily hydrolyzed and are preferentially lost during early stages of litter decomposition (Hedges and Weliky 1989; Haddad et al. 1992). The p-hydroxycinnamic acid is highly concentrated in sweet gum leaf body (Fig. 3; Table 6) and selective loss of this compound and/or tissue could also be responsible for the decrease in Ci/V in POM compared to initial leaf litter, particularly in the mid successional forests.
Overall, PCA indicated that tree species was not the predominant driver of differences in chemical composition within our dataset (Fig. 3). A generalized pattern distinguishing the biopolymer composition of leaves by successional stage did emerge. However, this pattern was opposite from the one distinguishing POM from mid and late succession sites.
The aliphatic components of suberin and cutin are composed of similar compounds that are present in different abundances in root and leaf tissue (i.e., Kögel-Knabner et al. 1989; Otto and Simpson 2006; Filley et al. 2008b). On a global level, across ecosystems and vegetation types, many compounds are found in both suberin and cutin polyesters (Otto and Simpson 2007); however, on a site level we identified distinct groups of compounds that were predominant in either root or leaf tissue. Among the species present at our site, suberin- and cutin acids were identified that were ~90% exclusive of each other in root and foliar inputs (Table 3).
Some of the cutin-derived acids identified at SERC were preserved in POM whereas others were preferentially lost during decomposition. Of the three cutin-derived acids, 9,10,ω-C18:1 was high in foliar tissues (especially for sweet gum, red maple, and tulip poplar) but was not present in high abundance in POM (Table 6). This result is consistent with previous research that has shown hydroxy acids with double bonds and more than one hydroxyl group are preferentially degraded relative to other cutin-derived acids (Goñi and Hedges 1990c; Kögel-Knabner et al.1989; Otto and Simpson 2006; Feng and Simpson 2007), although this pattern is environment-specific and not always the case (Riederer et al.1993). The other two cutin-derived acids remained prevalent in POM (Table 6) and the total amount of cutin-derived acids was significantly greater in the old sites than the young sites (Table 4). Oak spp. were characteristically high in 9,10,ω-C18 and this compound accumulated in POM only in the late successional stands where oaks played an increasingly dominant role in the canopy, contributing in large part to the difference in total cutin-derived acids. Increased abundance of oak spp. in forests could provide a source of stabilized aliphatic compounds to soil and is an example of the impact a specific species could potentially have on POM chemistry.
Lignin and aliphatic components such as cutin and suberin polyesters are often considered refractory in the litter layer and can be major contributors to stabilized soil carbon (Nierop 1998; Nierop et al. 2003; Mikutta et al. 2006). In the simplest sense, if factors controlling decomposition (e.g., decomposer community, moisture, temperature) are similar across a successional gradient, then the general chemical trajectory of litter decay and POM formation also should be consistent. That is, POM chemical composition should generally resemble the initial litter unless an additional factor is present to substantially alter the decay trajectory. We found that generalized patterns in POM plant biopolymer chemistry between sites were not consistent with patterns in chemistry among litter inputs along a successional gradient (Fig. 2a–d). Further, we did not find that tree species was the predominant factor driving patterns in chemical composition overall within our dataset (Fig. 3). These data suggest an important difference in the factors controlling the chemical trajectory of litter decay and POM formation in mid- and late successional forests exists that is independent of tree species.
Earthworm activity as a driver of POM chemistry
The body of chemical evidence presented herein suggests that in the mid succession sites, where invasive earthworms are abundant, petioles appeared to be a predominant source of POM. For example, syringyl-based lignin phenols were more concentrated (Table 2) and more predominant in POM (Fig. 4) from the younger sites compared to the older sites. Syringyl-based lignin phenols were characteristically high in petiole tissue (Table 2; Fig. 3) and are likely the predominant source of these compounds in POM from the mid successional stands. Further, POM Ci/V and Σ SFA/SV-Lignin from the younger sites were similar in value to the petiole tissue of the tree species in that age class, also indicating that this tissue potentially played a larger role in POM composition than leaf body. In contrast, the relative amount of these plant biopolymers in POM at the older sites was more similar to the whole leaf than to petioles, suggesting that POM at these sites contains organic matter from the leaf body as well (Fig. 2a, d).
