Abstract
The creation of mega-hydropower dams inundates vast lowland areas, causing widespread environmental impacts in tropical forest regions. Few studies, however, have taken advantage of these newly fragmented landscapes to examine the effects of habitat insularization on arthropod faunas. Here, we assess how dung beetle assemblages respond to 30 years of post-isolation history in forest islands within a major hydroelectric reservoir in Central Amazonia. We sampled 30 of the 3546 islands created by this reservoir, and three neighbouring forest sites. We collected a total of 865 individuals representing 34 dung beetle species and 15 genera. Remarkably, one third of all islands had been entirely defaunated of dung beetles in terms of overall occupancy. Isolation was the single best predictor of dung beetle species richness, followed by the interaction between isolation and island area, and these variables were key determinants of the relict species composition. Isolation was the most important predictor of dung beetle abundance, but area alone was the main predictor of abundance when the dominant species was excluded. We predicted species richness across all 3546 islands, indicating that 61.5% of all islands likely retain only a single ‘super-tramp’ species (Onthophagus osculatii). These community disassembly patterns were likely aggravated by the marked hostility of the open-water matrix combined with the poor flight dispersal capacity of dung beetles over wide gaps between insular forests. As such, the overwhelming number of small, isolated islands created by major dams has profound effects on regional forest biodiversity, including wholesale local extinctions in detritivore assemblages and their ecosystem functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and degradation due to land-use change is the primary cause of biodiversity decline throughout terrestrial ecosystems worldwide (Pereira et al. 2010). Forest loss almost invariably leads to habitat fragmentation, which results in the overall reduction in habitat area, increased isolation and greater amounts of remaining habitat area under new biotic and abiotic edge-dominated conditions (Fahrig 2003). The effects of habitat fragmentation are species-specific, but in tropical forest regions typically have a detrimental impact on overall species richness, abundance, species composition and subsequent ecological processes within forest remnants (Haddad et al. 2015; Pfeifer et al. 2017). Fragmentation studies usually focus on anthropogenic landscapes formed by expanding croplands and cattle pastures (Laurance et al. 2014). However, the implementation of major hydropower dams flooding vast upstream areas has become a key emergent driver of tropical forest biodiversity loss (Finer and Jenkins 2012; Lees et al. 2016).
Upland inundation caused by river dams results in several social, economic, environmental, and hydrological impacts (Zarfl et al. 2015; Fearnside 2016), but the exact mechanisms of biodiversity loss are still poorly understood. Hydropower dams modify animal and plant communities, isolate remaining habitat patches and profoundly affect the newly created habitat matrix. This has been shown for birds (Bueno and Peres 2019), plants (Rocha-Santos et al. 2017), primates (Benchimol and Venticinque 2014), large vertebrates (Cosson et al. 1999; Benchimol and Peres 2015a, b), fishes (Agostinho et al. 2011), bats (Meyer and Kalko 2008), harvestmen (Tourinho et al. 2019) and bees (Storck-Tonon and Peres 2017). Hydroelectric reservoirs can transform large tracts of primary forests into vast lakes with many forest islands on higher elevation forming an archipelago of forest patches. This pattern of fragmentation leads to more hostile isolation due to the dominant inhospitable open-water matrix, which is far more difficult for forest organisms to permeate compared to terrestrial habitat matrices elsewhere (Mendenhall et al. 2014). However, hydropower reservoirs provide an excellent opportunity for quasi-experimental fragmentation ecology studies on the drivers of local extinctions that are otherwise difficult to quantify (Diamond 2001; Terborgh et al. 2001).
Dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaidae) have a near-global distribution but their highest diversity is found in tropical forests (Scholtz et al. 2009; Frank et al. 2018). They make up an extremely important functional group in performing several ecological roles, such as nutrient cycling, bioturbation, plant growth enhancement and secondary seed dispersal (Nichols et al. 2008). Furthermore, due to their high sensitivity to habitat transformation, straightforward sampling, well-documented alpha-taxonomy, broad distribution and congruent landscape responses with other co-occurring taxa, dung beetles are an ideal group to study the impacts of landscape alteration and ecosystem functions (Spector 2006). Due to their almost strict dependence on mammalian fecal resources for nesting, dung beetles are more susceptible to the effects of forest loss through trophic cascades induced by losses in mammal diversity (Nichols et al. 2009). Therefore, they are considered an excellent bioindicator of habitat transformation and have become an important group for conservation ecology studies (Spector 2006; Nichols and Gardner 2011).
Researches on the effects of habitat loss and landscape fragmentation have used dung beetles as a model system for decades (Nichols et al. 2007; Filgueiras et al. 2016; Silva et al. 2016). Among the main predictors of alpha and beta diversity in fragmented landscapes are habitat patch size (Filgueiras et al. 2011, 2015; Larsen et al. 2008), the structure and quality of the remaining habitat (Halffter and Arellano 2002; Silva et al. 2016; Costa et al. 2017, Bitencourt et al. 2019), and the combination of patch size, landscape forest cover, and matrix composition (Sánchez-de-Jesús et al. 2016; Pinto Leite et al. 2018; da Silva et al. 2019).
Despite a large body of literature on dung beetle responses to habitat degradation and/or fragmentation, few studies have focused on dung-beetle responses to forest fragmentation in landscapes dominated by an uniform aquatic matrix (but see Feer and Hingrat 2005; Larsen et al. 2008; Qie et al. 2011; Nunes et al. 2014). Here, we assess how local dung beetle assemblages responded to ~ 30-years of forest patch isolation in a vast man-made reservoir formed by the Balbina Hydroeletric Dam in Central Brazilian Amazonia. We sampled 30 of the 3546 islands contained by this 312,900-ha reservoir, and three undisturbed neighbouring forests in the adjacent mainland areas. Specifically, we examine the following related questions: (i) how do spatial attributes at both the patch and landscape scale (amount of surrounding forest, degree of isolation and forest structure) affect dung beetle species richness and abundance? (ii) Is there an island size and isolation threshold governing the extinction dynamics of dung beetles? and (iii) How does dung beetle community structure on forest islands diverge from those in mainland continuous forests? Finally, we show the scale of defaunation and attendant projected losses of key ecosystem services, which may be induced by large-scale habitat loss and fragmentation associated with hydroelectric dams in tropical forest regions.
Methods
Study area
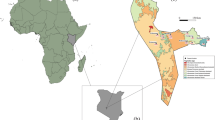
The Balbina Hydroeletric Reservoir (BHR) is located ~ 80 km northeast of Manaus within the Uatumã river basin, a tributary of the Amazon (1°48′S; 59°29′W; Fig. 1, Table S1). The BHR Dam was closed in October 1987 (Fearnside 1989) and inundated 312,900 ha of primary forests, forming 3,546 land-bridge islands with sizes ranging from < 1 to 4878 ha (Benchimol and Peres 2015b). The 940,000-ha Uatumã Biological Reserve, the largest of its kind in Brazil, was then created in 1990 to protect most islands and the right bank of the Uatumã river. The dominant vegetation is sub-montane closed-canopy dense forests. The average annual rainfall and temperature is ~ 2776 mm [range = 2113–2716 mm] and 28 °C [range = 21–35 °C], respectively (Benchimol and Peres 2015b).
