Abstract
Tributyl phosphate (TBP) is an organophosphorous compound, used extensively (3000–5000 tonnes/annum) as a solvent for nuclear fuel processing and as a base stock in the formulation of fire-resistant aircraft hydraulic fluids and other applications. Because of its wide applications and relative stability in the natural environment TBP poses the problem of pollution and health hazards. In the present study, fifteen potent bacterial strains capable of using tributyl phosphate (TBP) as sole carbon and phosphorus source were isolated from enrichment cultures. These isolates were identified on the basis of biochemical and morphological characteristics and 16S rRNA gene sequence analysis. Phylogenetic analysis of 16S rRNA gene sequences revealed that two isolates belonged to class Bacilli and thirteen to β and γ-Proteobacteria. All these isolates were found to be members of genera Alcaligenes, Providencia, Delftia, Ralstonia, and Bacillus. These isolates were able to tolerate and degrade up to 5 mM TBP, the highest concentration reported to date. The GC–MS method was developed to monitor TBP degradation. Two strains, Providencia sp. BGW4 and Delftia sp. BGW1 showed respectively, 61.0 ± 2.8% and 57.0 ± 2.0% TBP degradation within 4 days. The degradation rate constants, calculated by first order kinetic model were between 0.0024 and 0.0099 h−1. These bacterial strains are novel for TBP degradation and could be used as an important bioresource for efficient decontamination of TBP polluted waste streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus compounds are toxic compounds; they inhibit the enzyme acetylcholine esterase (AChE), an extremely important enzyme in neurotransmission in animals. Tributyl phosphate (TBP) is one such organophosphorus compound. The inhibition of AchE by TBP leads to neurotoxicity and cholinergic toxicity (Berne et al. 2007; Raushel 2002). In addition, hyperplasia of kidney and urinary bladder epithelium, hepatomegaly, papillomas and transitional cell carcinomas are also caused by TBP (Arnold et al. 1997; Auletta et al. 1998). TBP causes irritation of skin, eyes and respiratory tract (Nakamura 1991). Also acute toxicity to freshwater organisms such as fish and algae is reported to occur at concentrations as low as 2 to 10 ppm (Hernandez 2002; Michel et al. 2004).
TBP is widely mainly used as a solvent for the conventional nuclear fuel processing, solvent extractant of rare earth metals from ore, as a solvent for lacquers, natural gums and cellulose esters. It is also extensively used as an antifoaming agent, as a base stock in the formulation of fire-resistant aircraft hydraulic fluids, as a plasticizer in the manufacture of plastics and vinyl resins, in the manufacturing of herbicides (Nakamura 1991) and in the formulation of fluorescent dye carriers (Schulz and Navratil 1987). These wide applications of TBP have led to its worldwide production of 3000–5000 tonnes/annum (Hernandez 2002).
TBP poses the problem of environmental pollution, since it is relatively resistant to degradation in the natural environments such as soil and water leading to its long persistence therein (US EPA 1992). TBP pollution has been reported in indoor air from domestic and occupational environments (Marklund et al. 2005a), aquatic environments (Fries and Puttmann 2003) and in the influent and effluent of wastewater treatment plants (Meyer and Beser 2004; Marklund et al. 2005b).
Despite its persistence in the environment and toxicity, biodegradation of TBP has been scarcely investigated especially in comparison to other organophosphorus compounds. A few studies on TBP degradation by Acinetobacter sp. (Stoner and Tien 1995), Citrobacter sp. (Owen et al. 1992), Serratia odorifera and Rhodopseudomonas palustris (Berne et al. 2004, 2005) have been reported. Likewise, Thomas et al. (1997b) demonstrated TBP degradation using mixed cultures of Pseudomonads. In all these studies, TBP degradation up to 2 mM was reported. However, to our knowledge there are no reports on TBP degradation at higher concentrations (>2 mM). On this background of wide usage of TBP, its toxicity and persistence in environment, studies on biodegradation of TBP are becoming increasingly important. We report here the isolation, molecular characterization and phylogenetic analysis of bacteria degrading TBP at higher concentration (5 mM). In addition, the novel GC–MS method to estimate TBP in the investigation of kinetics of TBP degradation is also reported.