Both S and V lignin classes are also abundant in woody tissue, on the order of twice what were found in petioles (Hedges and Mann 1979). However, woody debris has not been an important input at SERC in the recent past due to agricultural land use at the research sites. In addition, sites were chosen specifically to avoid as much large wood debris as possible and woody inputs have been minimized since the beginning of the experiment. Nonetheless, observational data have shown that woody debris is more common in the late successional stands compared to the mid-successional stands at SERC and is thus opposite of the pattern we found in POM.
Earthworms have the capacity to decompose many classes of compounds because of internal flora that are thought to have enzyme activities reflective of the microbes in the ingested soil and litter (Lattaud et al. 1998; Li et al. 2002). Cutin and suberin may be degraded or rapidly hydrolyzed to monomers by earthworms through the action of enzymes such as serine-proteases, which have been shown to have esterase activity (Nakajima et al. 2005). These hydroxy fatty acids may then be better able to associate with mineral surfaces and follow a different input mechanism to SOM than the petiole-sourced and lignin-rich POM. It is likely that the leaf body is not entirely mineralized but becomes fragmented and incorporated into casts and microaggregates with immediate mineral associations (Bossuyt et al. 2006). The soil fractionation method used herein disrupted aggregate structure and does not allow for such distinctions. Nonetheless, if earthworm feeding on leaf bodies effectively reduces leaf body tissue as an input to POM, then the SOM chemistry should reflect those changes in inputs to some degree.
Plant tissue type was a more important driver than tree species of overall differences in chemical composition among POM in both age classes. The PCA showed that leaf bodies and petioles were chemically distinct and, in the same manner, POM from the older and younger sites also was distinct (Fig. 3). We found that 9&10,ω-C16 was characteristically high in all leaf body tissue compared to petioles and roots. This compound is abundant in angiosperm cutin (Baker and Holloway 1970) and, at least in ocean sediments, is thought to be preferentially degraded to a certain degree during initial decomposition and sedimentation before stabilizing (Goñi and Hedges 1990c). Even so, these compounds were more prevalent in POM from the older sites compared to the younger sites and further support other data that have indicated greater overall leaf body inputs to soil in the late successional stands compared to the mid successional stands.
If aliphatic-rich leaf body inputs were reduced, then root-derived aliphatic compounds may become more prevalent in POM chemistry. Of the four suberin-derived acids, ω-C18:1, and C18:1DA were high in roots but were not preserved in POM relative to the other plant biopolymers (Table 6). Conversely, the relative abundance of ω-C16 and C16DA was high in roots and even greater in POM (Table 6). The concentration of suberin-derived acids was not different between age classes (Table 4). However, POM from the younger sites had a significantly greater proportion of total SFA that were suberin-derived than the older sites (Table 4), indicating that leaf inputs play a less important role in overall SFA composition in the young sites.
Several lines of evidence suggest that selective loss or relative gain of a specific tissue component drove differences in POM chemistry and composition between the age classes. Although for the same nutritional reasons that earthworms and other invertebrates prefer leaf litter such as sweet gum, maple, and tulip poplar to oak and beech (which initially have higher concentrations of polyphenols and lower N and P concentration) (Satchel and Lowe 1967; Zicsi 1983; Curry 2004), decay rates even in the absence of earthworms are likely greater for the mid successional species than the late successional species. However, given previous evidence of the physical impact of earthworm feeding activity on leaf inputs (Filley et al. 2008a) and our collective results, it seems that earthworm feeding on leaf body tissue preferentially to petioles can be an important mechanism driving differences in POM chemistry between successional stage forest soils.
The impact of earthworm feeding habits
In addition to feeding habits resulting in lignin-rich litter inputs, high earthworm activity has been associated with a shift away from lignin degrading fungi (Li et al. 2002). Fungi are important food sources for earthworms and a number of studies have shown that they preferentially feed on litter extensively colonized by fungi (see reviews by Brown 1995; Brown et al. 2004). Earthworm feeding and mixing activities may also shift the decomposer community towards being bacteria dominated (McLean et al. 2006). At the SERC sites phenol oxidase enzyme activity in bulk surface soil was greater in the young forest sites compared to the old sites (Filley et al. 2008a) perhaps in response to the high availability of lignin-derived residue remaining after earthworm feeding.
