Abstract
Deforestation is a major driver of biodiversity loss in the Tropical region, but the role of upstream refugia and dispersal ability on the community response to this disturbance is unknown. We assessed the relevance of undisturbed upstream patches (“refugia”) on the responses of benthic communities to forest cover loss. We selected four Andean rivers with a well-protected forest in their upstream section and different degree of forest cover loss downstream and evaluated the dissimilarity patterns of three benthic communities (diatoms, Chironomidae, and the assemblage of Ephemeroptera, Plecoptera and Trichoptera—‘EPT’) along their water courses. We evaluated the dissimilarity by using Euclidean (environment), Sørensen (incidence data) and Morisita–Horn (abundance data) pairwise distances. We found that diatom beta-diversity, as organisms with passive but higher dispersal ability, significantly tracked the environmental changes caused by forest loss. However, insect communities, whose a priori are active dispersers and can track for suitable conditions, were weakly affected by deforestation. These results provide evidences that the existence of well-preserved upstream reaches along patched corridors may allow non-tolerant species to remain extant throughout dispersal-driven feedbacks. This being the case in the Andean streams, effects of deforestation on benthic communities were small, and not uniform. Our results reinforce the strategy of preserving upstream sections in order to achieve successful restoration or rehabilitation goals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although deforestation is one of the major drivers of biodiversity loss in the Tropical region (Barlow et al. 2016), its effects on biodiversity structural and functional components have been scarcely evaluated in the Tropics (see on this regard the meta-analysis evidences by Richardson and Béraud 2014). Deforestation effect studies have mostly focused on comparing changes on alpha-diversity (i.e. local richness) and bioassessment-derived metrics (e.g. the Biological Monitoring Working Party, ‘BMWP’; see Hawkes 1998) between forested and non-forested streams (e.g. Bojsen and Jacobsen 2003; Lorion and Kennedy 2009; Iñiguez-Armijos et al. 2014). As these alpha-diversity and bioassessment-derived metrics do not take into account the potential influence of dispersal (Hitt and Angermeier 2008), they cannot quantify the relevance of refugia occurrence (i.e. sites where the absence of an impairment have enabled the native communities to survive) among the stream network in structuring local communities and basin-level metacommunities (i.e. a set of communities potentially linked by dispersal; Leibold et al. 2004). Addressing this knowledge gap is crucial to improve bioassessment protocols as well as the performance of mitigation plans and conservation practices.
The use of alpha-diversity and bioassessment-derived metrics assumes that community composition will reflect the environmental change linked to one or more impairments (Siqueira et al. 2014; Buss et al. 2015). Under this assumption, species at impaired sites will be those with greater resistance to the new environmental conditions (e.g. species more tolerant to increased input of sediments covering the streambed, reduced inputs of allochthonous organic material or input of fertilizers in deforest segments). In this way, environmental sorting would be the main process underlying community and metacommunity structure (i.e. species composition and distribution across local communities). However, streams are dendritic and highly connected ecosystems (Campbell Grant et al. 2007; Altermatt et al. 2013), where dispersal-driven processes may override the effect of environmental sorting on the structure of local communities and metacommunities. This is mostly related to two situations (e.g. Tonkin et al. 2014, 2018; Kitto et al. 2015; Sarremejane et al. 2017): when species have high dispersal ability, through which they can virtually establish everywhere; or when there exists a high connectivity between source (e.g. refugia) and sink patches (e.g. impaired sites). Considering that undisturbed headwater segments can serve as refugia for species under human-related disturbances (Sedell et al. 1990), both species dispersal ability and the distance to the refugia cannot be ignored when assessing the effects of impairment.
One approach to explore the influence of dispersal and connectivity on metacommunity structure is to focus on characterizing the beta diversity patterns of communities possessed of species with distinct dispersal abilities (Kärnä et al. 2015; Tonkin et al. 2018). The beta diversity summarizes the variability in species composition between sampling units or ‘local communities’ (Anderson et al. 2006). Therefore, analyzing the beta diversity patterns along stress gradients may help disentangling the effects of environmental heterogeneity, network spatial structure, and/or dispersal-driven processes on metacommunity structure (Gutiérrez-Cánovas et al. 2013; Heino et al. 2015). By decomposing beta diversity in its turnover and nestedness components (Baselga 2010), it is also possible to infer the main effects of a particular stressor on metacommunity structure (Gutiérrez-Cánovas et al. 2013; Socolar et al. 2016). That is, if nestedness is the major driver, the stressor may promote assemblage disaggregation and leads communities at impaired zones to be subsets of the undisturbed ones (Gaston et al. 2000; Tornés and Ruhí 2013). On the contrary, if turnover is the main driver, the stressor may promote the replacement of some species by others according to their stress tolerance (Qian et al. 2004).
We therefore used beta-diversity metrics to assess the effect of deforestation on the distribution patterns of benthic communities, and to determine how it is modulated by the occurrence of upstream refugia and dispersal ability. So forth, we evaluated beta-diversity patterns of high-elevation Tropical benthic communities within four Andean streams, all having an upstream section with well-protected forest and two downstream sections with varying degrees of forest cover loss (Fig. 1a). As populations of high-dispersal species may persist at impaired reaches through colonization from upstream refugia—in a source-sink dynamics (Leibold et al. 2004; Leibold and Chase 2018), we expected communities possessed of species with low dispersal ability to track better the local environment changes linked to deforestation. Thus, we hypothesized the relationship between beta-diversity and environmental dissimilarity between reaches of each stream to be weaker as dispersal ability increases (Fig. 1b). Besides, if local environmental conditions change in line with forest cover, we also hypothesized that within-stream beta-diversity will change accordingly to the degree of forest cover loss along each stream: a lower beta-diversity should be expected when the forest cover is similar along the stream, as local environment is similar between reaches (Fig. 1b, stream “S1”); a gradual increase in beta-diversity had to be expected when the stream had a gradual loss of forest cover (Fig. 1b, streams “S2” and “S3”); and, finally, when the forest cover would abruptly decrease (Fig. 1b, stream “S4”), beta-diversity should be expected to increase abruptly as well.