Materials and methods
Chemicals
Tributyl phosphate (99%) was obtained from Aldrich and dibutyl phosphate (97%) from Fluka, USA. The organic solvents obtained from Merck were of highest purity and were filtered through 0.45 μm Nylon membrane filter prior to use. Primary Secondary Amine (Bondesil-PSA, 40 μm) was obtained from Varian, USA. The components used for bacteriological media, minimal media and inorganic phosphate assay were purchased from Hi-Media, SRL, India and Sigma, USA.
Sampling
Four locations in the Pune, India (18°32′N, 73°55′E) were selected for sampling on the basis of the history of their previous exposures to various xenobiotics. Two of the sampling sites, Band Garden Bridge and Sangam Bridge were from heavily polluted Mutha river. One sampling site was agriculture college field with the history of pesticide applications. The samples were also collected from primary and secondary waste treatment plants of Padamji paper and pulp industry. All these samples were used immediately after the collection.
Enrichment and isolation of TBP degrading bacteria
Enrichment of TBP degrading bacteria was carried out for each sample separately in the minimal medium (Thomas and Macaskie 1996) comprised (g l−1) of MOPS, 5.23; CaCl2·2H2O, 0.025; MgSO4·7H2O, 0.2; NaCl, 0.1; KCl, 0.075; (NH4)2SO4, 5.0; Na2-EDTA, 0.015; ZnSO4·7H2O, 0.0066; MnCl2·4H2O, 0.00171; FeSO4·7H2O, 0.0015; CoCl2·6H2O, 0.000483; CuSO4·5H2O, 0.000471; NaMoO4·2H2O, 0.000453 and yeast extract, 0.001 as a growth factor. The pH of the medium was adjusted to 7.0 ± 0.2. The enrichment was performed in two steps: In the first step, the soil or water sample (1 g soil or 1 ml water) was added in the 250 ml Erlenmeyer flasks containing 100 ml minimal medium supplemented with glucose (1%) as a source of carbon and TBP (0.53 g l−1 i.e. 2 mM) as a sole source of phosphorus. The flasks were incubated for seven days on rotary shaker (150 rpm) at 30°C and the resultant culture (1 ml) was used to inoculate fresh minimal medium containing 2 mM TBP and 1% glucose. Further, this inoculum transfer from previous flask (1 ml) to the fresh minimal medium (100 ml in 250 ml flask) was carried out for subsequent eight times at regular (7 days) interval. In the second step, the initially obtained enrichment culture was transferred to the glucose deficient minimal medium with higher TBP concentration (5 mM). Thereafter, the inoculum was transferred from previous flask (1 ml) to the fresh minimal medium containing only TBP (5 mM) for subsequent six times at regular (7 days) interval. Finally, the aliquots (100 μl) of this secondary enrichment culture were inoculated on minimal agar medium containing 5 mM TBP as the sole source of both carbon and phosphorus and after incubation at 30°C for 24 h, morphologically distinct colonies were isolated and purified.
Biochemical characterization and plasmid profile of the isolates
The colony characteristics (colony size, colour, margin, opacity, consistency, elevation etc.) of selected 15 isolates were noted after the growth on nutrient agar for 24 h at 30°C. The morphological (Gram test, cell morphology and motility tests) and biochemical (catalase, oxidase, Gram and motility tests) characterization was carried out according to standard methods (Collins et al. 1995). Plasmid DNA was isolated by Qiagen plasmid purification kit according to manufacturer’s protocol and subsequently electrophoresed on 0.8% agarose containing ethidium bromide (0.5 μg ml−1). Plasmids of Escherichia coli V517 (Macrina et al. 1978) were used as molecular size markers.
PCR amplification and sequencing of 16S rRNA genes
The genomic DNA of 15 TBP degrading bacteria was extracted using Gen-Elute DNA isolation kit (Sigma, USA). The 16S rRNA genes were amplified by PCR using 16S rDNA specific universal primers 16F27N (5′-AGA GTT TGA TCC TGG CTC AG-3′) (Brosius et al. 1978) and 16R1488 (5′-CGG TTA CCT TGT TAC GAC TTC ACC-3′) (Lane 1991) hybridizing respectively at positions 8–27 and 1488–1511 relative to E. coli 16S rDNA numbering. The PCR reactions were carried out in PE 9700 thermal cycler (Perkin Elmer, USA) using following amplification conditions: an initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, extension at 72°C for 1 min and the final extension at 72°C for 10 min. PCR was carried out in 25 μl reaction mixture consisted of 10× Taq polymerase buffer (New England Biolabs), 2 mM dNTPs, 10 pM primers, 1 unit Taq polymerase (New England Biolabs), and 10 ng DNA. The PCR products were purified using the QIAquick PCR purification kit (Qiagen, Germany) following the manufacturer’s protocol and were sequenced on both strands on ABI 3730 DNA analyzer using the Big Dye terminator kit (Applied Biosystems, USA). The near full length 16S rRNA gene sequences were deposited to GenBank under the consecutive accession numbers: EU195864–EU195878.
