Abstract
The purpose was to investigate a simultaneous biodegradation of the recalcitrant monoazo dye Reactive Orange 16 (RO16) in a mixed culture consisting of a biofilm of Pleurotus ostreatus–colonizing polyamide carrier and a suspension of the yeast Candida zeylanoides to see their biological interactions and possible synergistic action during degradation. Decolorization in the mixed culture was more effective than in the fungal monoculture, the respective decolorizations reaching 87.5% and 70% on day 11. The proliferation of yeast was reduced compared with the C. zeylanoides monoculture but enabled the yeast to participate in decolorization. The interaction of P. ostreatus with the yeast resulted in a gradual decrease of fungal manganese-dependent peroxidase (MnP) and laccase activities. Gas chromatography-mass spectrometry (GC-MS) analysis of the degradation products brought evidence that P. ostreatus split the dye molecule asymmetrically to provide 4-(ethenylsulfonyl) benzene whose concentration was much decreased in the mixed culture suggesting its increased metabolization in the presence of the yeast. In contrast, C. zeylanoides split the azo bond symmetrically producing the metabolites 4-(ethenylsulfonyl) aniline and α-hydroxybenzenepropanoic acid. Those metabolites were rapidly degraded in the mixed culture. A novel aspect is represented by the evidence of a mutual cooperative action of the fungal and yeast microorganisms in the mixed culture resulting in rapid decolorization and degradation of the dye.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial consortia and mixed cultures of different microorganisms are able to degrade organic pollutants, including recalcitrant synthetic dyes, and often exhibit better performance than single strains. Microbial interactions in such co-cultures consist of concomittant mechanisms ranging from positive to negative effects, but our knowledge of these mechanisms, that may include mutual microbial cooperation, is rather poor (Mikesková et al. 2012; Wang et al. 2014; Wang et al. 2019).
Broad biodegradation potential of ligninolytic fungi (LF) has been established with Pleurotus ostreatus serving as one of model organisms, and its biochemical and physiological behavior under various conditions was well documented (Gadd 2008; Svobodová et al. 2016; Ceci et al. 2019). In this group of microorganisms, extracellular lignin-modifying enzymes are implicated in oxidative degradation of organopollutants such as industrial dyes (Knapp et al. 2008). Yeasts are another large group of microorganisms that exhibit important biodegradation capacities, e.g., those able to decolorize recalcitrant azo dyes (Yang et al. 2005; Tan et al. 2016). Various reductases, such as azoreductase or NADH-dichlorophenolindophenol reductase, are implicated in reductive cleavage of the azo bond of the dye-producing metabolites that are further degraded to aliphatic amines by the action of oxidative enzymes (Ramalho et al. 2002, 2005; Jadhav et al. 2007; Saratale et al. 2009).
Biodegradation potential of defined consortia of fungi with other microorganisms has been documented, e.g., a fungal-bacterial consortium comprising Penicillium sp. QQ strain in azo dye degradation (Gou et al. 2009); consortium consisting of Aspergillus ochraceus and Pseudomonas sp. in degradation of textile dyes (Kadam et al. 2011); co-cultures of P. ostreatus with bacteria Pseudomonas fluorescens or Bacillus licheniformis degrading anthraquinone dye Remazol Brilliant Blue R (Válková et al. 2017); or fungal consortia of Aspergillus lentulus, A. terreus and Rhizopus oryzae, or Dichotomomyces cejpii and Phoma tropica used for decolorization of various azo dyes (Mishra and Malik 2014; Krishnamoorthy et al. 2018). Both positive and negative effects of the other organisms on biodegradation by fungal cultures have been reported, the factors generally involving pH, extracellular enzymes, and competition for nutrients (Libra et al. 2003; Spina et al. 2014; Li et al. 2015).
Levels of extracellular enzymes, namely peroxidases and laccase that are involved in degradation of organopollutants in LF, can be affected by interaction with other microorganisms resulting in an increase of laccase activity as observed in Trametes versicolor and P. ostreatus by Hiscox et al. (2010) and Válková et al. (2017), respectively. Such an increase, however, did not occur in the case of MnP (Válková et al. 2017). The increased enzyme activity may or may not result in higher biodegradation rates (Baldrian 2004; Novotný et al. 2004; Hiscox et al. 2010; Válková et al. 2017).
Both positive and adverse effects of microorganisms on the degradation process by LF have been reported, for instance, fixed-film cultures of Phanerochaete chrysosporium exhibited a high and stable degradation efficiency in the presence of bacteria (Gao et al. 2008) or the efficiency of decolorization of recalcitrant Remazol Brilliant Blue R (RBBR) dye by mature biofilms of P. ostreatus was not restrained by populations of P. fluorescens or B. licheniformis. On the other hand, degradation of polycyclic aromatic hydrocarbons by various LF in soil and in submerged cultures was negatively affected by the presence of bacteria (Borchert and Libra 2001; Borràs et al. 2010). Yeasts were reported to be responsible for failures of bioremediation realized by LF as they exhibited good growth at low pH and high growth rates in carbohydrate-based media (Boekhout and Robert 2003; Knapp et al. 2008). However, little is known about the behavior of yeasts in mixed cultures with LF and how the partners influence the biodegradation process of the other partner fungal microorganism. White rot fungi and yeasts have been reported to use different degradation pathways when decomposing azo dyes (Erkurt et al. 2010; Jafari et al. 2014).