Percentage of forest cover at each stream reach (a) and conceptual scheme representing the expected biotic and environmental dissimilarity among reference and impact stream reaches (b). The dotted rectangles represent the expected position of each stream in the relationship between community and environmental dissimilarity. The dotted circles represent S4 stream reaches
We tested these hypotheses by using three benthic communities possessed of taxa with specific dispersal abilities, since they show differences in their body size and dispersal strategy (De Bie et al. 2012; Padial et al. 2014). The first community is made up by benthic diatoms, which are passively dispersing organisms. Diatoms are sessile microorganisms whose dispersal is mostly passive and confined to the stream network (Liu et al. 2013). However, the small body size of diatoms is usually related to a high dispersal capacity (Padial et al. 2014). The second community selected was the non-biting midges (Chironomidae), which hardly disperse actively due to their small body size but can passively reach longer distances in both aerial and aquatic pathways (Serra et al. 2017). And the third community is that made up by Ephemeroptera, Plecoptera and Trichoptera (EPT), which are taxa with active dispersal through aquatic and aerial pathways (Malmqvist 2000; Graham et al. 2017). Given their high dispersal ability, EPT taxa tend to seek for the most suitable places for inhabiting or laying eggs (Thompson and Townsend 2006; Lancaster and Downes 2017). Overall, these three communities represent a great set to assess for the role of dispersal on structuring benthic metacommunities. All three communities are frequently used in bioassessments due to their sensitivity to environmental changes (e.g. Barbour et al. 1999; Leira and Sabater 2005; Buss and Vitorino 2010; Lencioni et al. 2012; Milošević et al. 2013; Tornés et al. 2018); and therefore, can be accurately used for assessing the effects of deforestation on river integrity and health.
Methods
Study design and site selection
The Andean mountains are one of the most diverse regions in the world, but also highly impaired by historical land-use changes (Myers et al. 2000; Etter et al. 2008). About 62% of the natural ecosystems had been cleared by 2000, and causes are multiple and include human settlement expansion, cattle rising, illegal cropping, and the expansion of agricultural frontiers (Rodríguez Eraso et al. 2012). Although Andean countries have laws for protecting the forest, the minimum width—30 m in general—is usually not maintained along stream corridors. This has caused that Andean streams exhibit a patched pattern of forest along their corridors: the most cleared zones are located near human settlements, and the most preserved are confined to protected or inaccessible areas, such as headwaters or stepper valleys (Armenteras et al. 2011). We used this pattern as an opportunity to study the relevance of these patches on the response of three different benthic communities to deforestation.
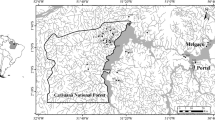
We selected four second-order streams from the high-Andean region which differed to each other on the patterns of riparian forest cover loss. All four streams drain to the Meta River basin and are located between 2300 and 2800 m a.s.l (department of Cundinamarca, Colombia; Fig. 2a). Their waters drain areas mainly cover by high-Andean forest ecosystems but some of them (S1 and S4) flow partially through Páramo ecosystems. Páramo regions are endemic to the Neotropical Andean Mountains and are recognizable by their extreme temperature variations (intraday variations of more than 20 °C), soils with a high water retention capacity (such as Andisols), and vegetation chiefly comprised of giant rosette plants (Espeletia), shrubs, and grasses (Buytaert et al. 2006).
Using aerial photographs (Google Maps Pro 2017, version 7.1), we estimated the forest cover along the stream channel within buffers of 30 m-width (representing the minimum width established by the Colombian Ministry of Environment: decree 2245 of 2017). After scanning photographs, we selected one segment of minimum 2 km-long per stream and established three sampling reaches of 100 m-long. Stream reaches, which consisted of at least three riffles, were separated to each other by ca. 1 km (Fig. 2b). Although some studies have demonstrated that insect dispersal may be as high as 5 km (reviewed by Bohonak and Jenkins 2003), recent evidence points out that dispersal of Diptera, Plecoptera, Ephemeroptera and Trichoptera individuals may be lesser than 1 km within a generation (Macneale et al. 2005; Poff et al. 2006; Winking et al. 2014; Graham et al. 2017). Therefore, this distance was considered sufficient to reliably assess the influence of dispersal ability on community responses to deforestation. Particularly, in our study streams, where we found at least one waterfall between the sampling reaches of each stream.
Environmental and biological data collection
The sampling was conducted during the dry season (10th to 22nd February 2017), when biological communities (insects and diatoms) were well developed. Environmental and biological samples were collected in the riffle sections of each stream segment. We measured 11 environmental variables in order to characterize the environmental conditions of each of the reaches (Table 1). Conductivity, pH, oxygen, and temperature were recorded using a HANNA HI98194 water quality meter. Canopy cover (%) was estimated from an upward photo taken through a fisheye lens. Channel width, depth and current velocity were measured at two cross-channel transects located at the beginning and ending sections of each riffle. Depth and water velocity were measured at 0.25 m intervals, and the average value per riffle section was used in the analyses. The total suspended solids (TSS) were determined by filtering 500 ml of stream water in a pre-weighted GFF filter and drying the filter for 1 h at 105 °C. Organic matter was collected from each Surber sample after sorting all invertebrates (see below), dried for 48-72 h at 70 °C, and oven-dried for 4 h at 500 °C to obtain the ash-free dry weight (Elosegi and Sabater 2009). The ash-free dry weight of each sample was used as a proxy of the Benthic Organic Matter (BOM) collected at each sample.