Identification and phylogenetic analysis of the isolates
The 16S rRNA gene sequences of the isolates were compared with known sequences in NCBI-GenBank database (http//:www.ncbi.nlm.nih.gov) using homology search tool BLAST. For phylogenetic analysis, the 16S rRNA gene sequences of isolates reported here and the sequences of isolates from database were aligned using the CLUSTAL-W multiple sequence alignment tool. Phylogenetic relationships between the genera were inferred using the neighbor-joining method (Saitou and Nei 1987) by MEGA v. 4.0 software (Tamura et al. 2007).
Biodegradation studies
All 15 isolates were grown separately in LB broth for 12–15 h under shaking conditions (150 rpm) at 30°C. Subsequently, the cell pellet of each isolate was obtained by centrifugation at 5000×g for 5 min and was washed twice with sterile minimal medium. Then, 1 ml of cell suspension with cell density adjusted to OD600 ≈ 1 was inoculated to 9 ml minimal medium (in 50 ml flasks) containing 5 mM TBP as a sole source of carbon and phosphorus. For each isolate, ten such sets were prepared in duplicate and incubated at 30°C for 96 h under shaking conditions (150 rpm). The uninoculated medium with TBP and medium without TBP inoculated with each isolate were used as controls. During 96 h incubation, one flask of each isolate after every 12 h interval was used for the estimation of growth (OD600), inorganic phosphate content and residual TBP. Inorganic phosphate was estimated with Katewa and Katyare (2003) method using KH2PO4 as a standard. The residual TBP was extracted and estimated by GC–MS as described in the following section. The residual TBP was calculated by following formula:
The growth was also monitored with 5 mM dibutyl phosphate (DBP) in similar set of experiments to trace the metabolism of TBP.
Extraction of TBP and its analysis by GC–MS method
TBP from the sample was extracted thrice using equal volume of hexane. The solvent extract was appropriately diluted with hexane to bring TBP concentration in the linear range of GC–MS analysis (50–500 ppb). Further, 2 ml of diluted extract was mixed with 25 mg Primary Secondary Amine (PSA) in order to absorb the matrix components from the extract, centrifuged at 10,000×g for 4 min at 4°C and the supernatant was used for GC–MS analysis. Working standards were prepared by appropriate serial dilutions of stock solution of TBP with hexane.
Residual TBP analysis was carried out using the GC–MS system, consisting of Thermo Finnigan Trace GC Ultra equipped with TriPlus autosampler and PolarisQ Ion Trap MS/MS detector controlled by Xcalibur software (Thermo Electron Corporation, Italy). Using Triplus autosampler 15 μl of sample was injected with four pre-injection and post injection washes with hexane. The autosampler and injector parameters were as described in our earlier study (Ahire et al. 2008). The chromatographic separation of TBP was performed on a Mega-5-MS column (Mega Capillary Columns Laboratory, Italy) with 30 m × 0.32 mm i.d. and 0.5 μm film thickness. Helium (99.999% purity) was used as carrier gas at flow rate of 1 ml min−1. The oven temperature was programmed at 50°C initially, followed by a 25°C min−1 ramp to 285°C. Electron Impact (EI) ionization was achieved by 70 eV ionization energy with source temperature 230°C and auxiliary temperature 285°C. Positive mode full scan was performed in the mass range m/z 50–450 with the solvent delay of 5 min.