Our aim was to estimate the simultaneous action of the two different biodegradation processes carried out by P. ostreatus and C. zeylanoides during decolorization of the model recalcitrant azo dye RO16 in their mixed culture where the yeast suspension was added to a preformed biofilm of P. ostreatus immobilized on a plastic carrier. In the mixed culture, the growth of both organisms was followed, the activities of extracellular enzymes involved in degradation were monitored, and the analysis of biodegradation products using GC-MS was carried out to check whether both partners contributed to decolorization.
Materials and methods
Microorganisms
P. ostreatus was obtained from the Culture Collection of Basidiomycetes of ASCR, Prague, Czech Republic. The yeast Candida zeylanoides was acquired from the Spanish Type Culture Collection, University of Valencia, Spain. The fungus was maintained on malt extract-glucose (MEG) agar (malt extract 5 g/L, glucose 10 g/L, agar powder 20 g/L), grown at 28 °C for 7 days and stored at 4 °C. The yeast was preserved on complex medium plates containing glucose 40 g/L, mycopeptone 10 g/L, and agar 15 g/L (GMA). The yeast was grown at 28 °C for 2 days and then stored at 4 °C.
Chemicals
2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,6 dimethoxyphenol (DMP), 96% 3,4-dimethoxyphenol, malonic acid, EDTA, and Reactive Orange 16 were purchased from Sigma-Aldrich. All chemicals were of analytical grade.
Culture conditions
The fungal inoculum was prepared by transferring ten agar plugs of P. ostreatus grown on MEG agar into 250-mL flasks containing 100 mL of liquid MEG medium and incubated at 28 °C for 7 days. Then the cultures were homogenized (Ultra-Turrax T25, IKA) and used as the inoculum (10% V/V) to inoculate 250-mL flasks containing a liquid growth medium and polyamide mesh carrier (wire wool: fiber thickness, 2 mm; mesh size, 3 mm) to prepare immobilized fungal cultures. A growth medium (glucose 20 g/L, (NH4)2SO4 2.5 g/L, yeast extract g/L, KH2PO4 5 g/L, MgSO4·7H2O 0.5 g/L, CaCl2·2H2O 0.13 g/L) was used for this purpose.The fungal cultures were then grown under static conditions for 7 days to form biofilm on the polyamide carrier and were further used as preformed biofilms in the mixed cultures and control fungal cultures.
A yeast inoculum culture was prepared by growing the microorganism overnight in MEG medium on a rotary shaker (DOS-20L, ELMI) (80 rpm). The final concentration of the yeast in the mixed culture and in the control yeast monoculture was adjusted to achieve a final value of 106 colony forming units (CFU) per mL.
RO16 azo dye was added to the mixed or control cultures at a final concentration of 150 mg/L to start the decolorization that took place at 28 °C under shaking (80 rpm) to ensure a sufficient aeration for the dye degradation by the yeast. Samples of the liquid medium were removed in time to determine the dye removal, extracellular enzyme activities, and, where applicable, the yeast cell counts.
The control monocultures of the fungus and the yeast were prepared in a similar way but without the presence of the other microbial partner and were used for decolorization of RO16.
All experiments were carried out in triplicates.
Decolorization and enzymatic assay
Decolorization of RO16 was measured spectrophotometrically at 494 nm using a microplate method (Epoch Microplate Spectrophotometer, Bio-Tek, USA; Program Gen5). Yeasts, bacteria, and fungal fragments were removed by centrifugation before the measurement.
The activities of extracellular enzymes implicated in dye degradation were measured spectrophotometrically: manganese-dependent peroxidase (MnP) using the oxidation of DMP (De Jong et al. 1994), lignin peroxidase (LiP) with veratryl alcohol as the substrate (Tien and Kirk 1988), and laccase using the oxidation of ABTS (Matsumura et al. 1986). One unit of enzyme activity (U) was defined as an amount of the enzyme oxidizing 1 μmol of substrate per min.
Fungal biomass and yeast cell counts measurements
Fungal biomass colonizing the polyamide carrier was determined gravimetrically as dry biomass at the end of experiments. The polyamide carrier covered with the colonizing fungal biofilm was removed from the cultivation flask, gently washed with distilled water, and dried at 105 °C until constant weight. Then the pre-weighed mass of the carrier was subtracted to obtain the dry biofilm mass. Yeast cell counts were determined by plating on GMA medium.
