Abstract
Home gardens have received increasing attention and have been insistently presented as hotspots for agro-biodiversity over the last decades. However, apart from their exceptional high plant species diversity, there is little quantitative evidence of the effectiveness of plant species conservation in home gardens. This study examined this issue by assessing (i) the size and membership of garden flora and the contribution to the maintenance of the national flora, (ii) how home garden flora connects to the larger ecosystem it belongs to and (iii) the conservation status of plant species at the home garden level. 360 home gardens distributed in three agro-ecological zones and nine phyto-geographical districts in Benin were visited and inventoried. Diversity parameters at different taxonomic levels were calculated. Species accumulation and spatial occupancy, multivariate methods and rarity index were also used for data analysis. Findings showed that the 360 studied home gardens hosted up to 14.21% of plant species and 44.32% of plant families of the national flora. Home garden flora was constantly dominated by exotic plant species but strongly connected to their surrounding ecosystems, being composed of at least 60% of plant species from their phyto-geographical districts. Finally, home garden plant species were mostly rare and threatened at the home garden level. In this study, we acknowledge the contribution of home gardens to the maintenance of plant species diversity at regional and global levels than local level. Based on the observed prevalence of exotic species, HG effectiveness in sustainably conserving native plant species biodiversity remains questionable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity loss is a major driver of ecosystem change (Hooper et al. 2012) and an issue of high concern in conservation sciences. All around the world, human-influenced ecosystems are experiencing an acceleration at a rate that crops and wild plant species are diminishing (Cardinale et al. 2012), mainly due to human alteration of ecosystems (Baillie et al. 2004; Green et al. 2005). Over the last twenty years, considerable attempts were made towards understanding how biodiversity loss affects ecosystem services provision and human well-being (Díaz et al. 2006; Cardinale et al. 2012). Until now, an innovative and sustainable approach to biological resource conservation remains a challenge, as pointed out in the top hundred unanswered questions of importance in agriculture (Pretty et al. 2010) and conservation (Sutherland et al. 2009). In line with the pressing need for biodiversity conservation, among others, the on-farm conservation approach has received important attention (Dawson et al. 2013). Thus, agroforestry systems and particularly home gardens (HGs) are receiving increasing attention. Home gardens (HGs) are traditional farming systems, presumably the oldest land use system (Pushpakumara et al. 2012) in the world. These cultivation systems are generally adjacent to homesteads or slightly further away but easily accessible (Sunwar et al. 2006). In spite of being human influenced and controlled ecosystems, HGs have been abundantly reported to have prospective added value for agro-biodiversity conservation (Watson and Eyzaguirre 2002; Sunwar et al. 2006; Galluzzi et al. 2010; Calvet-Mir et al. 2012a, b; Amberber et al. 2014; Salako et al. 2014; Heraty and Ellstrand 2016; Junqueira et al. 2016). Agro-biodiversity refers to the subset of natural biodiversity which includes the plant genetic resources used for food and agriculture (Negri 2005) as well as wild edible and non-edible plant that are maintained in home gardens for different purposes.
Yet, apart from their exceptional high plant species diversity, there is little quantitative evidence on the capacity of HGs to sustainably conserve this biodiversity, especially the local plant diversity. This issue has led to daunting questions: Could HGs provide long-term biodiversity conservation services in addition to other ecosystem services? Do HGs represent a viable conservation alternative to other forms of agroforestry?
In Benin, home gardening is a common cultivation activity in addition to twelve other farming systems (Igue et al. 2000). Home gardening is encountered in both rural and urban regions and contributes to household food systems (Gerard Grubben et al. 2014; Perrin et al. 2015) and traditional home care (Quiroz et al. 2014) among other functions. They have, for a long time, been neglected by researchers but have received increasing attention these last five years. We now know more about their spatial configuration, ownership, structure, plant diversity, functions and associated traditional knowledge (Idohou et al. 2014; Salako et al. 2014; Gbedomon et al. 2015). Salako et al. (2014) showed the conservation benefits of HGs in Benin stressing their high agro-biodiversity including important crop wild relatives and red-listed species. However, the quality of the conservation benefits is still questionable, and important questions still need to be elucidated:
-
1.
The Benin flora is about 2807 plant species (Akoègninou et al. 2006; Neuenschwander et al. 2011). Therefore, the pool of species available to gardeners is large. So, what proportion of this flora is maintained in HGs? How do native and exotic plant species contribute to HG flora?
-
2.