Results and discussion
Enrichment and isolation of TBP degrading bacteria
Enrichment cultures were obtained from seven different soil and water samples after around 9 weeks of enrichment with TBP as an only phosphorus source. The acclimatization of these cultures to utilize TBP as the sole carbon and phosphorus source resulted in further enrichment of potent TBP degrading cultures. After rigorous enrichment, 22 morphologically different bacteria capable of growing on minimal media containing TBP were isolated. The ability of individual bacterial strain to degrade TBP was tested by the monitoring their growth spectrophotometrically for four days, which showed the substantial increase in the biomass. Amongst 22 isolates, 15 isolates showed OD600 more than 0.5 in minimal medium containing TBP. All the soil and water samples yielded at least one TBP degrader (Table 1).
Biochemical characteristics and plasmid profile of the isolates
The cultural and biochemical characteristics and plasmid profile of TBP degrading bacteria are shown in Table 2. All 15 isolates were catalase positive, 13 isolates were oxidase positive and 13 isolates were Gram negative rods. Analysis of plasmid DNA profile revealed that all 15 isolates harbored plasmids (1–3) of molecular size ranging from 4.9 to 18.4 kb (Fig. 1). Plasmids of 6.7 and 4.9 kb were most frequently detected and were found in 11 (73.33%) and 8 (53.33%) isolates respectively, whereas plasmid of 18.4 kb was detected in only one isolate. The multiple plasmids might have role in the TBP degradation. A similar phenomenon was noted previously by Thomas et al. (1997a, b), wherein the loss of degradability due to loss of one of the plasmids was observed.
Identification and phylogenetic analysis of the isolates
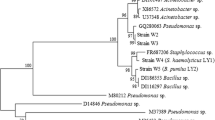
The BLAST analysis of 16S rRNA gene sequences revealed that nine out of 15 isolates belonged to subclass β-Proteobacteria, four to γ-Proteobacteria and two to Bacilli. All isolates showed 95–100% similarity to the sequences of Alcaligenes, Providencia, Delftia, Ralstonia, and Bacillus genera available in the GenBank database (Table 3). Amongst all, isolate PTP1 showed the least homology with Bacillus subtilis (95%), whereas isolate BGW1 showed the highest homology with Delftia sp. (100%). The phylogenetic analysis revealed that the seven isolates SBW1, SBW2, BGW3, STP11, STP12, STP13 and ACS1 clustered together and found to be closely related to genus Alcaligenes. The isolate BGW1 and BGS2 were closely related to Delftia sp. and Ralstonia sp. respectively (Fig. 2). Moreover, members of γ-Proteobacteria found to be clustered with Providencia sp. (Fig. 3). The isolates PTP1 and PTP2 from class Bacilli showed clustering with Bacillus subtilis and Bacillus cereus respectively (Fig. 4). Although the isolates were obtained from different environmental samples (Table 1), their phylogenetic analysis demonstrated that some isolates such as ACS1, BGW3 and STP12 were very closely related irrespective of their sampling site (Table 3). In previous studies, the involvement of Alcaligenes sp. in the degradation of organophosphorus pesticides such as glyphosate, chlorpyrifos and other xenobiotic compounds (Tolbot et al. 1984; Singh and Walker 2006) has been reported. Likewise, in our studies seven out of 15 isolates were Alcaligenes sp. enriched on TBP further confirmed their ability of organophosphorous degradation. Although Providencia sp. have been reported for the reduction of explosives like RDX and HMX (Kitts et al. 1994), there is no report on the involvement of this genus in the degradation of organophosphorus compounds. However, some of our isolates which were identified as Providencia sp. were observed to be potent degraders of TBP. Although, Ralstonia sp. has been reported for carbendazim, chlorobenzene, 2,6-dichlorophenol and 3,5,6-trichloro-2-pyridinol degradation (Zhang et al. 2005; Li et al. 2010), to the best of our knowledge this is the first report on its involvement in TBP degradation. Bacillus is well known for the degradation of organophosphorus pesticides such as parathion, glyphosate, monocrotophos etc. (Singh and Walker 2006), however, two Bacillus sp. (PTP1 and PTP2) in our study showed comparatively poor TBP degradation than other isolates.