Gas chromatography-mass spectrometry analysis
The culture medium was filtered (cellulose wadding), centrifuged (2000 rpm, 5 min, laboratory temperature), and directly extracted with dichloromethane (pesticide grade). The acid-base liquid-liquid extraction at pH 2 and 12 was used. When measured before extraction, all samples had pH values of approximately 6.5. A volume of 100 mL of each sample was extracted in separatory funnels (200 mL) using 5 mL of dichloromethane three times. First, extraction was performed using any sample as-received (pH neutral). Then, the same sample was serially extracted with dichloromethane at pH 12 and, subsequently, at pH 2. All extracts were mixed together, dried using Na2SO4, and concentrated to a volume of 1 mL under nitrogen. Finally, the extracts were analyzed by GC-MS (7890N/5975C, Agilent Technologies, USA). GC-MS was equipped with a capillary column DB-XLB (30 m × 0.25 mm × 0.25 μm). The operating conditions for screening analysis were the injection port was maintained at 290 °C and the dichloromethane extracts were injected in splitless mode. The program column temperature started at 40 °C for 2 min, then increased by 5 K/min to 300 °C, and was held for 10 min at 300 °C. The ion source of MS detector was operated at 230 °C. The scan mode was employed, and the experimental data were measured in the range of 50–550 amu. The mass spectra library NIST011 was used for evaluation of the mass spectra obtained. Only compounds with a high or moderately high confidence in structure were identified, for which an excellent (RSI > 900) or good (RSI = 800–900) match between the mass spectrum and that of electron ionization mass spectra library was found (Plachá et al. 2017).
Results and discussion
Growth and decolorization in mixed cultures of P. ostreatus and C. zeylanoides
In order to investigate the process of decolorization of RO16 dye (150 mg/L) in the mixed culture of P. ostreatus and C. zeylanoides,7-day-old cultures of the immobilized P. ostreatus were exposed to a yeast suspension and the decolorization compared with that of the control monocultures (Fig. 1). The decolorization in the mixed culture was more rapid than in the fungal monoculture, especially between days 5 and 11, the decolorization reaching a value of 87.5% on day 11, whereas in the fungal monoculture the decolorization was only 70%. Similar positive effect of the partner organism was observed on decolorization of the anthraquinone RBBR dye in mixed cultures of immobilized P. ostreatus with Rhodococcus erythropolis or activated sludge (Svobodová et al. 2016) or in mixed cultures of Trametes sp. SQ01 and Chaetomium sp. R01 degrading triphenylmethane dyes (Yang et al. 2011). Evidently, preformed fungal biofilms are rather resistant to adverse effects of other microorganisms as was also confirmed by works where biofilms of various white rot fungi were exposed to other microorganisms such as Gram-negative or Gram-positive bacteria or the yeast Saccharomyces cerevisiae without influencing the fungal degrading capacity (Gao et al. 2008; Válková et al. 2017; Šlosarčíková et al. 2017).
The decolorization by the shaken submerged culture of C. zeylanoides was more efficient than that of the fungal culture and reached 100% decolorization within 5 days (Fig. 1). However, the decolorization by the yeast was strongly inhibited by the presence of the fungus even though the yeast was still able to significantly contribute to the total decolorization capacity of the mixed culture by increasing the decolorization rate and the total percentage of the decolorized dye, compared with the fungal monoculture (Fig. 1). A relative shortage of nutrients in the mixed culture resulting from the consumption by the two organisms could be responsible for both the lower yeast cell counts observed (Table 1) and reduced efficiency of decolorization by the yeast (Knapp et al. 2008).
The inoculation of the fungal culture immobilized on the solid support with a massive suspension of C. zeylanoides followed by further incubation of the mixed culture at 28 °C for 11 days resulted only in a negligible decrease of the fungal dry biomass (Table 1) which was in agreement with the observations of the effects of bacteria P. fluorescens or B. licheniformis and of S. cerevisiae on biofilms of P. ostreatus and I. lacteus, respectively (Válková et al. 2017; Šlosarčíková et al. 2017).
In the control yeast monoculture, the yeast cell counts inoculated to the level of 1.106 CFU increased by two orders of magnitude within 9 days and the yeast growth was not affected by the presence of the dye (Table 1). When the yeast was added to the culture of P. ostreatus, the yeast cell numbers increased only about five times within 9 days, probably due to the competition for nutrients in the culture. This behavior was similar to that of the yeast S. cerevisiae when in coexistence with biofilms of I. lacteus (Šlosarčíková et al. 2017) but was in contrast to the behavior of P. fluorescens, B. licheniformis, and activated sludge bacteria whose CFU values were decreasing when added to preformed P. ostreatus biofilms (Svobodová et al. 2016; Válková et al. 2017).
Manganese-dependent peroxidase and laccase activities during decolorization of RO16
MnP and laccase activities have been shown to be involved in degradation of synthetic dyes by white rot fungi (Knapp et al. 2008). In the mixed culture of P. ostreatus and C. zeylanoides containing RO16, the initial level of laccase of about 30 U/L was maintained for 4 days and then the enzyme activity gradually decreased to attain a level of about 10 U/L at the end of the decolorization experiment (Fig. 2). A similar behavior was observed also in the absence of RO16 dye in the mixed culture. In comparison, the levels of laccase in P. ostreatus monocultures were increasing during the experiment, two-fold in the monoculture with RO16 and four-fold in the monoculture without RO16 (Fig. 2). This result clearly demonstrated a negative effect of the presence of the yeast on the fungal laccase activity in the mixed culture. This is in contrast to the observations of other studies that often reported an increase in the level of laccase as a result of the interaction with other microorganisms (Hiscox et al. 2010; Svobodová et al. 2016; Válková et al. 2017). The fact that the effect appeared in later phases of the experiment might suggest that lack of nutrients caused by a higher demand of the two organisms in the mixed culture could be responsible for this behavior (Knapp et al. 1997; Zhang et al. 1999). The results also demonstrated a negative effect of RO16 on the synthesis of laccase by P. ostreatus (Fig. 2).