HGs as ecosystems are part of a larger ecosystem e.g. phyto-geographical districts (PDs); and agro-ecological zones (AEZs). Thus, how does the flora of HGs connect to the flora of the PDs they belong to?
-
3.
The main added value of HGs for biodiversity conservation is related to their capacity to represent agro-biodiversity at multiple levels over small spaces (Hodgkin et al. 2002). Thus, how widespread might individual plant species be? Finally, HGs were reported to be hotspots for agro-biodiversity (Salako et al. 2014). Thus, what is the plant species conservation status at the HG level? What is the situation of plant species already threatened and red listed by IUCN and Benin? What are the priority sites for active conservation actions?
Materials and methods
Study area
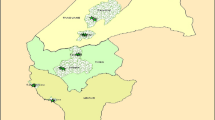
The study was carried out in Benin (Fig. 1). The rainfall distribution in Benin shows two types of climate with a region of transition. In the southern part (humid and sub humid zones), the climate is tropical humid with two rainfall maxima corresponding to two rainy seasons: March–July and September–November. In the Northern part (semi-arid), the climate is Sudanian with one rainy season covering May to October and a long dry season covering November to May (Adomou 2005; Neuenschwander et al. 2011).
The native vegetation is composed of fallows and small forest patches of less than 5 ha in the humid zone (Sinsin et al. 2004). The sub-humid zone consists of mosaics of woodlands, whereas the semi-arid zone is dominated by savannas and gallery forests with trees and shrubs slightly covering the ground (Sinsin et al. 2004). Based on a meso-scale analysis of these vegetation patterns, Benin has been divided into 10 PDs (Fig. 1), which also differ with regard to floristic composition, climatic conditions and soil types (Adomou 2005). The humid zone comprises 4 PDs: a costal PD; the PD of Pobe; the Oueme Valley PD and the plateau. The sub humid zone includes 3 PDs: the PD of Bassila; the Zou PD and the Southern Borgou PD. The semi-arid zone is composed of 3 PDS: the Northern Borgou, the Atacora Chain PD and the Mekrou–Pendjari PD (Table 1).
The resident population of Benin is of about 9,983,884 inhabitants and slightly female-biased (51.2%) (INSAE 2013). The economy is based mostly on agriculture (INSAE 2013). In order to fulfil their daily needs, people in both rural and urban areas usually maintain and cultivate the relatively small space surrounding their houses. These systems of production, called “home gardens”, are traditional farming systems in addition to approximately twelve other farming systems known in Benin (Igue et al. 2000). Even if ethnic groups are specific to agro-ecological regions, population and especially some ethnic groups used to migrate across the regions for many purposes including for agriculture. These ethnic migrations contribute to the propagation and persistence of some plant species (Assogbadjo et al. 2012).
Concept clarification
Some concepts have been used in this paper including agro-biodiversity and the different status of its constituent plant species. Agro-biodiversity includes diversity of crops species (Improved cultivars and landraces), crop wild relatives and wild plants. Apart from taxonomic patterns (family, genus and species), agro-biodiversity of gardens could be characterised through different status of the constituent plant species: Cultivated plant species versus wild plant species; native plant species versus exotic plant species; In-PD plant species versus Out-PD plant species (Table 2).
Sampling and data collection
The data used in this study are compiled from a larger data set collected in the framework of an ongoing research project on HGs in Benin (Idohou et al. 2014; Salako et al. 2014; Gbedomon et al. 2015). The overall methodology developed for sampling and data collection included a participative diagnosis, field observations (walking and visits of gardens), gardens inventories as well as individual and group interviews. The sampling design included both probabilistic and non-probabilistic approaches. First, a rapid rural appraisal was carried out in all three AEZs by a multidisciplinary team (agronomists, socio-economists and ecologists), along with agricultural extension services and local farmers’ organizations to identify PDs of interest with regards to home gardening. The main question during the interview was: where are important areas of home gardening in your region? An exploratory survey was conducted on 100 randomly selected informants in each AEZ to determine a proportion p of household with home gardens and consequently the sample size (n) in each AEZ zone. The estimation of n was done using the Normal approximation of the binomial distribution (Dagnelie 1998).
In Eq. (1), U 21−α/2 is the value of the Normal random variable at a probability value of 1 − α/2. For α = 0.05, U 21−α/2 = 3.84; d is the margin error of the estimation of any parameter value computed from the survey, assumed to be 8% (Idohou et al. 2014; Gbedomon et al. 2015); estimated values of p were 20, 31 and 34% for the humid, the sub-humid and the semi-arid zone, respectively. Based on the extent of PDs as well as the importance of home gardening (assessed during the interview with local agricultural extension services and local farmers’ organizations), the estimated value of n for a given AEZ was partitioned among PDs of that AEZ (Table 3).