Study landscape showing the spatial distribution of the trapping sites (solid circles) throughout the Balbina Hydroelectric Reservoir (BHR) landscape of Central Brazilian Amazonia. Three mainland sites (blue triangles) and 30 [of the 3546] islands (red circles) across the reservoir archipelago were sampled. Surveyed islands, unsurveyed islands, and surrounding areas of undisturbed continuous forest are shown in dark grey, light grey and dark green, respectively
We sampled a subset of 30 islands and three mainland continuous forest sites adjacent to the reservoir that were spaced apart by at least 1 km. Islands were selected to span the widest possible range of size and degree of isolation (Benchimol and Peres 2015a). The area of mainland sites sampled was arbitrarily defined as one order of magnitude larger than our largest study island. Forest islands sampled in this study ranged from 0.83 to 1466 ha, and straight-line isolation distances to the nearest mainland forest ranged from 44 m to 17,400 m.
Dung beetle sampling
Dung beetles were sampled within the wider BHR landscape between July and August 2016. We used a standardized pitfall trap protocol (1 L plastic container, 15-cm diameter and 8-cm depth) baited with 20 g of a homogeneous mixture of 70% pig and 30% human dung (Marsh et al. 2013). Traps were operated in the field for 48 h. We installed 10 pitfall traps on each of the 30 island and three mainland areas, amounting to 330 traps. To minimize interference between traps, pitfall traps were spaced apart by at least 100 m (da Silva and Hernández 2015). However, on small islands that did not permit between-trap spacing of > 100 m, we reduced the distance between traps in proportion to island size, aiming to retain the same overall sampling effort on every island. All collected specimens were preserved in 97% alcohol, and then sorted from other arthropods, dried and identified to at least the genus level. Species level identifications were subsequently implemented on the basis of vouchers deposited at the Scarabaeinae Collection at the Universidade Federal de Mato Grosso (UFMT), Cuiabá, Brazil (CEMT), the largest dung beetle collection in South America. Voucher specimens were also deposited at the Entomological Collection of Universidade do Estado de Mato Grosso (UNEMAT) in Tangará da Serra, with replicate specimens deposited at the CEMT collection.
Landscape and patch variables
We obtained local habitat variables to describe vegetation structure and habitat quality based on floristic surveys within 87 plots (0.25 ha) plots established at each sampled island and mainland site (Table S2; Benchimol and Peres 2015a). We also measured a set of environmental variables at both the habitat patch and landscape scales using an optimized 6980-km2 RapidEye® seamless mosaic (5-m pixel resolution) of georeferenced satellite imagery overlapping the entire BHRL study area, including 28 juxtaposed tiles obtained from March 2011 to September 2012. Using a semi-supervised classification, we classified four land cover classes: closed-canopy forest, open-canopy forest, bare ground and open water.
At the patch scale, we quantified total island area; island forest area; the proportional area (%) of closed-canopy forest; and island shape. Within floristic plots at each island and mainland site, we also quantified the degree of forest fire disturbance detected by char marks on standing dead and live trees (> 30 cm DBH), the abundance, basal area, species richness, density and Simpson diversity index of live tree species, and the percentage of old-growth trees that had been retained from the pre-flooding primary forest.
In terms of landscape metrics, we quantified the shortest straight-line distance between each island and the nearest mainland site. Within external buffer areas with a radius of 500-m, 1000-m and 2000-m, we calculated a proximity index that considers both area and isolation of each land mass within these buffers. We then quantified the percentage of closed-canopy forest, the number of neighbouring forest patches larger than 10 ha and the percentage of closed-canopy forest within these buffers. However, each of these landscape metrics were highly correlated (r > 0.9) across the three buffer sizes, so we used a 500-m buffer area, which provided the best overall explanatory power across all models. For further details on our landscape metrics, see Benchimol and Peres (2015a).
Data analysis
Species richness and abundance
We initially ordered all species sampled on islands and continuous forest sites by decreasing abundance. These results are displayed in bar plots only. Multicollinearity among variables was tested using variation inflation factors (VIF) (Dormman et al. 2013) using the ‘HH’ package (Heiberger, 2016), removing the least moderately redundant or collinear variables (VIF > 6). Using Generalized Linear Models (GLMs), we then attempted to explain patterns of dung beetle species richness, abundance and species composition using on the basis of the following covariates: forest patch area (log10 + 1) (Area), isolation distance from the nearest mainland site (Isol), the interaction between these two variables (Area × Isol), the proportion of forest cover within a 500-m buffer outside the perimeter of each site (F. Cover), the incidence and severity of fires measured as four categorical classes from 0 to 3 (Fire), the proximity among islands within the 500-m buffer (Prox), and the percentage of old-growth trees (OGT) retained within each 0.25-ha plot. For GLMs used to examine overall species richness and abundance we used either a Poisson or a Quasi-Poisson error structure when over-dispersion had been detected. All candidate model sets were further assessed using all combinations of the six main explanatory variables retained, plus the Area × Isol interaction. Models were subsequently ranked according to their Akaike Information Criterion for small sample sizes (AICc), using the MuMIn R package (Barton 2018).
We selected the most parsimonious “best” models (ΔAICc < 2.0) based on a multi-model approach and the AICC (or QAICC for Quasi-Poisson) (Burnham et al. 2011). We defined variables as significant within candidate models if their estimated 95% confidence intervals that did not include zero (Kiffner et al. 2013). To further demonstrate the contribution of each variable to overall model variance, we adopted the hierarchical partition method (Murray and Conner 2009). During the analysis we noted that our abundance GLM results had been affected by the occurrence of Onthophagus osculatii, a hyper-abundant species (51.9% of the overall abundance). For this reason, we also performed GLM analyses using the abundance of O. osculatii as an additional response variable. In addition, we performed all GLMs both including and excluding the incidence and abundance data for O. osculatii. Due to the significant importance of fires in the GLMs, we also examined the interaction between Fire and Area, as well as Fire and measures of isolation using GLMs. We note that Fire showed a significant interaction only with island isolation (Isol). Finally, when the interaction between any two variables was significant (95% CIs did not include zero), we performed subsequent ‘clean-models’ with only the significant variables. This occurred only for models based on species richness and the Area × Isol interaction, and the abundance of O. osculatii with the Isol × Fire interaction. These results were displayed by inserting the GLM coefficients onto the plots. To better visualize these results, we log-transformed (log10 x + 1) the abundance of O. osculatii.
Finally, we used the predict.glm function of the stats R package on the basis of the species richness as a function of the interaction between patch area and isolation (Area × Isol) for all 30 surveyed islands and tree mainland sites to predict the unknown species richness for all 3546 islands across the wider Balbina reservoir. This scaling-up exercise therefore enabled us to predict the current status of dung beetle defaunation across all islands within the Balbina Hydroelectric Reservoir, and examine how this may be related to other patch and landscape variables.