If litter dwelling earthworms impact soil chemistry by selectively feeding on the SFA-rich, SV-Lignin-poor leaf body and leaving behind SFA-poor and SV-Lignin-rich petioles, then tree species with (1) a high ratio of total SFA to lignin in leaves and (2) a large difference between leaf body and petiole chemical composition will have greater potential to impact soil chemistry. We found that the species with the greatest potential for impacting soil chemistry in this manner (sweet gum and tulip poplar) were consistent with species that are dominant during early forest succession. Even if earthworms continue to ingest leaf bodies of later successional species, because the relative amount of plant biopolymers is not very different between foliar tissues the potential impact of selective feeding on soil organic matter chemistry is inherently reduced.
It is not clear why earthworm abundance differs among successional stages at SERC or how long the invasive species have been present. It is possible that earthworms invaded recently and the late successional forests have never had high earthworm abundance or that late successional forests at one point went through a period of high earthworm abundance that declined as the forest matured. The patches of forests in various stages of succession are adjacent at SERC and there are no physical barriers between stands of different ages keeping earthworms from migrating, which suggest a natural decline in earthworm abundance occurs with stand maturation (Szlavecz and Csuzdi 2007). Further, other studies in France and the Czech Republic showed that earthworm abundance declines along a successional gradient (Pižl 1992; Bernier et al. 1993). Historic land-use, propagule pressure, or small differences in pH may reduce populations in later successional forests.
At SERC, tulip poplar is still abundant and providing a preferred food source to the earthworm assemblages in the older sites. Yet, as forests at SERC enter later successional stages (~150 years since abandonment) high abundances of litter-consuming earthworms are no longer supported (Szlavecz and Csuzdi 2007). As tulip poplar begins to disappear over time, the impact of earthworms on the chemical trajectory of litter residue may also continue to decline in concert.
Summary
Our hypotheses addressed whether patterns observed in the chemical trajectory of decomposition of tulip poplar litter at the same study sites at SERC by Filley et al. (2008a) would persist in the underlying soil organic matter. Specifically, our earlier work showed that high earthworm activity in the mid-successional stands resulted in degraded tulip poplar litter that was depleted in Ci/V and SFA and enriched in SV-Lignin compared to litter exposed to low earthworm activity in the late successional stands. Tulip poplar litter at the old sites with low earthworm activity had a greater ratio of SFA-to-SV-Lignin and degree of lignin decay than at the young sites with high earthworm activity. We found that the chemical trajectories of plant biopolymers in tulip poplar litter following earthworm processing persisted in soil POM.
In response to our original questions: (1) Patterns previously documented in the chemical trajectory of tulip poplar litter decay driven by earthworm abundance persisted in the soil POM indicating that similar processes were likely impacting the formation of soil POM as was observed during litter decomposition. (2) Stand age, tree species, and selective feeding on leaf body tissue by invasive earthworms all impacted POM composition in different manners and should be considered together as aspects of forest succession that affect soil C chemistry and thus potentially soil C dynamics.
Overall, differences in the soil POM chemistry were not driven by differences between the successional species. Instead, the data indicated that much dissimilarity in chemistry between plant tissues, e.g., leaf body versus petiole and leaf versus root, was also present between soil POM chemistry in mid and late successional forests. Invasive earthworms that feed preferentially on one tissue over the other potentially are important drivers of forest litter disappearance and soil C dynamics in regions where native species assemblages exist and not just in originally earthworm-free forests. Our results are consistent with our hypothesis that selective feeding on leaf body tissue contributes to a shift in the aliphatic and aromatic composition of litter residue that is transferred belowground to POM in early and mid successional forests at SERC where non-native, litter-feeding, earthworms are abundant.