We collected brush-scraped material from a 0.008 m2 surface of 30 boulders of similar size at each riffle for the diatom samples. Samples were preserved in a Transeau solution (1:1 ratio) and taken to the laboratory where diatom frustules were cleaned from the organic material using hydrogen peroxide. We mounted frustules on permanent slides using Naphrax® medium. Slides were observed with immersion oil at a 1000 × magnification under a light microscope and identified at genus-level using specialized monographs (Krammer and Lange-Bertalot 1986, 1988, 1991a, b; Metzeltin and Lange-Bertalot 2007; Bellinger and Sigee 2015). At least 400 valves were counted for each slide.
The invertebrates were collected following a multi-habitat sampling procedure. This was performed by collecting 5 Surber (mesh size 350 Mm, area 0.09 m2) samples in stream substrata selected according to the corresponding habitat coverage. For instance, if a riffle was composed by 60% of boulders, 30% gravels, and 10% cobbles, 3 Surber samples of the first, 1 of the second, and 1 of the third substrate type were collected. A total of 60 samples were taken for this study (4 streams/segments × 3 riffles × 5 samples), each sample being preserved in a plastic bottle with 96% alcohol. In the laboratory, all the individuals were sorted and identified at genus-level following specialized keys (Trivinho-Strixino and Strixino 1995; Merritt and Cummins 2008; Domínguez and Fernández 2009; Prat et al. 2011, 2014).
Data preparation
The grain sizes for insect and diatom communities were a Surber sample and a composed sample per riffle, respectively. To create comparable datasets, Chironomidae and EPT abundances were weighed according to the substrate coverage at each riffle. Thus, all three datasets had a grain size equivalent to a riffle section; and therefore, a total of 36 rows (4 stream segments × 3 reaches—1 reference, 2 downstream impairments— × 3 riffles).
Data analysis
We tested the abovementioned hypotheses by measuring the relationship strength between community and environmental dissimilarities within each stream. Community dissimilarity, estimated by using Sørensen (Sørensen 1948) and Morisita–Horn (Horn 1966) distances, tell us how much community composition changed between reaches with different degrees of forest loss. Meanwhile, environmental dissimilarity, estimated by using a Euclidean distance, tell us how much the local environment changed due to the loss of forest cover. Thus, if environmental sorting is determining community composition, we expected this relationship to be stronger in streams with a greater degree of forest cover loss along their course. The greater the loss, the greater should be the impact on the local environment; and therefore, the greater should be the change in community composition.
To evaluate and compare the strength of this relationship between streams and communities, we used the regression coefficients obtained from linear regressions of the Sørensen and Morisita–Horn pairwise distances against the Euclidean pairwise distances. Pairwise-Euclidean distances were estimated by using uncorrelated variables (Pearson correlation coefficient < 0.6; Booth et al. 1994) to minimize collinearity: BOM, pH, conductivity, temperature, TSS, Canopy cover, oxygen saturation, depth, current velocity, and width. On the other hand, a matrix of incidence data (presence-absence) was used for estimating Sørensen distances, and a matrix of relative abundances was used for estimating Morisita–Horn distances. These two distances allow us to evaluate how much of the dissimilarity was driven by differences in species composition (Sørensen) or by changes in species relative abundance (Morisita–Horn). Besides, the Morisita–Horn distances shows a high resistance to under-sampling by giving higher weight to the most abundant species. Since the Morisita–Horn is related to the Sørensen index, two communities having the same species and analogous abundances distribution, will provide the same values in the two indices (Jost et al. 2011). This property is useful for disentangling in which way riparian loss affects species composition or their abundances. Additionally, we decomposed the Sørensen index (βSOR) in their Turnover (βSIM) and Nestedness (βNES) components (Baselga 2010).
As our aim was to evaluate how the occurrence of upstream refugia can modulate community-level responses to deforestation, we only estimated pairwise distances within-streams but not among them (Fig. 2b). Moreover, as it has been found in other Tropical zones (Múrria et al. 2015), our streams were significantly different in terms of their community composition (see Fig. S1; Table S1). Thus, we considered that estimating distances between streams could add unnecessary noise to the analysis, while not providing additional evidences for testing our hypotheses.
We estimated regression coefficients for each stream (“within-stream”) and for all streams (“among-streams”) (Fig. 2b). Within-streams, we quantified the relationship using a simple linear regression of the pairwise distances between reaches of each stream (n = 27 pairs for stream). We used the coefficients of these regressions to evaluate if the community-environment relationship change at each stream and depending on the community. For quantifying the among-streams relationship, we pooled the pairwise distances between reaches of each stream (n = 108 pairs) and performed generalized least squares (GLS) regressions. The coefficients of these regressions allow us to evaluate if the responses to deforestation varied depending on the community. We used GLS since these models can deal with data violating the heteroskedasticity assumption—heterogeneous variances among groups (streams). The heterogeneity of each stream was included in the random term of the model as suggested by Zuur et al. (2009). All analyses and graphical outputs were performed with the packages vegan (Oksanen et al. 2013), ade4 (Chessel et al. 2004), betapart (Baselga and Orme 2012), and ggplot2 (Wickham 2010) of R Statistical software v 3.4.1 (R Core Team 2018). All the assumptions were previously tested by following Zuur et al. (2009) suggestions.