Species composition
The multivariate pattern of dung beetle species composition across all islands and continuous forest sites was explored using Principal Coordinates Analysis (PCoA). We used log10 (x + 1) standardized abundance data and the Bray–Curtis dissimilarity index in all analyses of species composition. Despite the renowned efficiency of our pitfall trapping technique, we failed to record any dung beetles at 10 small islands, so to demonstrate this defaunation effect, we created a “dummy species” to force completely defaunated islands to show a single common ‘species’, with the same abundance, with the aim of representing them as identical in terms of species composition and show the same difference in relation to areas any other surveyed site (Clarke et al. 2006). All analyses were performed using the vegan R package (Oksanen et al. 2018). We further examined the effects of environmental variables on species composition by assuming the first axis of PCoA as an independent variable. This first axis explained 49% of the overall variation in the data. We followed the same analytical approach using a global GLM model including all environmental covariates. We then simplified those models (ΔAICc < 2) using the hierarchical partition approach.
We checked for spatial autocorrelation using the Mantel test in the vegan package (Oksanen et al. 2018). These tests were performed using the log10 (x + 1) abundance data based on the Bray–Curtis distance, and using incidence (presence/absence) data, based on the Jaccard distance. Mantel tests failed to detect any meaningful spatial autocorrelation whether we considered the abundance (r = 0.04, P > 0.05) or presence/absence data (r = 0.03, P > 0.05).
We further examined whether the species composition on small islands containing dung beetles were a subset of that in large islands, and whether species on all islands were a subset of those in the mainland sites. To do so, we used nestedness indices based on NODF values (nestedness based on overlap and decreasing fills, ranging from 0.0 to 1.0) to measure the level of overall nestedness in the dung beetle assemblages (Almeida-Neto et al. 2008; Almeida-Neto and Ulrich 2011). NODF values were then tested using a computer simulation with 1000 randomizations. All analyses were performed using the vegan package (Oksanen et al. 2018) in R v.3.5.1 (R Development Core Team 2018).
Results
Insular dung-beetle faunas
On the basis of the 330 pitfall traps operated throughout the Balbina Hydroelectric Reservoir landscape, we collected a total of 865 dung beetles representing 15 genera and 34 species. Considering all 30 islands surveyed, we collected a total of 678 individuals belonging to 28 species and 14 genera, including 11 species that were restricted to forest islands: Canthidium deyrollei, C. sp.1, C. sp.2, Canthon aff. juvencus, Cryptocanthon peckorum, Dichotomius mamillatus, D. robustus, D. subaenus, Ontherus carinifrons, Onthophagus sp.1 and Oxysternon festivum viridanum. We failed to record any dung beetles in one third of the surveyed islands, all of which were smaller than 100 ha, and only five of all islands contained more than five dung beetle species. Considering the three mainland sites, we collected 187 individuals belonging to 23 species and 12 genera, including five species that were not recorded in any of the islands: Deltochilum sp.2, Eurysternus ventricosus, Phanaeus cambeforti, Scybalocanthon cyanocephalus and Sinapisoma sp. A total of 18 species were recorded both on islands and mainland sites (see Fig. 2). Onthophagus osculatii was by far the most abundant species, accounting for over half (51.9%) of all individuals sampled across all island and mainland sites (Fig. 2).
Species richness was negatively affected by both forest patch isolation and size, and we detected a positive interaction between island isolation and size (Fig. 3a, Table S3). This interaction indicates that the negative effects of distance from any mainland continuous forest on species richness are reduced within increasingly larger islands. Conversely, small and highly isolated islands contained fewer dung beetle species than similar-sized islands that were less isolated (Fig. 3b), and this relationship becomes apparent on increasingly larger islands.
Model averaging with candidate models within AICc < 2, for species richness of dung beetles (a). Mean ± 95% confidence intervals of regression coefficients obtained from GLMs are presented on the left side and the independent effects contribution are presented on the right side. Relationship between the interaction of area and isolation and dung beetle species richness (b). Solid line = null isolation; dotted line = low-isolation islands, and dashed lines = highly isolated islands. Red circles = fully defaunated islands; dark green = mainland forest sites; grey and dotted line = largest island size. Area = Island Area (ha, log10 x + 1), Isol = distance to the nearest mainland continuous forest site; Prox = Proximity index, F.Cover = Percentage of forest cover within a 500-m buffer
Isolation distance (Isol) also had the strongest negative effect on the overall numerical abundance of dung beetles, explaining 33.6% of the variation. In contrast, three other variables—fire severity, percentage of old-growth trees, and the amount of surrounding forest cover—had a positive effect on abundance (Fig. 4a, Table S3). However, only forest island size showed a marked effect on overall abundance (accounting for 83.6% of the explanatory power) when the most hyper-abundant species (Onthophagus osculatii) was excluded from GLMs (Fig. 4b). Considering the abundance of O. osculatii alone, fire severity had a positive effect (explaining 48% of the variance), followed by a negative effect of isolation and a positive effect of old-growth tree abundance and the interaction between isolation and fire severity (Fig. 4c). The most severely burnt forest islands contained the highest abundance of this hyper-dominant species. However, the importance of fires on O. osculatii abundance decreased with increasing isolation.
Model averaging of candidate models within AICc < 2, considering the abundance (log10 x + 1) of all dung beetle species (a); abundance of all species except for Onthophagus osculatii (b); abundance of O. osculatii only (c). Mean ± 95% confidence intervals of regression coefficients obtained from GLMs are presented on the left side and the independent effects contribution are presented on the right side. Relationship between the interaction of fire and isolation for O. osculatii abundance (d). Solid line = sites with high incidence of fires; dotted lines = unburnt and rarely burnt sites. Red circles = defaunated islands; dark green = mainland sites; Area = Island area (ha, log10 x + 1), Isol = distance to the nearest mainland area; Prox = Proximity index, F.Cover = Percentage of forest cover in a 500-m buffer; Fire = fire incidence index (ranked 0 to 3)
Three variables had the strongest effects on dung beetle species composition: isolation, percentage of surrounding forest cover and the island size × isolation interaction (Fig. 5a). The clear difference between entirely defaunated islands and other sites was captured along the first PCoA axis. The variation in species composition between mainland sites and islands retaining at least some dung beetles was captured along the second PCoA axis. These two axes captured 50% of the overall variation in species composition (Fig. 5b).
a Model averaging of candidate models within AICc < 2, explaining the variation in dung beetle species composition, summarized as the first PCoA axis. Averaged coefficients are presented on the left side and the independent effects contributions are presented on the right side. Area = Island area (ha, log10 x + 1), Isol = distance to the nearest mainland area, OGT = Percentage of Old Growth Trees within each area; Fire = fire incidence index. b Principal Coordinate Analysis ordination based on the Bray–Curtis similarity matrix of dung beetle species composition on islands (light green circles), mainland sites (dark green circles) and defaunated islands (red circles) sampled at the BHR landscape. Size of circles represents the island size. c Nested matrices of all dung beetle species on islands and mainland sites
The 72.1-ha Relógio island was the largest island on which we failed to record any dung beetles, whereas all other defaunated islands were smaller than 14 ha. However, Relógio was a highly isolated island, at least 8089 m from any mainland continuous forest. With the exception of Jiquitaia, all entirely defaunated islands showed high levels of isolation, and were at least 2900 m from the mainland (Fig. 5c). In terms of species composition, dung beetle assemblages had low levels of nestedness across sites (matrix size: 1054, fill = 0.11; NODFrow = 28.93, P < 0.05, Fig. 5c).