References
Baker EA, Holloway PJ (1970) Constituent acids of angiosperm cutins. Phytochemistry 9:1557–1562. doi:10.1016/S0031-9422(00)85275-9
Beare MH, Coleman DC, Crossley DA et al (1995) A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170:5–22. doi:10.1007/BF02183051
Bernier N, Ponge JF, Andre J (1993) Comparative-study of soil organic layers in 2 bilberry spruce forest stands (Vaccinio-Piceetea)—relation to forest dynamics. Geoderma 59:89–108. doi:10.1016/0016-7061(93)90064-R
Bohlen PJ, Groffman PM, Fahey TJ et al (2004a) Ecosystem consequences of exotic earthworm invasion of north temperate forests. Ecosystems (N Y, Print) 7:1–12. doi:10.1007/s10021-003-0126-z
Bohlen PJ, Scheu S, Hale CM et al (2004b) Non-native invasive earthworms as agents of change in northern temperate forests. Front Ecol Environ 2:427–435
Bohlen PJ, Pelletier DM, Groffman PM et al (2004c) Influence of earthworm invasion on redistribution and retention of soil carbon and nitrogen in northern temperate forests. Ecosystems (N Y, Print) 7:13–27. doi:10.1007/s10021-003-0127-y
Bossuyt H, Six J, Hendrix PF (2006) Interactive effects of functionally different earthworm species on aggregation and incorporation and decomposition of newly added residue carbon. Geoderma 130:14–25. doi:10.1016/j.geoderma.2005.01.005
Brown GG (1995) How do earthworms affect microfloral and faunal community diversity. Plant Soil 170:209–231. doi:10.1007/BF02183068
Brown GG, Doube BM, Edwards CA (2004) Functional interactions between earthworms, microorganisms, organic matter and plants. In: Edwards CA (ed) Earthworm ecology, 2nd edn. CRC Press LLC, Boca Raton, pp 213–239
Cambardella CA, Elliott ET (1992) Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci Soc Am J 56:777–783
Cambardella CA, Elliott ET (1993) Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils. Soil Sci Soc Am J 57:1071–1076
Chapin SF, Matson PA, Mooney HA (eds) (2002) Principles of terrestrial ecosystem ecology. Springer-Verlag, New York
Curry JP (2004) Factors affecting the abundance of earthworms in soils. In: Edwards CA (ed) Earthworm ecology. CRC Press, Boca Raton, pp 91–113
Curry JP, Schmidt O (2007) The feeding ecology of earthworms–a review. Pedobiologia (Jena) 50:463–477. doi:10.1016/j.pedobi.2006.09.001
Dalzell BJ, Filley TR, Harbor JM (2005) Flood pulse influences on terrestrial organic matter export from an agricultural watershed. J Geophys Res 110:G02011. doi:10.1029/2005JG000043
Dignac MF, Rumpel C (2006) Relative distributions of phenol dimers and hydroxy acids in a cultivated soil and above ground maize tissue. Org Geochem 37:1634–1638. doi:10.1016/j.orggeochem.2006.06.019
Eisenhauer N, Partsch S, Parkinson D et al (2007) Invasion of a deciduous forest by earthworms: changes in soil chemistry, microflora, microarthropods and vegetation. Soil Biol Biochem 39:1099–1110. doi:10.1016/j.soilbio.2006.12.019
Feng XJ, Simpson MJ (2007) The distribution and degradation of biomarkers in Alberta grassland soil profiles. Org Geochem 38:1558–1570. doi:10.1016/j.orggeochem.2007.05.001
Filley TR, McCormick MK, Crow SE et al (2008a) Comparison of the chemical alteration trajectory of Liriodendron tulipifera L. leaf litter among forests with different earthworm abundance. J Geophys Res Biogeosci 113:G01027. doi:10.1029/2007JG000542
Filley TR, Boutton TW, Liao JD et al (2008b) Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J Geophys Res Biogeosci 113:G03009. doi:10.1029/2007JG000564
Frelich LE, Hale CM, Scheu S et al (2006) Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol Invasions 8:1235–1245. doi:10.1007/s10530-006-9019-3
Goñi MA, Hedges JI (1990a) Cutin-derived cuo reaction-products from purified cuticles and tree leaves. Geochim Cosmochim Acta 54:3065–3072. doi:10.1016/0016-7037(90)90122-2