Phylogenetic relationships between nine TBP degrading isolates (in bold) and other members belonging to β-Proteobacteria, inferred from 16S rRNA gene sequences using the Neighbor-Joining method. Bacillus subtilis was used as an outgroup. The number at each node indicates the percentage of 500 bootstrap replicates and bar represents the number of base substitutions per site
Novel GC–MS method for TBP analysis
Among six organic solvents (hexane, acetone, ethyl acetate, dichloromethane, iso-octane, diethyl ether, and toluene) used for TBP extraction, the extraction efficiency with hexane was maximum (96.92 ± 2.69% RSD) (data not shown) and hence it was used as a solvent for further experiments. The % recovery was calculated by following formula:
The previously reported GC–MS method for analysis of TBP was for portable field GC–MS system (Makas and Troshkov 2004). The parameters and working principles (such as multiple module GC system, compact magnetic mass-spectrometer and multicollector ion detector) of portable field GC–MS system are far different than those used in regular research and analytical laboratories. Moreover, this system is rarely used in regular research laboratories. Therefore, the efforts were made to develop new GC–MS method for the analysis of TBP. The calibration curve of TBP verses peak area obtained with developed GC–MS method showed excellent linearity in the concentration range of 50–500 ppb (r2 = 0.995). Although, other than dilution no offline sample preparation was carried out, the matrix effects caused by salts and other biomolecules were least. In addition, the developed method has the strength of rapid, accurate and reproducible analysis of TBP at trace levels with higher sensitivity (LOD = 1 ppb, S/N more than 3) than the previously reported methods (Lamouroux et al. 2000 and references therein; Dodi and Verda 2001; Makas and Troshkov 2004).
Furthermore, attempts were made to investigate the degradation products of TBP from the medium by GC–MS method. However, the degradation products of TBP could not be recovered, most probably due to their concentrations below the detection limit in the extract. This might be because of their utilization by bacteria for the growth, as TBP was the only carbon and phosphorus source in the medium. Therefore, further investigations to explore the intracellular enzyme systems of these bacteria are proposed.
Biodegradation of TBP
All 15 isolates showed 21–61% degradation of TBP with the initial concentration of 5 mM within 4-days of incubation (Fig. 5). Kinetics of TBP degradation by Providencia sp. BGW4 showed the growth with concurrent depletion of TBP from the minimal medium (Fig. 6). In addition, the gradual increase in inorganic phosphate clearly indicated the degradation of TBP. Furthermore, all isolates showed OD600 more than 0.5 (Fig. 5), which was significantly more than that in control flasks lacking any substrate (OD600 ≈ 0.1). Two isolates, Providencia sp. BGW4 and Delftia sp. BGW1 showed very low residual TBP as 1.95 mM (61.0 ± 2.8% degradation) and 2.15 mM (57.0 ± 2.0% degradation) respectively (Fig. 5). The TBP degradation rate constants (k) for all isolates, calculated using first-order kinetic equation of the form Ct = C0e−kt (Xu et al. 2005 and references therein) (where C0 is concentration of TBP at time 0, Ct is concentration of TBP at time t) ranged from 0.0024 to 0.0099 h−1 (Table 4). The degradation rates of TBP by all isolates were four to tenfold more than that reported for Acinetobacter sp. (0.8 μmol ml−1 h−1) by Stoner and Tien (1995). Berne et al. (2004) reported inability of S. odorifera to degrade TBP unless the media was supplemented with 1 mM glucose and 50 mM phosphate. In comparison to this, the isolates reported here were able to use TBP as sole source of carbon and phosphorus. Thomas and Macaskie (1998) reported degradation of TBP by Pseudomonas sp. (15 μmol ml−1 h−1), which was slightly higher than the degradation rates of isolates in this study. However, the spontaneous loss of TBP degrading ability after eight sub culturing was evident in their studies. The authors further mentioned that it was not possible to stabilize the cultures and DNA profile with respect to TBP utilization. In contrast, the isolates reported in our study were stable for their TBP degradation ability even after several subculturing (over 2 years) in liquid and solid media (data not shown). The estimated half-life of TBP degradation was 70–288 h for all isolates (Table 4). The isolates obtained in this study exhibited higher TBP tolerance (5 mM) than earlier reported 2 mM concentration (Thomas et al. 1997a, b; Berne et al. 2004, 2005). Additional studies with DBP (one of the proposed intermediates of TBP degradation) revealed that only five isolates viz., ACS1, BGW4, SBS2, SBW1, STP13 could grow well on DBP. However, other isolates showed moderate or no growth on DBP (data not shown). These observations suggest the possible involvement of alternative pathways in TBP degradation and/or differential TBP/DBP transport mechanism across the cell membrane. To elucidate this further studies on transport of TBP/DBP across the cell membrane are desirable.