How defaunated are forest islands?
Our study indicates that this archipelagic forest landscape has become severely impoverished of its dung beetle fauna over the ~ 30 year history of post-flooding habitat fragmentation, which was almost certainly induced by pervasive and widespread local extinctions within islands. We predicted the local dung beetle species richness for all > 3500 islands that we did not survey on the basis of the model interaction between island size and isolation. This indicates that 61.5% of all islands across the Balbina Reservoir are either completely defaunated or could only retain a single small-bodied dung beetle species (Onthophagus osculatii). We predicted that 69.8% of all islands could support four dung beetle species or fewer, and only two of all 3546 islands in the entire reservoir could retain at least 50% of all species detected in this study (Fig. 6a). However, even small to medium-sized islands could support a meaningful fraction of the overall species richness, provided that they were relatively near mainland areas of continuous forest (Fig. 6b). Finally, assuming that in their original pre-flooding condition all forest island sites sampled contained the full set of dung beetle species found throughout the Balbina landscape, we estimated that an approximate total of 109,223 local extinctions occurred across all 3546 islands, representing an overall level of extirpation of 90.6% of all 120,564 insular dung beetle populations across the Balbina Reservoir.
Discussion
This study reveals a marked erosion of the overall abundance and species diversity of tropical forest dung beetles induced by a large hydroelectric dam following a ~ 30-year post-isolation period. We highlight that the best predictor of dung beetle diversity was an interaction between island size and degree of isolation, which is consistent with the general principles of island biogeography theory (MacArthur and Wilson 1967). Our metacommunity model shows that island size in itself was insufficient to best explain the number of dung beetle species they retained, with island isolation making a even greater contribution to patterns of species diversity than models based on island area alone (Leibold et al. 2004). Habitat patch area and isolation have been identified as the most important determinants of dung beetle communities on fragmented forest landscapes across the globe (Nichols et al. 2007). However, other habitat patch and landscape variables are also considered to be important, including matrix quality, landscape-scale forest cover (Sánchez-de-Jesús et al. 2016), successional stages (Bitencourt et al. 2019), anthropogenic fires (Andrade et al. 2011, 2014; Smith et al. 2018), selective logging (França et al. 2016), and trophic-cascades involving depletion of mammalian fecal resources (Culot et al. 2013; Nichols et al. 2013a, b; Bogoni et al. 2019).
At the Balbina landscape, both the overall abundance and species richness of dung beetles were lower than those reported by neotropical studies elsewhere assessing the effects tropical of forest fragmentation on dung beetle assemblages (Scheffler 2005; Horgan 2007; Nichols et al. 2007; Quintero and Halffter 2009; Filgueiras et al. 2015, 2016; Silva et al. 2016). However, the vast majority of habitat fragmentation studies on dung beetles have focused on landscapes where forest remnants are embedded within a terrestrial habitat matrix exhibiting varying degrees of permeability. These studies found that dung beetles defined as forest specialists rarely traverse through an open-habitat matrix (Silva et al. 2016; Pinto Leite et al. 2018), and matrices dominated by closed-canopy habitat are more permeable to dung beetles moving between remnants (Sánchez-de-Jesús et al. 2016). For example, da Silva et al (2019) found no significant effects of either habitat patch or landscape metrics on dung beetle community structure, but they found strong effects on forest specialists and concluded that matrix-tolerant species can mask the effects on forest-specialists species. However, the high-elevation forest remnants they studied were surrounded by grasslands that were more permeable compared to an equally hostile non-habitat matrix consisting of open water as in this study. In our study, 11 species were only detected on islands. We believe that these species are present in the mainland continuous forest but failed to be detected due to lower trap density. Therefore, had the number of pitfall traps been proportional to the area of each study site, species richness in mainland areas would have been greater and the effects of island size and isolation on the beetle assemblages would be even stronger, thereby rendering our results conservative.
Man-made forest archipelagos created by hydroelectric dams provide a rare opportunity to address the ecological effects of forest fragmentation, while controlling for both the degree of matrix permeability and history of isolation (Diamond 2001). As such, the structurally uniform aquatic matrix at Balbina has clear advantages compared to other fragmentation studies in terrestrial landscapes in which high levels of matrix heterogeneity can substantially mitigate isolation effects (Prugh et al. 2008). Additionally, all Balbina islands were isolated simultaneously imposing comparable lag times as forest organisms readjust to a new landscape configuration at all remnant sites.
We documented a strong effect of isolation from the freshwater matrix on insular dung beetle assemblages. We found that either isolation alone or the interaction between isolation and island size were the best predictors of dung beetle species richness. This indicates that the most isolated islands were unlikely to be recolonized in the event of any local extinctions, regardless of their size. This is reflected in the high level of βturn-diversity (79%), and further associated with the inherently low capacity of dung beetles to move across large aquatic matrix gaps, translating low levels of structural connectivity into high levels of functional isolation in dung beetle assemblages (da Silva and Hernández 2015). These disjunct assemblages form the first step towards inbreeding depression, which can aggravate local to regional-scale extinctions in small isolated populations within a larger non-equilibrium metapopulation (Harrison and Hastings 1996). Where gene flow is severed across habitat fragments, populations tend to gradually become more extinction-prone, and in this case no longer compensated for by even rare recolonization events. This process can eventually lead to severe landscape scale erosion of terrestrial biodiversity (Akçakaya et al. 2007).
Dung beetle defaunation at Balbina was related to several drivers related to island size, isolation, or both. These include (i) abrupt changes in microclimatic conditions of novel forest habitat on small islands, which render several forest-affiliated species much more extinction-prone (Klein 1989); (ii) extinction of medium to large bodied terrestrial and arboreal mammals (Benchimol and Peres 2015b; Palmeirim et al. 2018), which are the principal sources of fecal resources, particularly for large dung beetles; and (iii) severe reductions in population size associated with small islands and/or lack of recolonization from the mainland and large islands on more isolated islands.
In addition, the marked importance of island isolation on both species richness and overall abundance is almost certainly reflected in the low gap-crossing capacity of dung beetles over open-water as they are inherently unable to fly long distances (da Silva and Hernández 2015). While some species may travel long distances (e.g. a mark-recapture study showed that Oxysternon conspicillatum can move at least 1 km (Peck and Forsyth 1982), larger-bodied species typically exhibit fast cruise flights, which hinder flight maneuverability (Chittka et al. 2009). The erratic feature of the flight dynamics in large dung beetles (Howden and Nealis 1975) can increase their mortality rate due to incidental falls on water during any attempt to reach neighbouring islands. Small-bodied species, on the other hand, typically fly short distances (e.g. Onthophagus and Canthon, da Silva and Hernández 2015) and exhibit different foraging strategies, such as perching on understorey leaves while “waiting” for fecal resources to become available in the immediate vicinities (Howden and Nealis 1975). The foraging strategies and reduced flight capacity of small-bodied dung beetles likely renders them more prone to occupy even small islands. Conversely, large-bodied species are more susceptible to rapid extirpation following forest isolation (Larsen et al. 2008), which is consistent with our results showing that the vast majority of dung beetle species we sampled on islands were small-bodied.