Laccase activities in the mixed culture of P. ostreatus and C. zeylanoides and in the P. ostreatus monoculture during RO16 decolorization compared with the corresponding control cultures without the dye. Mixed culture of P. ostreatus and C. zeylanoides with RO16, ; mixed culture of P. ostreatus and C zeylanoides without RO16,
; mixed culture of P. ostreatus and C zeylanoides without RO16,  ; P. ostreatus monoculture with RO16,
; P. ostreatus monoculture with RO16,  ; P. ostreatus monoculture without RO16
; P. ostreatus monoculture without RO16
MnP activity in the P. ostreatus monocultures was steadily increasing or fluctuating in the range of 1–14 U/L, whereas in the presence of the yeast in the mixed cultures, the initial level of MnP was slowly decreasing to reach a negligible activity after day 7 (Fig. 3). Comparable results from other studies obtained with LF when confronted with other microorganisms are varied, ranging from a decrease of MnP (Rhodococcus erythropolis, Svobodová et al. 2016), no significant effect (activated sludge, Svobodová et al. 2016; P. fluorescens, B. licheniformis, Válková et al. 2017; S. cerevisiae, Šlosarčíková et al. 2017) to a five-fold increase (Chaetomium sp., Yang et al. 2011). The effect was depending on the culture medium (Svobodová et al. 2016) or the physical properties of the carrier material (Gao et al. 2008) where nonsterile conditions resulted either in an increase (polyurethane foam) or decrease (reticulated material) of the MnP level. An increase in MnP activity was also observed in majority of interactions of T. versicolor with other basidiomycetes on a solid medium (Hiscox et al. 2010). Similar to laccase, a negative effect of RO16 on the synthesis of MnP by P. ostreatus was demonstrated (Fig. 3).
Manganese-dependent peroxidase activities in the mixed culture of P. ostreatus and C. zeylanoides and in the P. ostreatus monoculture during RO16 decolorization compared with the corresponding control cultures without the dye. Mixed culture of P. ostreatus and C. zeylanoides with RO16, ; mixed culture of P. ostreatus and C. zeylanoides without RO16,
; mixed culture of P. ostreatus and C. zeylanoides without RO16,  ; P. ostreatus monoculture with RO16,
; P. ostreatus monoculture with RO16,  ; P. ostreatus monoculture without RO16
; P. ostreatus monoculture without RO16
The C. zeylanoides monoculture growing in the presence of RO16 was checked for the production of LiP, MnP, and laccase, but no activities were detected. No LiP activity was found in P. ostreatus cultures either. In spite of the decrease of the MnP and laccase activities during decolorization in the mixed culture, our results showed that both laccase and MnP activities were present during a significant part of the decolorization experiment with the mixed culture of P. ostreatus and C. zeylanoides, suggesting that they could take part in the degradation process.
GC-MS analysis of RO16 degradation products
The degradation of the dye RO16 by the fungus P. ostreatus and the yeast C. zeylanoides were followed by the analysis of degradation products by GC-MS. The characteristics of the products are listed in Table 2. In the monoculture of the fungus, the dye molecule was split into two parts. Three degradation products were found, namely 4-(ethenylsulfonyl) benzene, (methylsulfonyl) benzene, and 2-(phenylsulfonyl) ethanol. Additional data are given in Electronic Supplementary Material (Online Resource 1). The asymmetric cleavage of RO16 molecule and subsequent removal of hydrazine in the form of N2 probably provided 4-(ethenylsulfonyl) benzene (cf. Svobodová et al. 2007). The asymmetrical cleavage of azo bonds in azo dyes by fungal peroxidase and laccase was described in 1990’s (Spadaro and Renganathan 1994; Chivukula and Renganathan 1995). (Methylsulfonyl) benzene and 2-(phenylsulfonyl) ethanol were probably formed by demethylation and hydrolysis of the ethenyl double bond in 4-(ethenylsulfonyl) benzene, respectively (Fig. 5). Demethylase activities were found in various fungi having function in tolerance to toxic plant phytoalexins (Delserone et al. 1999; Coleman et al. 2011). 2-(phenylsulfonyl) ethanol was detected as a product of degradation of the azo dye Remazol Orange 3R by plant consortium of Aster amellius and Glandularia pulchella using laccase, peroxidase, and oxidase activities (Kabra et al. 2011). The other part of the RO16 molecule including two condensed aromatic rings (cf. Spadaro and Renganathan 1994) was probably rapidly oxidized to small molecules as no relevant degradation product was detected (Table 2). In the monoculture of C. zeylanoides, two compounds resulting from dye decolorization were detected. The azo bond was symmetrically split as also observed in studies using Galactomyces geotrichum and other yeasts (Jadhav et al. 2008; Jafari et al. 2014), and 4-(ethenylsulfonyl) aniline and α-hydroxybenzenepropanoic acid were detected as degradation products in the samples removed on days 3, 6, and 9.