Growth and kinetics of TBP degradation by bacterial isolates. Growth (filled square) was estimated spectrophotometrically and residual TBP (square with upper right to lower left fill) was estimated by GC–MS after 4 days incubation at 30°C under shaking conditions (150 rpm). Control 1 was sterile minimal medium containing TBP and Control 2 was medium without TBP inoculated with isolate
Kinetics of TBP degradation by Providencia sp. strain BGW4 at 30°C in the minimal medium containing 5 mM TBP. The growth (filled square), residual TBP (filled circle) and the inorganic phosphate (filled triangle) in the medium are shown. The data points are mean ± SD (indicated by vertical bars) of duplicate or triplicate sets
Conclusions
In summary, the bacteria capable of degrading tributyl phosphate at higher concentrations (5 mM) were isolated by extensive enrichment culture technique. The prominent TBP degraders were identified as members of Delftia, Ralstonia, Providencia, Alcaligenes and Bacillus genera using biochemical, molecular and phylogenetic analysis. Further, biodegradation studies highlighted the novelty and stability of these bacterial isolates with respect to TBP degradation since, the isolates showed consistent ability to degrade TBP even after several subculturing. The developed GC–MS method demonstrated its feasibility and utility for quantitative estimation of TBP.
References
Ahire KC, Arora MS, Mukherjee SN (2008) Residue analysis of Lufenuron in milled wheat by gas chromatography–mass spectrometry (GC–MS) and monitoring its bio-efficacy against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Chromatogr B 861:16–21
Arnold LL, Christenson MC, John MK, Wahale BS, Cohen SM (1997) Tributyl phosphate effects on urine and bladder epithelium in male Sprague-Dawley rats. Fundam Appl Toxicol 40:247–255
Auletta CS, Weiner ML, Richter WR (1998) A dietary toxicity/oncogenicity study of tributyl phosphate in the rat. Toxicology 128:125–134
Berne C, Montjarret B, Guountti Y, Garcia D (2004) Tributyl phosphate degradation by Serratia odorifera. Biotechnol Lett 26:681–686
Berne C, Allainmat B, Garcia D (2005) Tributyl phosphate degradation by Rhodopseudomonas palustris and other photosynthetic bacteria. Biotechnol Lett 27:561–566
Berne C, Pignol D, Lavergne J, Garcia D (2007) CYP201A2, a cytochrome P450 from Rhodopseudomonas palustris, plays a key role in the biodegradation of tributyl phosphate. Appl Microbiol Biotechnol 77:135–144
Brosius J, Palmer ML, Kennedy PJ, Noller HF (1978) Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA 75:4801–4805
Collins CH, Lyne PM, Grange JM (1995) Collins and Lyne’s microbiological methods, 7th edn. Butterworth-Heinemann, London, U.K
Dodi A, Verda G (2001) Improved determination of tributyl phosphate degradation products (mono- and dibutyl phosphates) by ion chromatography. J Chromatogr A 920:275–281
Fries E, Puttmann W (2003) Monitoring of the three organophosphate esters TBP, TCEP and TBEP in river water and ground water (Oder, Germany). J Environ Monit 5:346–352
Hernandez O (2002) Tributyl phosphate—SIDS initial assessment report for 12th SIAM. UNEP Publications. http://www.chem.unep.ch/irptc/sids/OECDSIDS/126-73-8.pdf. Accessed 13 March 2008
Katewa SD, Katyare SS (2003) A simplified method for inorganic phosphate determination and its application for phosphate analysis in enzyme assays. Anal Biochem 323:180–187
Kitts CL, Cunningham DP, Unkefer PJ (1994) Isolation of three hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitramine explosive-contaminated soil. Appl Environ Microbiol 60:4608–4611
Lamouroux C, Virelizier H, Moulin C, Tabet JC, Jankowski CK (2000) Direct determination of dibutyl and monobutyl phosphate in a tributyl phosphate/nitric aqueous-phase system by electrospray mass spectroscopy. Anal Chem 72:1186–1191
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley & Sons Inc., Chichester, pp 115–175
Li J, Liu J, Shen W, Zhao X, Hou Y, Cao H, Cui Z (2010) Isolation and characterization of 3,5,6-trichloro-2-pyridinol-degrading Ralstonia sp. strain T6. Bioresour Technol 101:7479–7483