Although we failed to find that island size alone affected dung beetle species richness and abundance, we detected clear effects of the interaction between area and isolation. Accordingly, 90% of all surveyed islands that were completely defaunated were small and ranged in size from 0.83 to 13.41 ha, with the exception of only one medium-sized (72.1 ha) and highly isolated island (8 km from the mainland). Our results are entirely consistent with other studies reporting that small, isolated forest islands contain depauperate and low-abundance dung beetle assemblages (Larsen et al. 2008), which mirror other studies on other groups of insects in Balbina (orchid bees: Storck-Tonon and Peres 2017). Compared to dung beetle studies in man-made archipelagic landscapes elsewhere, we recorded a much stronger detrimental effect of island size. For example, none of the studies to date on tropical land-bridge islands showed such markedly strong defaunated effects (Feer and Hingrat 2005; Qie et al. 2011), in terms of the complete extirpation of dung beetles on most islands. This could be attributed to the marked difference in historical isolation and landscape configuration compared to this study. We believe that isolation effects in true islands are stronger than in forest fragments surrounded by a non-water matrix. Small forest remnants are associated with much stronger edge-related microclimatic effects (Murcia 1995), in which air and soil desiccation and increased solar radiation can induce high levels of larval mortality (Halffter and Edmonds 1982; Nichols et al. 2013a, b). Since the dung beetle fauna on Balbina islands are relictual populations of a previous ~ 30 year-old continuous matrix of undisturbed primary forest, we expected that forest loss and the creation of a water-matrix should have a much greater impact than a terrestrial matrix.
Our predictive model for the entire Balbina archipelago of more than 3,500 forest islands suggests that only a few islands likely retained at least 50% of all dung beetle species and most of them retained a single species, most likely the small-bodied Onthophagus osculatii, which is a habitat generalist. Similar results were found in the same set of Balbina islands for medium to large-bodied mammals, which were only able to retain a few species (Benchimol and Peres 2015a). Ours findings therefore suggest trophic cascade effects in which forest islands could not ensure the local persistence of two pivotal vertebrate and invertebrate taxa that play an important forest ecosystem role in detritus production, decomposition and cycling. We highlight that the likely failure of these important ecosystem functions can affect many other organisms and ecosystem services (Nichols et al. 2008).
Onthophagus osculatii and forest degradation
Onthophagus osculatii Guérin-Méneville, 1855 is a small-bodied dung beetle (6–8 mm in length) that is widely distributed across the Amazon, including Bolivia, Peru, Ecuador, Colombia, Brazil, Venezuela, Guyana, French Guiana and Surinam. O. osculatii is typical of forest habitats, and widely attracted to pitfall traps baited with human excrement or carrion (Rossini et al. 2018). This ‘supertramp’ species, occurred both on islands and the mainland, attaining very high abundance on islands between 10 and 1000 ha in size. This species exhibits high capacity to inhabit low forest-cover habitats and even open-water environments (Larsen et al. 2008; Rossini et al. 2018). Hence, the highly abundance of O. osculatti may be a function of source-sink dynamics between neighbouring habitats, suggesting high dispersal capacity across water barriers.
Broadly distributed species usually exhibit higher environmental plasticity (Wiens 2011), which appears to be the case of O. osculatii given its broad range and generalist feeding habits (Silva et al. 2014). Higher environmental plasticity in small-bodied dung beetles likely facilitates persistence of these species even in degraded environments (Filgueiras et al. 2015, 2016) such as the small Balbina islands that have been exposed to wildfires. In addition, small dung beetle species can use fecal resources from small mammals (e.g. rodents) for both food and nesting (Culot et al. 2013). Sites containing a reduced number of large mammal species and individuals may favor higher abundance of small mammals that are unaffected by hunting pressure (Peres and Palacios 2007). This could boost the abundance and diversity of small-bodied dung beetles that can exploit fecal resources from smaller vertebrates. On the other hand, large-bodied beetles, particularly large paracoprids (e.g. Coprophanaeus), require either dung or carrion from medium to large-bodied mammals for nesting (Edmonds and Zídek 2010). These large dung beetles cannot easily exploit small food patches, including fecal resources or carcasses produced by small mammals (such as rodents and marsupials) to meet their nesting requirements.
A recent study found that in fire-climax ecosystems, such as the Cerrado scrublands of Central Brazil, dung beetle communities are virtually unaffected by episodic fires (Nunes et al. 2019). However, Amazonian forests represent a fire sensitive ecosystem and both plant and animal communities are severely affected by fire disturbance (Barlow and Peres 2004). Balbina islands exposed to elevated fire incidence retained a very low diversity of medium-sized to large mammals, especially islands smaller than 10 ha (Benchimol and Peres 2015a), thereby aggravating the resource scarcity for large-bodied detritivore insects.
However, some small mammal, such as non-forest-dependent species, can increase their abundance in these areas due to reduced competition and lack of predators, thereby becoming hyper-abundant. Higher densities of these species can lead to greater availability of fecal resources and carcasses that can be used by O. osculatii. Thus, elevated abundance of O. osculatii in fire-prone forest sites should be related to higher abundance of generalist small mammals such as Hylaeamys megacephalus and Marmosa demerarae, which increased their abundance in small islands (Palmeirim et al. 2018).
Additionally, we can infer that an increase in fire severity may directly or indirectly lead to the extirpation of more susceptible dung beetles species. This would reduce resource competition favouring a compensatory increase in the abundance of O. osculatti. Forest wildfires, beyond reducing the diversity of dung beetles (Silveira et al. 2015), influence the overall structure of species composition (Andrade et al. 2011, 2014). All Balbina islands experienced a severe drought in 1997 and 1998, which induced patchy, accidental fires that impacted a large number of islands (Benchimol and Peres 2015b). These surface fires almost certainly contributed to the dramatic defaunation process of dung beetles within burnt Balbina islands.
Ecosystem implications of dung beetle extinctions
The local extinction of dung beetles on forest islands can result in the loss of several ecosystem services that are directly associated with the food resource allocation strategy of detritivorous beetles (Larsen et al. 2008). When dung beetles bury food resources and create nest balls, they trigger a series of beneficial effects on the ecosystem (Louzada et al. 2008; Nichols et al. 2008). These environmental services include incorporation of organic matter into the soil (nutrient cycling) (Yamada et al. 2007); aeration of the soil (bioturbation) (Braga et al. 2013); secondary seed dispersal (Andresen and Levey 2004); pollination (Nichols et al. 2008); control of detritivore and hematophagous fly populations (Braga et al. 2012), and control of gastrointestinal nematodes (Miller 1961). These ecosystem functions are therefore presumably discontinued or severely reduced when some critical number and diversity of dung beetles are extirpated. For example, the rate of seed germination, leading to inhibited forest regeneration can be reduced following the decline or extinction of dung beetles (Griffiths et al. 2016). In addition, communities showing greater functional simplification, induced by the extinction of key species, tend to reduce their environmental resilience capacity (Beiroz et al. 2018).