At PD level, 3–7 villages (for a total of 44 villages) selected as of high importance for home gardening were visited. To capture most of the variation, snowball technique (Goodman 1961) was coupled with HGs visits for the selection of households practicing home gardening. 3–5 key informants (head of household with HG) were recruited and joined the team. With their assistance the research team walked around the village and visited HGs. For each visited HG, we recorded the geographical coordinates using GPS Garmin 60, the name of the head of the household associated to the garden and took pictures. A list of HGs visited was established and each HG was assigned a number. Random table was used to select from the established list households that will participate in the study.
Data were collected between May 2014 and April 2015, and included garden inventories as well as individual interviews with the assistance of local translators. HGs were visited during both rainy and dry seasons to capture most of the variation in species composition. For each garden, an exhaustive inventory of plant species was carried out with the assistance of HG owner. Weeds were not inventoried. Inventoried plants were identified at the species level and named following the botanical nomenclature of Lebrun and Stork (1991). Vouchers of plants that could not be clearly identified on the fields were collected and preserved for final identification by botanists of Benin’s national herbarium. Native or exotic status of identified plant species were obtained using the analytical flora of Benin (Akoègninou et al. 2006), the online database on species distribution (http://www.catalogueoflife.org/) and with the assistance of the national herbarium of Benin.
Data analysis
Species richness and composition of the garden flora
All statistical analyses were performed in R software (R Core Team 2016). Descriptive statistics were used to describe occurrence of botanical families, native versus exotic species, cultivated versus wild species per PD and for the whole country. Proportion of native and exotic species were calculated per HG and compared between PDs using a generalized linear model (GLM) with beta distribution in the package “betareg” (Cribari-Neto and Zeileis 2009).
Size of HGs flora referred to species richness and completeness of the sampling. Species accumulation curves were thus established using 100 random permutations with the first order jackknife method in package “vegan” function specaccum, (Oksanen et al. 2016). The first order jackknife method is a non-parametric estimation which was shown to yield less biased estimation (Palmer 1990). This analysis was performed for the whole country and per PD, distinguishing native and exotic species.
Pearson correlation was used to test the link between the total plant species richness and the number of exotic plant species in HGs.
Home gardens flora, connection to and maintenance of local flora
In the context of this study, local flora referred to natural vegetation of each PD and was obtained from Adomou (2005) and from the ministry of agriculture of Benin (for cultivated species). Proportion of In-PD plant species within HG flora was calculated to assess to what extent HGs maintain the local flora and to what extent the local flora was influenced by flora from other PDs (out-PD). This data was submitted to a GLM with beta distribution to compare PDs. To assess similarity of floristic composition amongst PDs, a matrix describing for each PD, the percentage of HGs of that PD containing each species was established and submitted to a non-metric multidimensional scaling (NMDS), (Kruskal 1964; Salako et al. 2013). The NMDS was based on Jaccard dissimilarity index in package “vegan” (Oksanen et al. 2016). Additionally, a pairwise Pearson correlation was used to assess the magnitude of the similarity between PDs. Finally, Pearson correlation was used to test whether the level of influence by the flora from another PD was dependent on the distance to that PD.
Garden occupancy and conservation status of HGs plant species
Garden occupancy was used to assess the overall distribution of individual plant species. The garden occupancy of individual plant species was calculated by dividing the total number n of gardens where the species was found by the total number of gardens visited and expressed as %. The garden occupancy of each species was calculated for the whole Benin but also for each PD to capture to capture any variation that may occur. Garden occupancy was categorized as garden occupancy < 1%, 1% < garden occupancy < 5%, 5% < garden occupancy < 10%, 10% < garden occupancy < 25%, 25% < garden occupancy < 50%, 50% < garden occupancy < 75% and garden occupancy > 75%. Pearson Chi square test was used to test independence between occupancy and the type of species (native versus exotic) at country and PD levels. The number of singleton species (species found in only one garden), and doubleton species (species found in only two gardens from one or two PDs) were also determined.