Figure 4 showed that gradual decolorization of RO16 in the P. ostreatus monoculture between days 1–11 (Fig. 1) resulted in a transient accumulation of the degradation product 4-(ethenylsulfonyl) benzene that was further metabolized by the fungus using, for instance, hydrolytic or demethylation reactions (cf. Fig. 5).This behavior pointed to a more rapid early reactions producing 4-(ethenylsulfonyl) benzene, compared with those that further oxidized it in the degradation pathway, when working under the conditions of a relative abundance of the dye at the beginning of the decolorization process. Similar transient accumulation was also observed for 4-(ethenylsulfonyl) aniline, the product of degradation of the dye by C. zeylanoides. On day 6, when the dye was practically no more present in the medium, as evidenced by spectrophotometry (Fig. 1), the concentration of 4-(ethenylsulfonyl) aniline reached its maximum and was further transformed by the yeast metabolism between days 6–9, as documented by a decreased level of 4-(ethenylsulfonyl) aniline on day 11 (Fig. 4). Figure 4 thus documented that both degradation products were not end-products of degradation of RO16 by the fungal and yeast metabolism.
In the mixed culture of P. ostreatus and C. zeylanoides, only traces of 4-(ethenylsulfonyl) aniline and 4-(ethenylsulfonyl) benzene and no α-hydroxybenzenepropanoic acid were determined in the samples removed on days 3, 6, and 9, which suggested a more rapid metabolization of the fungal and yeast degradation products in the presence of the two microorganisms, compared with the two individual monocultures. Probably, the respective degradation activities of the partner microorganisms in the mixed culture contributed to a faster disappearance of the above-mentioned fungal and yeast products by working in a mutual cooperative mode. Generally, a fast degradation of RO16 by both microorganisms resulted in the detection of only a few metabolites resulting from the cleavage of the dye molecule. Possible pathways of RO16 biodegradation by the yeast C. zeylanoides and the fungus P. ostreatus were suggested (Fig. 5).
There were other organic compounds identified by GC-MS after the extraction of the culture liquid samples from the fungal and yeast cultures, i.e., benzenacetaldehyde, 4-methoxybenzaldehyde, benzaldehyde, 3-methyl-2(5H)-furanone, phenylethyl alcohol, 4,6-dimethoxy phthalide, and 1H-indol-3-ethanol. As they were detected also in the cultures where the dye was absent, they represented metabolites produced by the microorganisms in the growth medium used. Phenylethyl alcohol and 1H-indol-3-ethanol were described as volatile products in Pichia spp. (Mo et al. 2003), 4,6-dimethoxy phthalide was detected in the agaricomycete Lignomyces vetlinianus (Sazanova et al. 2018), fungal furanones are known to have antibiotic effects (Huff et al. 1994; Li et al. 2019), and benzeneacetaldehyde and 4-methoxybenzaldehyde are metabolites found in Pleurotus cultures (Kabbaj et al. 1997; Berger and Zorn 2004).
Conclusion
The mixed culture of the immobilized ligninolytic fungus P. ostreatus and the yeast C. zeylanoides exhibited a higher efficiency of decolorization of the recalcitrant RO16 monoazo dye in comparison with a monoculture of P. ostreatus. This finding, together with the products detected by GC-MS, showed that two parallel degradation processes were operating simultaneously, one realized by the fungus and the other by the yeast. A more rapid disappearance of the metabolites in the mixed culture, compared with the monocultures, documented a cooperative action of the two microorganisms probably resulting from their different enzyme activities involved in the dye degradation. The evidence of the cooperative action between the fungus and the yeast represents a novel finding as reports on such mixed cultures are rather rare. The compatibility of the two different biodegradation processes and resilience and stability of the mixed fungal-yeast culture support the concept of combining the activities of various microorganisms in biodegradation technology. The observed reduction of the yeast biodegradation capacity in the mixed culture however accentuated the importance of optimizing the conditions in the mixed culture for all microorganism partners.