Results
We identified 26 genera of diatoms, 28 genera of Chironomidae and 26 genera of EPT (9 genera of Ephemeroptera, 1 genus of Plecoptera, and 16 genera of Trichoptera) in the whole set of stream reaches (Supplementary Tables S2, S3, S4). The accumulation curves indicated that sampling was adequate, since curves tended to approach an asymptote (Fig. S2).
Deforestation effects on stream’s local environmental were evidenced 1 km and 2 km downstream from undisturbed reaches (Fig. 3). The physical and chemical characteristics tended to change in line with the percentage of forest cover loss. However, the magnitude of change varied depending on the stream. We observed a low dissimilarity between reference and impaired reaches at streams S1 and S2, but a high dissimilarity within reaches of the stream S3 (the most impaired stream). At the stream S4, the environmental conditions did not reflect the abrupt loss of forest coverage starting 2 km downstream of the undisturbed reach (Fig. 3c, d). Canopy cover, temperature, TSS, and conductivity were the variables that significantly changed within each stream (Table 1). Canopy cover diminished in disturbed reaches, while temperature, TSS and conductivity increased at undisturbed reaches.
On the left column, the mean and standard error (SE) of the Euclidean pairwise distances (representing environmental dissimilarity) between the undisturbed and the impaired reaches located 1 km (a) and 2 km (b) downstream. On the right column, the percentage of forest cover the undisturbed to the impaired reaches (1 km—C, 2 km—D) of each stream (S1–S4)
The relationships among the communities’ beta diversity and the environmental dissimilarity caused by deforestation among-streams are represented in Fig. 4, while, within-stream relationships are summarized in Table 2. We found that the diatom dissimilarity increased in line with environmental dissimilarity among-streams (Fig. 4a, regression coefficient = 0.089, SE = 0.008, p value < 0.001). Within-streams (Table 2), the most pronounced change occurred at S3, and in a lesser degree in S2, which are the streams with a gradual loss of riparian coverage downstream of the reference reach. The Chironomidae communities did not significantly respond to the environmental changes driven by deforestation, neither in the within- nor among-stream comparisons (Fig. 4b, regression coefficient = − 0.008, SE = 0.005, p-value = 0.16). Finally, the dissimilarity of EPT communities increased in line with the environmental dissimilarity (Fig. 4c, r = 0.045, SE = 0.009, p-value < 0.001). However, this among-stream trend disappeared within streams, although the Sørensen index (dissimilarity) increased accordingly to the total amount of forest loss among-streams, it remained similar within each stream.
Table 3 summarizes the relationships between the environmental dissimilarity and the nestedness and turnover within each stream of the three communities (i.e. diatoms, Chironomidae and EPT). Nestedness and turnover of diatom communities increased among-streams. The higher dissimilarity value and the larger regression coefficient suggest that diatom dissimilarity was mainly driven by processes promoting nestedness. The turnover and nestedness components of Chironomidae communities significantly decreased with environmental changes only within the stream S4. Finally, in the EPT communities only the turnover component responded significantly to the environmental dissimilarity among-streams. Within-streams, the turnover and the nestedness components responded significantly at streams S1 and S3, respectively.
The relationships between beta diversities and environmental dissimilarities became all significantly positive when considered the Morisita–Horn index (Fig. 5). Chironomidae communities exhibited the less pronounced changes in dissimilarity (Fig. 5b; r = 0.041, SE = 0.016, p-value = 0.013), followed by diatom (Fig. 5a; r = 0.043, SE = 0.013, p-value = 0.0019), and EPTs (Fig. 5c; r = 0.065, SE = 0.016, p-value ≤ 001). Overall, the dissimilarity of the three communities increased with environmental dissimilarity within the S3 stream. We also observed a positive correlation within S4 stream for diatom and Chironomidae communities, and only within S1 stream for diatoms (Table 4).
Discussion
The use of alpha-diversity and bioassessment-derived metrics relies on the assumption that biological communities track the environmental modifications caused by one or multiple stressors (Siqueira et al. 2014; Buss et al. 2015). Here, we showed that three benthic communities (diatoms, non-biting midges and EPT) may not necessarily track the loss of forest cover occurring along high-Andean streams, even when the local environmental conditions changed as affected by this stressor. The distinct dispersal abilities of species from each community and the occurrence of upstream refugia in all streams provide enough evidence to hypothesize that dispersal may override the effects of deforestation, even when the forest loss is overwhelming (in the way it occurs in stream S3 or S4). Well-preserved upstream zones may act as source patches (refugia) of non-tolerant species that can persist in unsuitable conditions (sink patches) through dispersal-driven feedbacks.
The influence of dispersal-driven processes on diversity and distribution patterns has been already evidenced (reviewed in Tonkin et al. 2018). Briefly explained, at one end, lower dispersal ability can constrain species to track their preferred environmental conditions; and at the other, higher dispersal ability can override the constrains imposed by local environment conditions (Leibold et al. 2004). So forth, our results support our hypothesis that dispersal ability can modulate the control exhorted by environmental filtering, and therefore the relationship between community (beta diversity) and environmental dissimilarities. However, it emerged in an unexpected way. We found that species with passive but higher dispersal ability (diatoms; Soininen 2007; Liu et al. 2013; Padial et al. 2014) were the best trackers of deforestation along the watercourse. Meanwhile, EPT and chironomid taxa, which a priori are active dispersers and can track for suitable conditions, were partially or not affected at all by deforestation.