Goñi MA, Hedges JI (1990b) Potential applications of cutin-derived cuo reaction-products for discriminating vascular plant sources in natural environments. Geochim Cosmochim Acta 54:3073–3081. doi:10.1016/0016-7037(90)90123-3
Goñi MA, Hedges JI (1990c) The diagenetic behavior of cutin acids in buried conifer needles and sediments from a coastal marine-environment. Geochim Cosmochim Acta 54:3083–3093. doi:10.1016/0016-7037(90)90124-4
Haddad RI, Newell SY, Martens CS, Fallon RD (1992) Earlydiagenesis of lignin associated phenolics in the salt marsh grass Spartinaalterniflora. Geochim Cosmochim Acta 56:3751–3764. doi:10.1016/0016-7037(92)90168-I
Hale CM, LE Frelich , Reich PB et al (2005) Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, USA. Ecosystems (N Y, Print) 8:911–927. doi:10.1007/s10021-005-0066-x
Hedde M, Bureau F, Akpa-Vinceslas M et al (2007) Beech leaf degradation in laboratory experiments: effects of eight detritivorous invertebrate species. Appl Soil Ecol 35:291–301. doi:10.1016/j.apsoil.2006.08.002
Hedges JI, Ertel JR (1982) Characterization of lignin by gas capillary chromatography of cupric oxide oxidation-products. Anal Chem 54:174–178. doi:10.1021/ac00239a007
Hedges JI, Mann DC (1979) Characterization of plant-tissues by their lignin oxidation-products. Geochim Cosmochim Acta 43:1803–1807. doi:10.1016/0016-7037(79)90028-0
Hedges JI, Weliky K (1989) Diagenesis of conifer needles in acoastal marine environment. Geochim Cosmochim Acta 53:2659–2673. doi:10.1016/0016-7037(89)90137-3
Hedges JI, Blanchette RA, Weliky K et al (1988) Effects of fungal degradation on the CuO oxidation-products of lignin—a controlled laboratory study. Geochim Cosmochim Acta 52:2717–2726. doi:10.1016/0016-7037(88)90040-3
Hobbie SE, Reich PB, Oleksyn J et al (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–2297. doi:10.1890/0012-9658(2006)87[2288:TSEODA]2.0.CO;2
Kögel I (1986) Estimation and decomposition pattern of the lignin component in forest humus layers. Soil Biol Biochem 18:589–594. doi:10.1016/0038-0717(86)90080-5
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. doi:10.1016/S0038-0717(01)00158-4
Kögel-Knabner I, Ziegler F, Riederer M et al (1989) Distribution and decomposition pattern of cutin and suberin in forest soils. Z Fur Pflanzenernahrung Und Bodenkunde 152:409–415. doi:10.1002/jpln.19891520502
Lajtha K, Crow SE, Yano Y et al (2005) Detrital controls on soil solution N and dissolved organic matter in soils: a field experiment. Biogeochemistry 76:261–281. doi:10.1007/s10533-005-5071-9
Lattaud C, Locati S, Mora P et al (1998) The diversity of digestive systems in tropical geophagous earthworms. Appl Soil Ecol 9:189–195. doi:10.1016/S0929-1393(98)00074-2
Li XY, Fisk MC, Fahey TJ et al (2002) Influence of earthworm invasion on soil microbial biomass and activity in a northern hardwood forest. Soil Biol Biochem 34:1929–1937. doi:10.1016/S0038-0717(02)00210-9
Lorenz K, Lal R, Preston CM et al (2007) Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules. Geoderma 142:1–10. doi:10.1016/j.geoderma.2007.07.013
McCune B, Mefford MJ (1999) Multivariate analysis of ecological data Version 4.14. MjM Software, Gleneden Beach
McLean MA, Migge-Kleian S, Parkinson D (2006) Earthworm invasions of ecosystems devoid of earthworms: effects on soil microbes. Biol Invasions 8:1257–1273. doi:10.1007/s10530-006-9020-x
Mikutta R, Kleber M, Torn MS et al (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77:25–56. doi:10.1007/s10533-005-0712-6
Nakajima N, Sugimoto M, Tsuboi S et al (2005) An isozyme of earthworm serine proteases acts on hydrolysis of triacylglycerol. Biosci Biotechnol Biochem 69:2009–2011. doi:10.1271/bbb.69.2009