References
Baldrian P (2004) Increase of laccase activity during interspecific interactions of white-rot fungi. FEMS Microb Ecol 50:245–253. https://doi.org/10.1016/j.femsec.2004.07.005
Berger RG, Zorn H (2004) Flavors and fragrances. In: Tkacz JS, Lange L (eds) Advances in fungal biotechnology for industry, agriculture, and medicine. Springer Science+Business Media, New York, pp 341–360. https://doi.org/10.1007/978-1-4419-8859-1
Boekhout T, Robert V (eds) (2003) Yeasts in food: beneficial and detrimental aspects. Behr's Verlag, Hamburg, p 322
Borchert M, Libra JA (2001) Decolorization of reactive dyes by the white rot fungus Trametes versicolor in sequencing batch reactors. Biotechnol Bioeng 75:313–321. https://doi.org/10.1002/bit.10026
Borràs E, Caminal G, Sarrà M, Novotný Č (2010) Effect of soil bacteria on the ability of polycyclic aromatic hydrocarbons (PAHs) removal by Trametes versicolor and Irpex lacteus from contaminated soil. Soil Biol Biochem 42:2087–2093. https://doi.org/10.1016/j.soilbio.2010.08.003
Ceci A, Pinzari F, Russo F, Persiani AM, Gadd GM (2019) Roles of saprotrophic fungi in biodegradation or transformation of organic and inorganic pollutants in co-contaminated sites. Appl Microbiol Biotechnol 103:53–68. https://doi.org/10.1007/s00253-018-9451-1
Chivukula M, Renganathan V (1995) Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl Environ Microbiol 61:4374–4377
Coleman JJ, Wasmann CC, Usami T, White GJ, Temporini ED, McCluskey K, VanEtten HD (2011) Characterization of the gene encoding pisatin demethylase (FoPDA1) in Fusarium oxysporum. Mol Plant Microbe Interact 24:1482–1491. https://doi.org/10.1094/MPMI-05-11-0119
Colin Slaughter J (1999) The naturally occurring furanones: formation and function from pheromone to food. Biol Rev Camb Philos Soc 74:259–276
De Jong E, Cazemier AE, Field JA, de Bont JAM (1994) Physiological role of chlorinated aryl alcohols biosynthesized de novo by the white rot fungus Bjerkandera sp. strain BOS55. Appl Environ Microbiol 60:271–277
Delserone LM, McCluskey K, Matthews DE, VanEtten HD (1999) Pisatin demethylation by fungal pathogens and nonpathogens of pea: association with pisatin tolerance and virulence. Physiol Mol Plant Pathol 55:317–326. https://doi.org/10.1006/pmpp.1999.0237
Erkurt EA, Erkurt HA, Unyayar A (2010) Decolorization of azo dyes by white rot fungi. In: Erkurt HA (ed) Biodegradation of azo dyes, Handbook of Environmental Chemistry. Springer-Verlag Berlin, Heidelberg, pp 157–167. https://doi.org/10.1007/698_2009_48
Gadd GM (ed) (2008) Fungi in bioremediation. Cambridge University Press, New York
Gao DW, Zeng YG, Wen XG, Qian Y (2008) Competition strategies for the incubation of white rot fungi under non-sterile conditions. Process Biochem 43:937–944. https://doi.org/10.1016/j.procbio.2008.04.026
Gou M, Qu Y, Zhou J, Ma F, Liang T (2009) Azo dye decolorization by a new fungal isolate, Penicillium sp QQ and fungal-bacterial cocultures. J Hazard Mater 170:314–319. https://doi.org/10.1016/j.jhazmat.2009.04.094
Hiscox J, Baldrian P, Rogers HJ, Boddy L (2010) Changes in oxidative enzyme activity during interspecific mycelial interactions involving the white-rot fungus Trametes versicolor. Fungal Genet Biol 47:562–571. https://doi.org/10.1016/j.fgb.2010.03.007
Huff T, Kuball H-G, Anke T (1994) 7-Chloro-4,6-dimethoxy-1(3 H)-isobenzofurane and basidalin: antibiotic secondary metabolites from Leucoagaricus carneifolia Gillet (Basidiomycetes). Z Natuforsch 49c:407–410
Jadhav JP, Parhetti GK, Kalme SD, Govindwar SP (2007) Decolorization of azo dye methyl red by Saccharomyces cerevisiae MTC 463. Chemosphere 68:394–400. https://doi.org/10.1016/j.chemosphere.2006.12.087
Jadhav SU, Kalme SD, Govindwar SP (2008) Biodegradation of methyl red by Galactomyces geotrichum MTCC 1360. Int Biodeterior Biodegradation 62:135–142. https://doi.org/10.1016/j.ibiod.2007.12.010
Jafari N, Soudi MR, Kasra-Kermanshahi R (2014) Biodegradation perspectives of azo dyes by yeasts. Microbiology 83:484–497. https://doi.org/10.1134/S0026261714050130
Kabbaj W, Bensonssan M, Roussos S (1997) Effets de la source d’azote sur la physiologie de croissance mycelienne et la production d’arome de champignons cultives sur support solide. In: Roussos S, Lonsane BK, Raimbault K, Viniegra-Gonzalez G (eds) Advances in solid state fermentation. Springer Science+Business Media, B.V, Dordrecht. https://doi.org/10.1007/978-94-017-0661-2
Kabra AN, Khandare RV, Waghmode TR, Govindwar SP (2011) Differential fate of metabolism of a sulfonated azo dye Remazol Orange 3R by plants Aster amellus Linn., Glandularia pulchella (sweet) Tronc. and their condortium. J Hazard Mater 190:424–431. https://doi.org/10.1016/j.jhazmat.2011.03.065