Beta diversity patterns suggest different drivers underlying the responses of each community to deforestation. Environmental sorting can be the main driver of dissimilarity across communities in organisms with passive but higher dispersal ability, such as diatoms. Their beta-diversity tended to increase proportionally to the percentage of forest loss: the greater the loss, the greater the diatoms beta-diversity. This trend and the relatively higher importance of nestedness on their distribution suggest that deforestation is promoting the increase in species diversity via greater resource availability and habitat heterogeneity. In deforested reaches, the longer periods of light availability, greater inputs of nutrients and diversity of microhabitats (i.e. filamentous algae) may facilitate species coexistence by niche differentiation (Tilman et al. 1982; Passy 2008). In fact, we observed diatom guilds to expand from prevalent low-profile taxa with short stalks, or cells completely adhered at the substratum (i.e. Achnanthidium) in the undisturbed reaches, to others of higher profile with longer stalks or developing in mucilaginous tubes (such as Gomphonema and Encyonema) in the disturbed sections.
The insect communities did not track the loss of forest cover in the same manner that diatoms did. Their beta diversity within-streams was weak or unrelated to the environmental changes caused by deforestation. Taking into account that the two communities were made up by taxa with moderate to high dispersal abilities, downstream populations of non-tolerant species can be maintained by the flux of individuals from the upstream refugia patches (e.g. Downes et al. 2016). Depending on the community, the fluxes of individuals may override partially or completely the impact of deforestation at downstream reaches. Chironomidae communities, for instance, did not exhibit a change in their beta-diversity when used incidence data. Although non-biting midges are weak flyers, their larvae—especially from Orthocladiinae tribe—disperse commonly through the drift (Williams 1989; Ríos-Touma et al. 2011). Precisely, the Orthocladiinae is one of the dominant groups in Andean streams (Acosta and Prat 2010; González-Trujillo and Donato-Rondon 2016), and their constant flux from the upstream reaches could allow the persistence of non-adapted taxa under adverse conditions, and consequently, keeping lower dissimilarity within our study streams. This ‘mass effect’ process (Leibold et al. 2004), can explain the null and weak changes in beta-diversity observed for incidence and abundance data, respectively. Mass effects maintain the occurrence of the major part of taxa along the river, but their abundances can diminish or increase according to their tolerance to the changes caused by deforestation. In addition, Chironomidae is a group composed by many species well-known as tolerant to environmental disturbance (e.g. Gresens et al. 2007), and consequently the stream impairment may affect less this assemblage than diatoms and EPT, driving a similar species composition among stream reaches.
The EPT communities exhibited a weaker relationship with environmental dissimilarity when used incidence data, but a stronger relationship when abundance data were used instead. It suggests that both the environmental conditions and the dispersal-ability are underlying the observed patterns. On one hand, the weaker relationship found when using incidence data provided some evidence that EPT communities are not dispersal-limited among- or within-streams. On the other hand, the strong relationship found by using the Morisita–Horn index indicates that, similar to chironomids, deforestation is probably not restricting taxa occurrence, but affecting their abundance according to their stress tolerance. The EPT communities are traditionally considered to be quite sensitive to environmental disturbances, because species usually track their preferred environmental conditions (Lenat 1993). This may justify the stronger effects of deforestation on EPT dissimilarity than in Chironomidae among stream reaches.
However, the results of the within-stream relationships also suggest that deforestation effects on EPT communities could be context-dependent. We observed that the Sørensen dissimilarity increased according to the total amount of forest loss among-streams, but not within each stream (Figs. 4, 5), and this suggests the existence of a threshold from which the composition of EPT taxa changes abruptly as affected by the environmental changes linked to deforestation. The absence of suitable places for laying eggs appears to be one of the major changes constraining EPT occurrence in deforested reaches. EPT taxa have been recorded in Andean, and worldwide streams, as active trackers of the best oviposition places, whose suitability is determined mainly by the substratum type, protruding and shading degree, and its associated water velocity (Hoffmann and Resh 2003; Encalada and Peckarsky 2006; Rios-Touma et al. 2012; Lancaster and Downes 2017). As those factors can be directly or indirectly affected by forest loss, the recruitment of EPT taxa can also be affected by the degree of deforestation. However, further understanding on oviposition patterns of these organisms is needed in order to disentangle the effect of deforestation on the recruitment process.
Implications for conservation
Our findings showed context-dependent responses to deforestation on high-Andean streams, where the type of community and the occurrence of upstream refugia can confound the assessment of this stressor. Along patchy corridors, as those of Andean streams, the existence of well-preserved upstream reaches can allow non-tolerant species to remain extant throughout dispersal-driven feedbacks. Therefore, not all communities might be adequate for assessing the impact of deforestation on high-Andean streams. Furthermore, considering both—the dispersal ability of bioindicators and the occurrence of refugia—is essential to achieve reliable conservation plans, given that they may mitigate negative local effects on biodiversity produced by human impacts such as deforestation (e.g. Bitušík et al. 2017).
The results reported here indicate three key aspects that need to be considered to enhance conservation and restoration efforts. Firstly, a deeper knowledge about the species traits (e.g. ability and mode dispersal) so our understanding of the effects of deforestation on biodiversity can be improved. Secondly, the conservation of upstream sections is a key aspect to consider for successful restoration or rehabilitation programs, as it has been previously suggested by Palmer et al. (1997). Thirdly, ecologists and conservationists should include beta-diversity measures incorporating species incidence and abundance, in addition to alpha-diversity metrics when studying deforestation effects on biodiversity.