The extinction of large-bodied dung beetles on small islands can also lead to severe degradation of environmental services. Large (> 1 cm) paracoprid and telecoprid dung beetles are the main providers of key forest ecosystem services (Horgan 2007; Andresen 2002; Louzada et al. 2008) due to their much greater capacity in both fertilizing the topsoil with buried dung and dispersing large-seeded plants (Braga et al. 2013). Large paracoprids such as Coprophanaeus parvulus and Dichotomius mamillatus were only observed in continuous forest areas and islands larger than 190 ha. Local extinctions of the large paracoprids on virtually all small islands can diminish the functional role of dung beetles in insular forest ecosystems.
Conclusions
This study clearly shows wholesale defaunation of dung beetle assemblages induced by both forest patch isolation and size across one of the world’s largest hydroelectric reservoirs, as evidenced by the conspicuous absence of dung beetles in a large number of small and medium sized islands. We showed that the vast majority of forest islands could not ensure the persistence of the dung beetle fauna, even if they had been protected by Brazil’s largest Biological Reserve. Island isolation in the Balbina archipelago was the best predictor of dung beetle species richness, followed by an interaction between island isolation and size. These drivers were also important in determining the multivariate patterns of species composition across all 33 sites sampled. Once we excluded the dominant species (Onthophagus oscullatii), however, we found that island size alone was the most important predictor of dung beetle abundance. We assume that these patterns are driven by the markedly low permeability of the wider open-water intervening matrix given that dung beetles are generally unable to perform long gap-crossing flights.
Additionally, the current spatial configuration of the Balbina archipelago led to the dramatic disassembly and simplification of the dung beetle metacommunity, which was further aggravated by trophic cascades induced by a pervasive process of large mammal extinctions (Benchimol and Peres 2015a, b). As such, the creation of a major dam in lowland Amazonia has led to profound effects on forest biodiversity, including the disruption of pivotal insect communities and the ecosystem services they provide. We highlight that the virtually complete defaunation of this important functional group of insects likely affects the whole process of detritus cycling into the topsoil, rather than just fecal decomposition. We therefore show that careful environmental planning is required for both licensing and developing new hydroelectric dams in lowland tropical forest regions, if the worst detrimental impacts on forest biodiversity are to be avoided.
References
Agostinho CS, Pelicice FM, Marques EE et al (2011) All that goes up must come down? Absence of downstream passage through a fish ladder in a large Amazonian river. Hydrobiologia 675:1–12. https://doi.org/10.1007/s10750-011-0787-0
Akçakaya HR, Mills G, Doncaster CP (2007) The role of metapopulations in conservation. In: Macdonald DW, Service K (eds) Key topics in conservation biology. Blackwell, Hoboken, pp 64–84
Almeida-Neto M, Guimarães P, Guimarães PR et al (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239. https://doi.org/10.1111/j.0030-1299.2008.16644.x
Almeida-Neto M, Ulrich W (2011) A straightforward computational approach for measuring nestedness using quantitative matrices. Environ Model Softw 26:173–178. https://doi.org/10.1016/j.envsoft.2010.08.003
Andresen E, Levey DJ (2004) Effects of dung and seed size on secondary dispersal, seed predation, and seedling establishment of rain forest trees. Oecologia 139:45–54
Barlow J, Peres CA (2004) Ecological responses to El Nino-induced surface fires in central Brazilian Amazonia: management implications for flammable tropical forests. Philos Trans R Soc B 359:367–380. https://doi.org/10.1098/rstb.2003.1423
Barton K (2018) MuMIn: multi-modal inference. Model selection and model averaging based on information criteria (AICc and alike). https://cran.rproject.org/web/packages/MuMIn/index.html R package version 1.42.1
Benchimol M, Peres CA (2015a) Predicting local extinctions of Amazonian vertebrates in forest islands created by a mega dam. Biol Conserv 187:61–72. https://doi.org/10.1016/j.biocon.2015.04.005
Benchimol M, Peres CA (2015b) Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLoS ONE 10(7):1–15. https://doi.org/10.5061/dryad.c301h
Benchimol M, Venticinque EM (2014) Responses of primates to landscape change in Amazonian land-bridge islands-a multi-scale analysis. Biotropica 46:470–478. https://doi.org/10.1111/btp.12122
Bieroz W, Sayer E, Slade EM, Audino LD, Braga RF, Louzada J, Barlow J (2018) Spatial and temporal shifts in functional and taxonomic diversity of dung beetles in a human-modified. Ecol Indic 95:518–526. https://doi.org/10.1016/j.ecolind.2018.07.062
Bitencourt BS, da Silva PG, Morato EF, Lima YG (2019) Dung beetle responses to successional stages in the Amazon rainforest. Biodivers Conserv 28:2745–2761. https://doi.org/10.1007/s10531-019-01791-y
Bogoni JA, da Silva PG, Peres CA (2019) Co-declining mammal-dung beetle faunas throughout the Atlantic Forest biome of South America. Ecography 42:1803–1818. https://doi.org/10.1111/ecog.04670
Braga RF, Korasaki V, Audino LD, Louzada J (2012) Are dung beetles driving dung-fly abundance in traditional agricultural areas in the Amazon? Ecosystems 15:1173–1181
Braga RF, Korasaki V, Andresen E, Louzada J (2013) Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. PLoS ONE 8:1–12
Bueno AS, Peres CA (2019) Patch-scale biodiversity retention in fragmented landscapes: reconciling the habitat amount hypothesis with the island biogeography theory. J Biogeogr 46:621–632
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65(1):23–35
Chittka L, Skorupski P, Raine NE (2009) Speed-accuracy tradeoffs in animal decision making. Trends Ecol Evol 24:400–407
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Bio Ecol 330:55–80. https://doi.org/10.1016/j.jembe.2005.12.017
Cosson JF, Ringuet S, Claessens O et al (1999) Ecological changes in recent land- bridge islands in French Guiana, with emphasis on vertebrate communities. Biol Conserv 91:213–222
Costa C, Oliveira VHF, Maciel R et al (2017) Variegated tropical landscapes conserve diverse dung beetle communities. PeerJ 5:e3125. https://doi.org/10.7717/peerj.3125
Culot L, Bovy E, Zagury Vaz-de-Mello F et al (2013) Selective defaunation affects dung beetle communities in continuous Atlantic rainforest. Biol Conserv 163:79–89. https://doi.org/10.1016/j.biocon.2013.04.004
da Silva PG, Hernández MIM (2015) Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS ONE 10:e0126112. https://doi.org/10.1371/journal.pone.0126112