Kadam AA, Telke AA, Jagtap AS, Govindwar SP (2011) Decolorization of adsorbed textile dyes by developed consortium of Pseudomonas sp. UK1 and Aspergillus ochraceus NCIM-1146 under solid state fermentation. J Hazard Mater 189:486–494. https://doi.org/10.1016/j.jhazmat.2011.02.066
Knapp JS, Zhang F, Tapley KN (1997) Decolourisation of Orange II by a wood-rotting fungus. J Chem Technol Biotechnol 69:289–296
Knapp JS, Vantoch-Wood EJ, Zhang F (2008) Use of wood-rotting fungi for the decolorization of dyes and industrial effluents. In: Gadd GM (ed) Fungi in Bioremediation. Cambridge University Press, New York, pp 242–304
Krishnamoorthy R, Jose PA, Ranjith M, Anandham R, Suganya K, Prabhakaran J, Thiyageshwari S, Johnson J, Gopal NO, Kumutha K (2018) Decolourisation and degradation of azodyes by mixed fungal culture consisted of Dichotomomyces cejpii MRCH 1-2 and Phoma tropica MRCH 1-3. J Environ Chem Eng 6:588–595. https://doi.org/10.1016/j.jece.2017.12.035
Lapadatescu C, Feron G, Vergoignan C, Djian A, Durand A, Bonnarme P (1997) Influence of cell immobilization on the production of benzaldehyde and benzyl alcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens. Appl Microbiol Biotechnol 47:708–714
Leon A, Del-Angel M, Avila JL, Delgado G (2017) Phthalides: distribution in nature, chemical reactivity, synthesis, and biological activity. Prog Chem Org Nat Prod 104:127–246. https://doi.org/10.1007/978-3-319-45618-8_2
Li X, de Toledo RA, Wang SP, Shim H (2015) Removal of carbamazepine and naproxen by immobilized Phanerochaete chrysosporium under non-sterile condition. New Biotechnol 32:282–289. https://doi.org/10.1016/j.nbt.2015.01.003
Li M-F, Li G-H, Zhang K-Q (2019) Non-volatile metabolites from Trichoderma spp. Metabolites 9:58. https://doi.org/10.3390/metabo9030058
Libra JA, Borchert M, Banit S (2003) Competition strategies for the decolorization of a textile-reactive dye with the white-tor fungi Trametes versicolor under non-sterile conditions. Biotechnol Bioeng 82:736–744. https://doi.org/10.1002/bit.10623
Lomascolo A, Asther M, Navarro D, Antona C, Delattre M, Lesage-Meessen L (2001) Shifting the biotransformation pathways of L-phenylalanine into benzaldehyde by Trametes suavelolens CBS 334.85 using HP20 resin. Lett Appl Microbiol 32:262–267. https://doi.org/10.1046/j.1472-765X.2001.0873a.x
Matsumura E, Yamamoto E, Nemata A, Kawano T, Shin T, Murano S (1986) Structure of the laccase-catalyzed oxidation products of hydroxylbenzoic acids in the presence of ABTS (2, 2′- azino-di-(3- ethylbenzothiazoline-6-sulfatic acid)). Agric Biol Chem 50:1355–1357. https://doi.org/10.1080/00021369.1986.10867576
Mikesková H, Novotný Č, Svobodová K (2012) Interspecific interactions in mixed microbial cultures in a biodegradation perspective. Appl Microbiol Biotechnol 95:861–870. https://doi.org/10.1007/s00253-012-4234-6
Mishra A, Malik A (2014) Novel fungal consortium for bioremediation of metals and dyes from mixed waste stream. Bioresour Technol 171:217–226. https://doi.org/10.1016/j.biortech.2014.08.047
Mo EK, Kang HJ, Lee CT, Xu BJ, Kim JH, Wang QJ, Kim JC, Sung CK (2003) Identification of phenylethyl alcohol and other volatile flavor compounds from yeasts, Pichia farinosa SKM-1, Pichia anomala, SKM-T, and Galactomyces geotrichum SJM-59. J Microbiol Biotechnol 13:800–808
Novotný Č, Svobodová K, Erbanová P, Cajthaml T, Kasinath A, Lang E, Šašek V (2004) Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biol Biochem 36:1545–1551. https://doi.org/10.1016/j.soilbio.2004.07.019
Plachá D, Vaculík M, Mikeska M, Dutko O, Peikertová P, Kukutschová J, Mamulová Kutláková K, Růžičková J, Tomášek V, Filip P (2017) Release of volatile organic compounds by oxidative wear of automotive friction materials. Wear 376:705–716. https://doi.org/10.1016/j.wear.2016.12.016
Ramalho PA, Scholze H, Cardoso MH, Ramalho MT, Oliveira-Campos AM (2002) Improved conditionsfor the aerobic reductive decolourisation of azo dyes by Candida zeylanoides. Enzym Microb Technol 31:848–854
Ramalho PA, Paiva S, Cavaco-Paulo A, Casal M, Cardoso MH, Ramalho MT (2005) Azo reductase activity of intact Saccharomyces cerevisiae cell is dependent on the Fre 1p component of plasma membrane ferric reductase. Appl Environ Microbiol 71:3882–3888. https://doi.org/10.1128/AEM.71.7.3882-3888.2005
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2009) Decolorization and biodegradation of textile navy blue HER by Trichosporon begelii NCIM- 3326. J Hazard Mater 166:1421–1428. https://doi.org/10.1016/j.jhazmat.2008.12.068