References
Acosta R, Prat N (2010) Chironomid assemblages in high altitude streams of the Andean region of Peru. Fundam Appl Limnol 177:57–79. https://doi.org/10.1127/1863-9135/2010/0177-0057
Altermatt F, Seymour M, Martinez N (2013) River network properties shape-diversity and community similarity patterns of aquatic insect communities across major drainage basins. J Biogeogr 40:2249–2260. https://doi.org/10.1111/jbi.12178
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Armenteras D, Rodríguez N, Retana J, Morales M (2011) Understanding deforestation in montane and lowland forests of the Colombian Andes. Reg Environ Chang 11:693–705. https://doi.org/10.1007/s10113-010-0200-y
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. US Environmental Protection Agency, Office of Water Washington, DC
Barlow J, Lennox GD, Ferreira J et al (2016) Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535:144–147. https://doi.org/10.1038/nature18326
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812. https://doi.org/10.1111/j.2041-210X.2012.00224.x
Bellinger EG, Sigee DC (2015) Freshwater algae: identification and use as bioindicators. Wiley, New York
Bitušík P, Svitok M, Novikmec M et al (2017) Biological recovery of acidified alpine lakes may be delayed by the dispersal limitation of aquatic insect adults. Hydrobiologia 790:287–298
Bohonak AJ, Jenkins DG (2003) Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol Lett 6:783–796
Bojsen BH, Jacobsen D (2003) Effects of deforestation on macroinvertebrate diversity and assemblage structure in Ecuadorian Amazon streams. Arch für Hydrobiol 158:317–342. https://doi.org/10.1127/0003-9136/2003/0158-0317
Booth GD, Niccolucci MJ, Schuster EG (1994) Identifying proxy sets in multiple linear regression: an aid to better coefficient interpretation
Buss DF, Vitorino AS (2010) Rapid bioassessment protocols using benthic macroinvertebrates in Brazil: evaluation of taxonomic sufficiency. J North Am Benthol Soc 29:562–571. https://doi.org/10.1899/09-095.1
Buss DF, Carlisle DM, Chon T-S et al (2015) Stream biomonitoring using macroinvertebrates around the globe: a comparison of large-scale programs. Environ Monit Assess 187:4132
Buytaert W, Célleri R, De Bièvre B et al (2006) Human impact on the hydrology of the Andean páramos. Earth Sci Rev 79:53–72. https://doi.org/10.1016/j.earscirev.2006.06.002
Campbell Grant EH, Lowe WH, Fagan WF (2007) Living in the branches: population dynamics and ecological processes in dendritic networks. Ecol Lett 10:165–175. https://doi.org/10.1111/j.1461-0248.2006.01007.x
Chessel D, Dufour AB, Thioulouse J (2004) The ade4 package-I-one-table methods. R news. 4:5–10
De Bie T, De Meester L, Brendonck L et al (2012) Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol Lett 15:740–747. https://doi.org/10.1111/j.1461-0248.2012.01794.x
Domínguez E, Fernández HR (2009) Macroinvertebrados bentónicos sudamericanos: sistemática y biología. Fundación Miguel Lillo Tucumán
Downes BJ, Lancaster J, Glaister A, Bovill WD (2016) A fresh approach reveals how dispersal shapes metacommunity structure in a human-altered landscape. J Appl Ecol. https://doi.org/10.1111/1365-2664.12759
Elosegi A, Sabater S (2009) Conceptos y técnicas en ecología fluvial. Fundación BBVA, Bilbao
Encalada AC, Peckarsky BL (2006) Selective oviposition of the mayfly Baetis bicaudatus. Oecologia 148:526–537. https://doi.org/10.1007/s00442-006-0376-5
Etter A, Mcalpine C, Possingham H (2008) Historical patterns and drivers of landscape change in Colombia Since 1500: a regionalized spatial approach annals of the association of American geographers. Ann Assoc Am Geogr 98:2–23. https://doi.org/10.1080/00045600701733911
Gaston KJ, Blackburn TM, Greenwood JJD et al (2000) Abundance–occupancy relationships. J Appl Ecol 37:39–59
González-Trujillo JD, Donato-Rondon JC (2016) Changes in invertebrate assemblage structure as affected by the flow regulation of a páramo river. Ann Limnol 52:307–316. https://doi.org/10.1051/limn/2016018
Graham SE, Storey R, Smith B (2017) Dispersal distances of aquatic insects: upstream crawling by benthic EPT larvae and flight of adult Trichoptera along valley floors. New Zeal J Mar Freshw Res 51:146–164. https://doi.org/10.1080/00288330.2016.1268175
Gresens SE, Belt KT, Tang JA et al (2007) Temporal and spatial responses of Chironomidae (Diptera) and other benthic invertebrates to urban stormwater runoff. Hydrobiologia 575:173–190
Gutiérrez-Cánovas C, Millán A, Velasco J et al (2013) Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Glob Ecol Biogeogr 22:796–805. https://doi.org/10.1111/geb.12060
Hawkes HA (1998) Origin and development of the biological monitoring working party score system. Water Res 32:964–968. https://doi.org/10.1016/S0043-1354(97)00275-3
Heino J, Melo AS, Bini LM (2015) Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw Biol 60:223–235. https://doi.org/10.1111/fwb.12502
Hitt NP, Angermeier PL (2008) Evidence for fish dispersal from spatial analysis of stream network topology. J North Am Benthol Soc 27:304–320. https://doi.org/10.1899/07-096.1
Hoffmann A, Resh VH (2003) Oviposition in three species of limnephiloid caddisflies (Trichoptera): hierarchical influences on site selection. Freshw Biol 48:1064–1077. https://doi.org/10.1046/j.1365-2427.2003.01074.x