da Silva PG, Nunes CA, Ferreira LF, Braga FR, Beiroz W et al (2019) Patch and landscape effects on forest-dependent dung beetles are masked by matrix-tolerant dung beetles in a mountaintop rainforest archipelago. Sci Total Environ 651:1321–1331. https://doi.org/10.1016/j.scitotenv.2018.09.195
De Andrade RB, Barlow J, Louzada J et al (2011) Quantifying responses of dung beetles to fire disturbance in tropical forests: the importance of trapping method and seasonality. PLoS ONE 6:e26208. https://doi.org/10.1371/journal.pone.0026208
De Andrade RB, Barlow J, Louzada J et al (2014) Tropical forest fires and biodiversity: dung beetle community and biomass responses in a northern Brazilian Amazon forest. J Insect Conserv 18:1097–1104. https://doi.org/10.1007/s10841-014-9719-4
Diamond J (2001) Ecology: dammed experiments! Science 294:1847–1848
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:027–046
Edmonds WD, Zídek J (2010) A taxonomic review of the neotropical genus Coprophanaeus Olsoufieff, 1924 (Coleoptera: Scarabaeidae, Scarabaeinae)
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Fearnside PM (1989) Brazil’s Balbina dam: environment versus the legacy of the Pharaohs in Amazonia. Environ Manag 13:401–423. https://doi.org/10.1007/BF01867675
Fearnside PM (2016) Environmental and social impacts of hydroelectric dams in Brazilian Amazonia : implications for the aluminum industry. World Dev 77:48–65. https://doi.org/10.1016/j.worlddev.2015.08.015
Feer F, Hingrat Y (2005) Effects of forest fragmentation on a dung beetle community in French Guiana. Conserv Biol 19:1103–1112. https://doi.org/10.1111/j.1523-1739.2005.00087.x
Filgueiras BKC, Iannuzzi L, Leal IR (2011) Habitat fragmentation alters the structure of dung beetle communities in the Atlantic Forest. Biol Conserv 144:362–369. https://doi.org/10.1016/j.biocon.2010.09.013
Filgueiras BKC, Tabarelli M, Leal IR et al (2015) Dung beetle persistence in human-modified landscapes : combining indicator species with anthropogenic land use and fragmentation-related effects. Ecol Indic 55:65–73. https://doi.org/10.1016/j.ecolind.2015.02.032
Filgueiras BKC, Tabarelli M, Leal IR et al (2016) Spatial replacement of dung beetles in edge-affected habitats: biotic homogenization or divergence in fragmented tropical forest landscapes? Divers Distrib 22:400–409. https://doi.org/10.1111/ddi.12410
Finer M, Jenkins CN (2012) Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. PLoS ONE 7:e35126. https://doi.org/10.1371/journal.pone.0035126
França F, Barlow J, Araújo B, Louzada J (2016) Does selective logging stress tropical forest invertebrates? Using fat stores to examine sublethal responses in dung beetles. Ecol Evol 6:8526–8533. https://doi.org/10.1002/ece3.2488
Frank K, Krell FT, Slade EM et al (2018) Global dung webs: high trophic generalism of dung beetles along the latitudinal diversity gradient. Ecol Lett 21:1229–1236. https://doi.org/10.1111/ele.13095
Griffiths HM, Bardgett RD, Louzada J, Barlow J (2016) The value of trophic interactions for ecosystem function: dung beetle communities influence seed burial and seedling recruitment in tropical forests. Proc R Soc Lond B 283:20161634
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:1–9. https://doi.org/10.1126/sciadv.1500052
Halffter G, Arellano L (2002) Response of dung beetle diversity to human-induced changes in a tropical landscape1. Biotropica 34:144–154. https://doi.org/10.1111/j.1744-7429.2002.tb00250.x
Halffter G, Edmonds WD (1982) The nesting behavior of dung beetles: an ecological and evolutive approach. Publicaciones del Instituto de Ecología de México 10:1–176
Harrison S, Hastings A (1996) Genetic and evolutionary consequences of metapopulation structure. Trends Ecol Evol 11:180–183
Heiberger RM (2016) HH: Statistical analysis and data display: Heiberger and Holland. R Package Version 3:1–31
Horgan FG (2007) Dung beetles in pasture landscapes of Central America: proliferation of synanthropogenic species and decline of forest specialists. Biodivers Conserv 16:2149–2165. https://doi.org/10.1007/s10531-006-9145-3
Howden HF, Nealis VG (1975) Effects of clearing in a tropical rain forest on the composition of the Coprophagous scarab beetle fauna (Coleoptera). Biotropica 7:77. https://doi.org/10.2307/2989750
Kiffner C, Stanko M, Morand S et al (2013) Sex-biased parasitism is not universal: evidence from rodent-flea associations from three biomes. Oecologia 173:1009–1022. https://doi.org/10.1007/s00442-013-2664-1
Klein BC (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70:1715–1725. https://doi.org/10.2307/1938106
Larsen TH, Lopera A, Forsyth A (2008) Understanding trait-dependent community disassembly: dung beetles, density functions, and forest fragmentation. Conserv Biol 22:1288–1298. https://doi.org/10.1111/j.1523-1739.2008.00969.x
Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29:107–116
Lees AC, Peres CA, Fearnside PM et al (2016) Hydropower and the future of Amazonian biodiversity. Biodivers Conserv 25:451–466
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Louzada JNC, Vieira LM, Spector S (2008) Effects of degradation and replacement of Southern Brazilian coastal sandy vegetation on the dung beetles (Coleoptera: Scarabaeidae). Biotropica 40:719–727
MacArthur R, Wilson E (1967) The theory of island biogeography. Princet Univ Press 1:202
Marsh CJ, Louzada J, Beiroz W, Ewers RM (2013) Optimising bait for pitfall trapping of Amazonian dung beetles (Coleoptera: Scarabaeinae). PLoS ONE 8:e73147. https://doi.org/10.1371/journal.pone.0073147
Mendenhall CD, Karp DS, Meyer CFJ et al (2014) Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 509:213–217. https://doi.org/10.1038/nature13139
Meyer CFJ, Kalko EKV (2008) Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: land-bridge islands as a model system. J Biogeogr 21:1–16. https://doi.org/10.1111/j.1365-2699.2008.01916.x
Miller A (1961) The mouth parts and digestive tract of adult dung beetles (Coleoptera: Scarabaeidae), with reference to the ingestion of helminth eggs. J Parasitol 47:735–744
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62. https://doi.org/10.1016/S0169-5347(00)88977-6
Murray K, Conner MM (2009) Methods to quantify variable importance: implications for the analysis of noisy ecological data KIM. Ecology 90:348–355
Nichols E, Gardner TA, Peres CA et al (2009) Co-declining mammals and dung beetles : an impending ecological cascade. Oikos 118:481–487. https://doi.org/10.1111/j.1600-0706.2009.17268.x
Nichols E, Larsen T, Spector S et al (2007) Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol Conserv 137:1–19. https://doi.org/10.1016/j.biocon.2007.01.023
Nichols E, Spector S, Louzada J et al (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
Nichols E, Uriarte M, Bunker DE et al (2013a) Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology 94:180–189