Nierop KGJ (1998) Origin of aliphatic compounds in a forest soil. Org Geochem 29:1009–1016. doi:10.1016/S0146-6380(98)00165-X
Nierop KGJ, Naafs DFW, Verstraten JM (2003) Occurrence and distribution of ester-bound lipids in Dutch coastal dune soils along a pH gradient. Org Geochem 34:719–729. doi:10.1016/S0146-6380(03)00042-1
Otto A, Simpson MJ (2006) Sources and composition of hydrolysable aliphatic lipids and phenols in soils from western Canada. Org Geochem 37:385–407. doi:10.1016/j.orggeochem.2005.12.011
Otto A, Simpson MJ (2007) Analysis of soil organic matter biomarkers by sequential chemical degradation and gas chromatography—mass spectrometry. J Sep Sci 30:272–282. doi:10.1002/jssc.200600243
Pierce JW (1974) Soil mineral analyses: % composition by mineral classes. In: Corell DL (ed) Environmental monitoring and baseline data, compiled under the Smithsonian Institution Environmental Sciences Program, Temperate Studies, vol 3. pp 1113–1129
Pižl V (1992) Succession of earthworm populations in abandoned fields. Soil Biol Biochem 24:1623–1628
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. doi:10.1007/s11104-004-0907-y
Reynolds JW (1994) The distribution of the earthworms (Oligochaeta) of Indiana: a case for the post quaternary introduction theory for Megadrile migration in North America. Megadrilogica 5:13–32
Riederer M, Matzke K, Ziegler F et al (1993) Occurrence, distribution and fate of the lipid plant biopolymers cutin and suberin in temperate forest soils. Org Geochem 20:1063–1076. doi:10.1016/0146-6380(93)90114-Q
Satchel JE, Lowe DG (1967) Selection of leaf litter by Lumbricus terrestris. In: Graff O, Satchell JE (eds) Progress in soil biology. North Holland Publishing Co., Amsterdam, pp 60–66
Suárez ER, Fahey TJ, Yavitt JB et al (2006) Patterns of litter disappearance in a northern hardwood forest invaded by exotic earthworms. Ecol Appl 16:154–165. doi:10.1890/04-0788
Szlavecz K, Csuzdi C (2007) Land use change affects earthworm communities in Eastern Maryland, USA. Eur J Soil Biol 43:S79–S85. doi:10.1016/j.ejsobi.2007.08.008
Trumbore S (2006) Carbon respired by terrestrial ecosystems—recent progress and challenges. Glob Change Biol 12:141–153. doi:10.1111/j.1365-2486.2006.01067.x
Zicsi A (1983) Earthworm ecology in deciduous forests in Central and Southeast Europe. In: Satchell JE (ed) Earthworm ecology: from Darwin to vermiculture. Chapman & Hall, London, pp 171–177
Zimmer M (2002) Is decomposition of woodland leaf litter influenced by its species richness? Soil Biol Biochem 34:277–284. doi:10.1016/S0038-0717(01)00173-0
Zimmer M, Kautz G, Topp W (2005) Do woodlice and earthworms interact synergistically in leaf litter decomposition? Funct Ecol 19:7–16. doi:10.1111/j.0269-8463.2005.00926.x
Acknowledgments
We thank Geoffrey Parker, Dennis Whigham, and John O’Neill for providing leaf and wood samples, Rhonda Graef for laboratory support, and Amanda Eggink for help with root-picking. Support from the editors and helpful comments from two anonymous reviewers improved the utility and clarity of the final manuscript. This research was supported by grants #DEB-0316523 and #EAR-0748746 from the National Science Foundation. This is Purdue Climate Change Center paper #0810.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 7.
Rights and permissions
About this article
Cite this article
Crow, S.E., Filley, T.R., McCormick, M. et al. Earthworms, stand age, and species composition interact to influence particulate organic matter chemistry during forest succession. Biogeochemistry 92, 61–82 (2009). https://doi.org/10.1007/s10533-008-9260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-008-9260-1