Horn HS (1966) Measurement of” overlap” in comparative ecological studies. Am Nat 100:419–424
Iñiguez-Armijos C, Leiva A, Frede HG et al (2014) Deforestation and benthic indicators: how much vegetation cover is needed to sustain healthy Andean streams? PLoS ONE. https://doi.org/10.1371/journal.pone.0105869
Jost L, Chao A, Chazdon RL (2011) Compositional similarity and beta diversity. Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford, pp 66–84
Kärnä OM, Grönroos M, Antikainen H et al (2015) Inferring the effects of potential dispersal routes on the metacommunity structure of stream insects: as the crow flies, as the fish swims or as the fox runs? J Anim Ecol 84:1342–1353. https://doi.org/10.1111/1365-2656.12397
Kitto JAJ, Gray DP, Greig HS et al (2015) Meta-community theory and stream restoration: evidence that spatial position constrains stream invertebrate communities in a mine impacted landscape. Restor Ecol 23:284–291. https://doi.org/10.1111/rec.12179
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds.) Süsswasserflora von Mitteleuropa. Bd. 2, Teil 1. Gustav Fischer, Stuttgart
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds.) Süsswasserflora von Mitteleuropa. Bd. 2, Teil 2. Gustav Fischer, Stuttgart
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliterarurverzeichnis. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds.) Süsswasserflora von Mitteleuropa. Bd. 2, Teil 4. Gustav Fischer, Stuttgart
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds.) Süsswasserflora von Mitteleuropa. Bd. 2, Teil 3. Gustav Fischer, Stuttgart
Lancaster J, Downes BJ (2017) Dispersal traits may reflect dispersal distances, but dispersers may not connect populations demographically. Oecologia 184:171–182. https://doi.org/10.1007/s00442-017-3856-x
Leibold MA, Chase JM (2018) Metacommunity ecology, 1st edn. Princeton University Press, Princeton
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Leira M, Sabater S (2005) Diatom assemblages distribution in catalan rivers, NE Spain, in relation to chemical and physiographical factors. Water Res 39:73–82
Lenat DR (1993) A biotic index for the southeastern United States: derivation and list of tolerance values, with criteria for assigning water-quality ratings. J North Am Benthol Soc 12:279–290
Lencioni V, Marziali L, Rossaro B (2012) Chironomids as bioindicators of environmental quality in mountain springs. Freshw Sci 31:525–541. https://doi.org/10.1899/11-038.1
Liu J, Soininen J, Han BP, Declerck SAJ (2013) Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. J Biogeogr 40:2238–2248. https://doi.org/10.1111/jbi.12160
Lorion CM, Kennedy BP (2009) Relationships between deforestation, riparian forest buffers and benthic macroinvertebrates in neotropical headwater streams. Freshw Biol 54:165–180. https://doi.org/10.1111/j.1365-2427.2008.02092.x
Macneale KH, Peckarsky BL, Likens GE (2005) Stable isotopes identify dispersal patterns of stonefly populations living along stream corridors. Freshw Biol 50:1117–1130. https://doi.org/10.1111/j.1365-2427.2005.01387.x
Malmqvist B (2000) How does wing length relate to distribution patterns of stoneflies (Plecoptera) and mayflies (Ephemeroptera)? Biol Conserv 93:271–276. https://doi.org/10.1016/S0006-3207(99)00139-1
Merritt RW, Cummins KW (2008) An introduction to the aquatic insects of North America. Kendall/Hunt, Dubuque
Metzeltin D, Lange-Bertalot H (2007) Tropical diatoms of South America II. Special remarks on biogeography disjunction. Iconogr Diatomol 18:1–877
Milošević D, Simić V, Stojković M et al (2013) Spatio-temporal pattern of the Chironomidae community: toward the use of non-biting midges in bioassessment programs. Aquat Ecol 47:37–55
Múrria C, Rugenski AT, Whiles MR, Vogler AP (2015) Long-term isolation and endemicity of Neotropical aquatic insects limit the community responses to recent amphibian decline. Divers Distrib 21:938–949. https://doi.org/10.1111/ddi.12343
Myers N, Fonseca GAB, Mittermeier R et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Oksanen J, Blanchet FG, Kindt R et al (2013) Package ‘vegan.’ Community Ecol Packag version 2
Padial AA, Ceschin F, Declerck SAJ et al (2014) Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9:1–8. https://doi.org/10.1371/journal.pone.0111227
Palmer MA, Ambrose RF, Poff NL (1997) Ecological theory and community restoration ecology. Restor Ecol 5:291–300. https://doi.org/10.1046/j.1526-100X.1997.00543.x
Passy SI (2008) Continental diatom biodiversity in stream benthos declines as more nutrients become limiting. Proc Natl Acad Sci USA 105:9663–9667. https://doi.org/10.1073/pnas.0802542105
Poff NL, Olden JD, Vieira NKM et al (2006) Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J North Am Benthol Soc 25:730–755. https://doi.org/10.1899/0887-3593(2006)025%5b0730:FTNONA%5d2.0.CO;2
Prat N, Rieradevall M, Acosta R, Villamarín C (2011) Guía para el reconocimiento de las larvas de Chironomidae (díptera) de los ríos altoandinos de Ecuador y Perú. Grup Investig FEM Dep Ecol Univ Barcelona, España, p 78
Prat N, González-Trujillo JD, Ospina-Torres R (2014) Key to chironomid pupal exuviae (Diptera: Chironomidae) of tropical high Andean streams. Rev Biol Trop 62:1385–1406