Nichols E, Uriarte M, Peres CA et al (2013b) Human-induced trophic cascades along the fecal detritus pathway. PLoS ONE 8:e75819. https://doi.org/10.1371/journal.pone.0075819
Nichols ES, Gardner TA (2011) Dung beetles as a candidate study taxon in applied biodiversity conservation research. In: Simmons LW, Ridsdill-smith TJ (eds) Ecology and evolution of dung beetles. Wiley, Oxford, pp 267–291
Nunes CA, Beiroz W, da Silva PG et al (2019) Fire? They don’t give a dung! The resilience of dung beetles to fire in a tropical Savanna. Ecol Entomol 44:315–323
Nunes RV, de Carvalho MSG, Vaz-de-Mello FZ et al (2014) Taxonomic composition of Scarabaeinae dung beetles (Coleoptera: Scarabaeidae) inhabiting fluvial islands in the southern Brazilian Amazon. Ann la Société Entomol Fr 50:407–413. https://doi.org/10.1080/00379271.2014.984955
Oksanen JFG, Blanchet R, Kindt P et al (2018) Vegan: community ecology package. R package version 2.4–1
Palmeirim AF, Benchimol M, Vieira MV, Peres CA (2018) Small mammal responses to Amazonian forest islands are modulated by their forest dependence. Oecologia 187:191–204. https://doi.org/10.1007/s00442-018-4114-6
Peck SB, Forsyth A (1982) Composition, structure, and competitive behaviour in a guild of Ecuadorian rain forest dung beetles (Coleoptera; Scarabaeidae). Can J Zool 60:1624–1634. https://doi.org/10.1139/z82-213
Pereira HM, Leadley PW, Proença V et al (2010) Scenarios for global biodiversity in the 21st century. Science 330:1496–1501. https://doi.org/10.1126/science.1196624
Peres CA, Palacios E (2007) Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39(3):304–315
Pfeifer M, Lefebvre V, Peres CA, Banks-Leite C et al (2017) Creation of forest edges has a global impact on forest vertebrates. Nature 551(7679):187–191
Pinto Leite CM, Mariano-Neto E, da Rocha PLB (2018) Biodiversity thresholds in invertebrate communities: the responses of dung beetle subgroups to forest loss. PLoS ONE 13:e0201368. https://doi.org/10.1371/journal.pone.0201368
Prugh LR, Hodges KE, Sinclair AR, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. PNAS 105:20770–20775
Qie L, Lee TM, Sodhi NS, Lim SL-H (2011) Dung beetle assemblages on tropical land-bridge islands: small island effect and vulnerable species. J Biogeogr 38:792–804. https://doi.org/10.1111/j.1365-2699.2010.02439.x
Quintero I, Halffter G (2009) Temporal changes in a community of dung beetles (insecta: coleoptera: scarabaeinae) resulting from the modification and frgamentation of tropical rain forest. Acta zoológica Mex 25:625–649
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rocha-Santos L, Benchimol M, Mayfield MM et al (2017) Functional decay in tree community within tropical fragmented landscapes: effects of landscape-scale forest cover. PLoS ONE 12:e0175545. https://doi.org/10.1371/journal.pone.0175545
Rossini M, Vaz-de-mello FZ, Zunino M (2018) A taxonomic revision of the New World Onthophagus Latreille, 1802 (Coleoptera: Scarabaeidae: Scarabaeinae) of the osculatii species-complex, with description of two new species from South America A taxonomic revision of the New World Onthophagus. J Nat Hist 52:541–586. https://doi.org/10.1080/00222933.2018.1437230
Sánchez-de-Jesús HA, Arroyo-Rodríguez V, Andresen E, Escobar F (2016) Forest loss and matrix composition are the major drivers shaping dung beetle assemblages in a fragmented rainforest. Landsc Ecol 31:843–854. https://doi.org/10.1007/s10980-015-0293-2
Scheffler PY (2005) Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. J Trop Ecol 21:9–19. https://doi.org/10.1017/S0266467404001683
Scholtz CH, Davis ALV, Kryger U (2009) Evolutionary biology and conservation of dung beetles. Pensoft Publishers, Bulgaria
Silva RJ, Coletti F, Costa D, Vaz-de-Mello FZ (2014) Rola-bostas (Coleoptera: Scarabaeidae: Scarabaeinae) de florestas e pastagens no sudoeste da Amazônia brasileira: Levantamento de espécies e guildas alimentares. Acta Amaz 44:345–352. https://doi.org/10.1590/1809-4392201304472
Silva RJ, Storck-Tonon D, Vaz-de-Mello FZ (2016) Dung beetle (Coleoptera: Scarabaeinae) persistence in Amazonian forest fragments and adjacent pastures: biogeographic implications for alpha and beta diversity. J Insect Conserv 20:549–564. https://doi.org/10.1007/s10841-016-9885-7
Silveira JM, Louzada J, Barlow J et al (2016) A multi-taxa assessment of biodiversity change after single and recurrent wildfires in a Brazilian Amazon forest. Biotropica 48:170–180. https://doi.org/10.1111/btp.12267
Smith BW, Dabbert BC, Verble RM (2018) Rangeland ecology & management prescribed fire effects on rangeland dung beetles (Coleoptera : Scarabaeinae, Aphodiinae) in the Southern Great Plains. Rangel Ecol Manag. https://doi.org/10.1016/j.rama.2018.07.00300
Spector S (2006) Scarabaeine dung beetles (coleoptera: Scarabaeidae: Scarabaeinae): an invertebrate focal taxon for biodiversity research and conservation. Coleopt Bull 60:71–83. https://doi.org/10.1649/0010-065X(2006)60[71:SDBCSS]2.0.CO;2
Storck-Tonon D, Peres CA (2017) Forest patch isolation drives local extinctions of Amazonian orchid bees in a 26 years old archipelago. Biol Conserv 214:270–277. https://doi.org/10.1016/j.biocon.2017.07.018
Terborgh J, Lopez L, Nuñez P et al (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926. https://doi.org/10.1126/science.1064397
Tourinho AL, Benchimol M, Porto W, Peres CA, Storck-Tonon D (2019) Marked compositional changes in harvestmen assemblages in Amazonian forest islands induced by a mega dam. Insect Conserv Divers. https://doi.org/10.1111/icad.12398
Wiens JJ (2011) The niche, biogeography and species interactions. Philos Trans R Soc B 366:2336–2350. https://doi.org/10.1098/rstb.2011.0059
Yamada D, Imura O, Shi K, Shibuya T (2007) Effect of tunneler dung beetles on cattle dung decomposition, soil nutrients and herbage growth. Grassl Sci 53:121–129
Zarfl C, Lumsdon AE, Tockner K (2015) A global boom in hydropower dam construction. Aquat Sci 77:161–170. https://doi.org/10.1007/s00027-014-0377-0
Acknowledgements
This study was funded by a NERC grant to CAP (NE/J01401X/1). We are especially grateful to Rafael Tonon, Mr. Celso and Mr. Chagas for fieldwork assistance. We also thank Diogo A. Costa for statistical advice. We are grateful to ICMBio for logistical and financial support, through the management of Reserva Biológica do Uatumã; and a Postdoctoral fellowship awarded to DS-T (CAPES/PNPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Clinton Jenkins.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Forest and plantation biodiversity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Storck-Tonon, D., da Silva, R.J., Sawaris, L. et al. Habitat patch size and isolation drive the near-complete collapse of Amazonian dung beetle assemblages in a 30-year-old forest archipelago. Biodivers Conserv 29, 2419–2438 (2020). https://doi.org/10.1007/s10531-020-01982-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-01982-y