Sazanova KV, Psurtseva NV, Shavarda AL (2018) Cultural and metabolomic studies of a new phthalides producer, Lignomyces vetlinianus (Agaricomycetes). Int J Med Mushrooms 20:1031–1045. https://doi.org/10.1615/IntJMedMushrooms.2018028687
Šlosarčíková P, Novotný Č, Malachová K, Válková H, Fojtík J (2017) Effect of yeasts on biodegradation potential of immobilized cultures of white rot fungi. Sci Total Environ 589:146–152. https://doi.org/10.1016/j.scitotenv.2017.02.079
Spadaro JT, Renganathan V (1994) Peroxidase-catalyzed oxidation of azo dyes: mechanism of disperse Yellow 3 degradation. Arch Biochem Biophys 312:301–307. https://doi.org/10.1006/abbi.1994.1313
Spina F, Romagnolo A, Prigione V, Tigini V, Varese GC (2014) A scaling-up issue: the optimal bioreactor configuration for effective fungal treatment of textile wastewaters. Chem Eng Trans 38:37–42. https://doi.org/10.3303/CET1438007
Svobodová K, Senholdt M, Novotný Č, Rehorek A (2007) Mechanism of reactive Orange 16 degradation with the white rot fungus Irpex lacteus. Process Biochem 42:1279–1284. https://doi.org/10.1016/j.procbio.2007.06.002
Svobodová K, Petráčková D, Kozická B, Halada P, Novotný Č (2016) Mutual interactions of Pleurotus ostreatus biofilms with bacteria of activated sludge in solid-bed bioreactors. World J Microbiol Biotechnol 32:94. https://doi.org/10.1007/s11274-016-2050-3
Tan L, He M, Song L, Shi S (2016) Aerobic decolorization, degradation and detoxification of azo dyes by a newly isolated salt-tolerant yeast Scheffersomyces spartinae TLHS-SF1. Bioresour Technol 203:287–294. https://doi.org/10.1016/j.biortech.2015.12.058
Tien M, Kirk TK (1988) Lignin peroxidase from Phanerochaete chrysosporium. Methods Enzymol 161:238–249. https://doi.org/10.1016/0076-6879(88)61025-1
Válková H, Novotný Č, Malachová K, Šlosarčíková P, Fojtík J (2017) Effect of bacteria on the degradation ability of Pleurotus ostreatus. Sci Total Environ 548-585:1114–1120. https://doi.org/10.1016/j.scitotenv.2017.01.171
Wang BC, Chua S-L, Cai Z, Sivakumar K, Zhang Q, Kjelleberg S, Cao B, Loo SCJ, Yang L (2014) A stable synergistic microbial consortium for simultaneous azo dye removal and bioelectricity generation. Bioresour Technol 155:71–76. https://doi.org/10.1016/j.biortech.2013.12.078
Wang Y, Shao H, Zhu S, Tian K, Qiu Q, Huo H (2019) Degradation of 17β-estradiol and products by a mixed culture of Rhodococcus equi DSSKP-R-001 and Comamonas testosteroni QYY20150409. Biotechnol Biotechnol Equip 33:268–277. https://doi.org/10.1080/13102818.2019.1568913
Yang Q, Yediler A, Yang M, Kettrup A (2005) Decolorization of an azo dye, Reactive Black 5 and MnP production by yeast isolate: Debaryomyces polymorphus. Biochem Eng J 24:249–253. https://doi.org/10.1016/j.bej.2004.12.004
Yang X, Wang J, Zhao X, Wang Q, Xue R (2011) Increasing manganese peroxidase production and biodecolorization of triphenylmethane dyes by novel fungal consortium. Bioresour Technol 102:10535–10541. https://doi.org/10.1016/j.biortech.2011.06.034
Zhang F, Knapp JS, Tapley KN (1999) Decolourisation of cotton bleaching effluent with wood rotting fungus. Water Res 33:919–928. https://doi.org/10.1016/S0043-1354(98)00288-7
Funding
This work was supported by EU programs (BIOCLEAN No. 312100, OPVK CZ.1.07/2.3.00/30.0019), Ministry of Education, Youth and Sports of the Czech Republic (Institutional Research Concept RVO 61388971, SP2019/23), University of Ostrava (SGS17/PřF/2017), and ERDF IET-Excellent Research (No. CZ.02.1.01/0.0/0.0/16_019/0000853).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 332 kb)
Rights and permissions
About this article
Cite this article
Šlosarčíková, P., Plachá, D., Malachová, K. et al. Biodegradation of Reactive Orange 16 azo dye by simultaneous action of Pleurotus ostreatus and the yeast Candida zeylanoides. Folia Microbiol 65, 629–638 (2020). https://doi.org/10.1007/s12223-019-00767-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00767-3


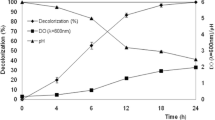


 ), monoculture of P. ostreatus (
), monoculture of P. ostreatus ( ), monoculture of C. zeylanoides (
), monoculture of C. zeylanoides ( ). Starting yeast concentration of 106 CFU/mL, the dye was used at 150 mg/L
). Starting yeast concentration of 106 CFU/mL, the dye was used at 150 mg/L


 and 4-(ethenylsulfonyl) aniline
and 4-(ethenylsulfonyl) aniline  in P. ostreatus and C. zeylanoides monocultures during decolorization of RO16
in P. ostreatus and C. zeylanoides monocultures during decolorization of RO16