Macrina FL, Kopecko DJ, Jones KR, Ayers DJ, McCowen SM (1978) A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid 1:417–420
Makas AL, Troshkov ML (2004) Field gas chromatography–mass spectrometry for fast analysis. J Chromatogr B 800:55–61
Marklund A, Andersson B, Haglund P (2005a) Organophosphorus flame retardants and plasticizers in air from various indoor environments. J Environ Monit 7:814–819
Marklund A, Andersson B, Haglund P (2005b) Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environ Sci Technol 39:7423–7429
Meyer J, Beser K (2004) Organophosphate flame retardants and plasticizers in wastewater treatment plants. J Environ Monit 6:599–605
Michel K, Brinkmann C, Hahn S, Dott W, Eisentraeger A (2004) Acute toxicity investigations of ester based lubricants by using biotests with algae and bacteria. Environ Toxicol 19:445–448
Nakamura A (1991) International programme on chemical safety—environmental health criteria 112-tri-n-butyl phosphate. World Health Organisation Publication, Geneva. http://whqlibdoc.who.int/ehc/WHO_EHC_112_eng.pdf
Owen S, Jeong BC, Poole PS, Macaskie LE (1992) Tributyl phosphate degradation by immobilized cells of Citrobacter sp. Appl Biochem Biotechnol 34:693–707
Raushel FM (2002) Bacterial detoxification of organophosphate nerve agents. Curr Opin Microbiol 5:288–295
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schulz WW, Navratil JD (eds) (1987) Science and technology of tributyl phosphate, vol 2, part B. CRC Press Inc., Boca Raton, FL
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30:428–471
Stoner D, Tien A (1995) Method and compositions for the degradation of tributyl phosphate in chemical waste mixtures. US Patent 5,453,375
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thomas RA, Macaskie LE (1996) Biodegradation of tributyl phosphate by naturally occurring microbial isolates and coupling to the removal of uranium from aqueous solution. Environ Sci Technol 30:2371–2375
Thomas RA, Macaskie LE (1998) The effect of growth conditions on the biodegradation of tributyl phosphate and potential for the remediation of acid mine drainage waters by a naturally-occurring mixed microbial culture. Appl Microbiol Biotechnol 49:202–209
Thomas RAP, Greated A, Lawlor K, Bailey M, Macaskie LE (1997a) Stabilisation of tributyl phosphate-biodegradative ability of naturally-occurring pseudomonads using ampicillin. Biotechnol Tech 11:781–785
Thomas RAP, Morby AP, Macaskie LE (1997b) The biodegradation of tributyl phosphate by naturally occurring microbial isolates. FEMS Microbiol Lett 155:155–159
Tolbot HW, Johnson LM, Munneck DM (1984) Glyphosate utilization by Pseudomonas sp. and Alcaligenes sp. isolated from environmental sources. Curr Microbiol 10:255–260
US EPA (1992) Chemical testing & data collection—tributyl phosphate test results. US Environmental Protection Agency Publication, Washington. http://www.epa.gov/opptintr/chemtest/pubs/tributph.html. Accessed 16 February 2009
Xu XR, Li HB, Gu JD (2005) Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate ester by Pseudomonas fluorescens B-1. Int Biodeter Biodegrad 55:9–15
Zhang GS, Jia XM, Cheng TF, Ma XH, Zhao YH (2005) Isolation and characterization of a new carbendazim-degrading Ralstonia sp. strain. World J Microbiol Biotechnol 21:265–269
Acknowledgments
Kedar Ahire acknowledges the Council of Scientific and Industrial Research (CSIR), Government of India and Board of College and University Development (BCUD), University of Pune for the financial assistance. Authors thank Dr. Vijay Kolekar, In-charge, Insecticide Residue Testing Laboratory, Pune (Govt. of India) for GC–MS facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahire, K.C., Kapadnis, B.P., Kulkarni, G.J. et al. Biodegradation of tributyl phosphate by novel bacteria isolated from enrichment cultures. Biodegradation 23, 165–176 (2012). https://doi.org/10.1007/s10532-011-9496-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-011-9496-7