Qian H, Ricklefs RE, White PS (2004) Beta diversity of angiosperms in temperate floras of eastern Asia and eastern North America. Ecol Lett 8:15–22. https://doi.org/10.1111/j.1461-0248.2004.00682.x
R Core Team (2018) R: A language and environment for statistical computing
Richardson JS, Béraud S (2014) Effects of riparian forest harvest on streams: a meta-analysis. J Appl Ecol 51:1712–1721. https://doi.org/10.1111/1365-2664.12332
Rios-Touma B, Encalada AC, Prat N (2012) Oviposition of aquatic insects in a tropical high altitude stream. Environ Entomol 41:1322–1331. https://doi.org/10.1603/EN12037
Ríos-Touma B, Prat N, Encalada AC (2011) Invertebrate drift and colonization processes in a tropical andean stream. Aquat Biol 14:233–246. https://doi.org/10.3354/ab00399
Rodríguez Eraso N, Armenteras Pascual D, Alumbrerosa R (2012) Land use and land cover change in the Colombian Andes: dynamics and future scenarios. J Land Use Sci 8:154–174
Sarremejane R, Mykrä H, Bonada N et al (2017) Habitat connectivity and dispersal ability drive the assembly mechanisms of macroinvertebrate communities in river networks. Freshw Biol 62:1073–1082. https://doi.org/10.1111/fwb.12926
Sedell JR, Reeves GH, Hauer FR et al (1990) Role of refugia in recovery from disturbances: modern fragmented and disconnected river systems. Environ Manag 14:711–724. https://doi.org/10.1007/BF02394720
Serra SRQ, Graça MAS, Dolédec S et al (2017) Chironomidae traits and life history strategies as indicators of anthropogenic disturbance. Environ Monit Assess 189:326. https://doi.org/10.1007/s10661-017-6027-y
Siqueira T, Durães L, de Roque FO (2014) Predictive modelling of insect metacommunities in biomonitoring of aquatic networks. Ecol Model Appl Entomol. Springer, Cham, pp 109–126
Socolar JB, Gilroy JJ, Kunin WE, Edwards DP (2016) How should beta-diversity inform biodiversity conservation? Trends Ecol Evol 31:67–80. https://doi.org/10.1016/j.tree.2015.11.005
Soininen J (2007) Environmental and spatial control of freshwater diatoms: a review. Diatom Res 22:473–490. https://doi.org/10.1080/0269249X.2007.9705724
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biol Skr 5:1–34
Thompson R, Townsend C (2006) A truce with neutral theory: local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. J Anim Ecol 75:476–484. https://doi.org/10.1111/j.1365-2656.2006.01068.x
Tilman D, Kilham SS, Kilham P (1982) Phytoplankton community ecology: the role of limiting nutrients. Annu Rev Ecol Syst 13:349–372. https://doi.org/10.1146/annurev.es.13.110182.002025
Tonkin JD, Stoll S, Sundermann A, Haase P (2014) Dispersal distance and the pool of taxa, but not barriers, determine the colonisation of restored river reaches by benthic invertebrates. Freshw Biol 59:1843–1855. https://doi.org/10.1111/fwb.12387
Tonkin JD, Altermatt F, Finn DS et al (2018) The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshw Biol 63:141–163. https://doi.org/10.1111/fwb.13037
Tornés E, Ruhí A (2013) Flow intermittency decreases nestedness and specialisation of diatom communities in Mediterranean rivers. Freshw Biol 58:2555–2566. https://doi.org/10.1111/fwb.12232
Tornés E, Mor J-R, Mandaric L, Sabater S (2018) Diatom responses to sewage inputs and hydrological alteration in Mediterranean streams. Environ Pollut 238:369–378. https://doi.org/10.1016/j.envpol.2018.03.037
Trivinho-Strixino S, Strixino G (1995) Larvas de Chironomidae (Diptera) do Estado de São Paulo: guia de identificação e diagnose dos gêneros
Wickham H (2010) ggplot2: elegant graphics for data analysis. J Stat Softw 35:65–88
Williams CJ (1989) Downstream drift of the larvae of Chironomidae (Diptera) in the River Chew, SW England. Hydrobiologia 183:59–72
Winking C, Lorenz AW, Sures B, Hering D (2014) Recolonisation patterns of benthic invertebrates: a field investigation of restored former sewage channels. Freshw Biol 59:1932–1944. https://doi.org/10.1111/fwb.12397
Zuur AF, Ieno EN, Walker NJ et al (2009) In: Gail R, Krickeberg K, Samet JM, Tsiatis A, Wong W (eds) Mixed effects models and extensions in ecology. Springer, New York
Acknowledgements
We would like to thank to the two anonymous reviewers who help to improve substantially our manuscript. We are grateful to Liz Alonso, Katherine Pallares, Laura Fernández and Daniel Amézquita for their help in the field and the laboratory. Special thanks to ‘La Chorrera’ Natural Park for providing access to ‘Chiflón’ stream, and to Miguel Rodriguez and his family for hosting us during our field work. The surveys were financed by the Universidad Nacional de Colombia—Sede Bogotá, through DIB project no. 34856 (CONVOCATORIA NACIONAL DE PROYECTOS PARA EL FORTALECIMIENTO DE LA INVESTIGACIÓN, CREACIÓN E INNOVACIÓN DE LA UNIVERSIDAD NACIONAL DE COLOMBIA 2016–2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González-Trujillo, J., Petsch, D., Córdoba-Ariza, G. et al. Upstream refugia and dispersal ability may override benthic-community responses to high-Andean streams deforestation. Biodivers Conserv 28, 1513–1531 (2019). https://doi.org/10.1007/s10531-019-01739-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-019-01739-2