Conservation status of HG plant species was assessed using on the one hand, a rarity index and on the other hand, the IUCN and Benin red lists of threatened species. Rarity index was deduced from garden occupancy at country level as 100—garden occupancy. Different thresholds of rarity index were used in the past to determine whether plant species are frequently found or rare, depending on the specificity of the studied flora (Ferrari et al. 2000). In this study, due to the high dynamic of HGs flora composition and based on garden occupancy, only plant species with rarity index >90%—occurring in less than 10% of garden—were considered as rare. Therefore plant species with rarity index <90% were assumed to be frequently found and thus not of priority for conservation actions.
Next, the geographic distribution of the rare plant species was assessed and a conservation status was assigned to plant species within HGs using IUCN criterion “b” (Nature et al. 2001) defined by the extent of the area of occurrence, population size, and population fragmentation. The criterion involves the number of locations (NL)—here number of PDs—in which individual plant species occurs. In this study, the plant species was considered as critically endangered (CR) when NL = 1, EN (endangered) when 2 ≤ NL ≤ 3, VU (vulnerable) when 4 ≤ NL ≤ 6, and LC when NL ≥ 7.
Finally, when available, the official conservation status of the rare plant species outside HGs was collected from IUCN Database version 2016-1 (IUCN 2016) and from the red list of species in Benin (Neuenschwander et al. 2011) for comparison purposes.
Results
Species richness and composition of the garden flora
3848 specimens were recorded, 323 plant species identified corresponding to 226 genera and 81 families. The most present families were Fabaceae (42 species and 31 genera), Euphorbiaceae (22 species and 11 genera) and Malvaceae (21 species and 12 genera). Half of the families’ richness (41) were made up of only one species (Fig. 1a, b in Supplementary material). Out of the 323 plant species, 185 (57.27%) were wild plant species while 138 (42.73%) were crops and planted species. 153 (47.37%) plant species were native, whereas 170 (52.63%) were exotic. Out of the 153 native plant species recorded, 37 (24.03%) were crops and planted species and 117 (75.97%) were wild plant species. Regarding the 170 exotic plant species, 101 (59.76%) were crops and planted species whereas 68 (40.24%) were wild plant species. Native and exotic plant species belonged approximately to the same number of families: 57 for native species and 56 for exotic species. The top ten families of exotic plants had on average (mean ± standard error) more species (9.9 ± 2.33) than those of native species (7.9 ± 1.32), though not significant (GLM with negative binomial error, z-value = −0.935, p value = 0.350). The top ten families of native species had 4–17 plant species, whereas those of exotic species contained 5–30 species (Table 4).
Although HG flora was nationally dominated by exotic plant species, the trend varied among PDs (GLM with beta distribution, z-value = 19.42, p-value <0.001, pseudo-R2 = 0.52). At PD level, there was a significant difference between the proportions of exotic and native plant species (p-value <0.05). The proportion of exotic species was in average greater than 60% (60 ± 11.35 to 69.78 ± 7.44) in Northern Borgou, Plateau, Pobe, Southern Borgou and Zou, and it was lower but greater than 50% (53.25 ± 12.18 to 55.41 ± 12.90) in the other PDs (Fig. 2).
Furthermore, there was a positive and significant correlation (r = 0.92, p-value <0.001) between HG total plant species richness and exotic plant species richness, indicating that the higher the species richness, the higher the number of exotic species.
When analysing the species richness of HG flora, the species accumulation curves (Fig. 2 in Supplementary material) did not reach the asymptote neither for the whole country at the 360 gardens nor for any of the PDs at the subsample size—number of gardens visited for that PD. The estimated asymptotic species richness (Table 5) for the whole country was 398.79 ± 11.81 (Table 5), suggesting a gap of ~75 plant species not yet recorded. The lowest gap was obtained for Plateau PD while the highest gap was obtained for Bassila PD (Table 5).
For the whole country and each PD, the species turnover was greater for exotic plants. The overall predicted plant species richness was 206.90 ± 6.98 for exotic plant species and about 191 ± 8.17 for native plant species.
Home garden flora and its connection to and maintenance of local flora
The capacity of HGs to maintain In-PD species, i.e. species of the PD they belong to, varied greatly across PDs (GLM with beta distribution, z-value = 12.91, p-value <0.001, pseudo-R2 = 0.35). There was a positive and significant correlation (r = 0.916, p-value <0.01) between the total plant species richness and the In-PD plant species richness in each HG (Fig. 3b). In contrast, the proportion of local species to overall species was negatively correlated with the overall plant diversity of HG (Person correlation = −0.195; p < 0.001). Thus, HGs with higher plant species richness were likely to maintain more In-PD plant species richness. HG flora was composed on average of 60% of local flora of the PD they belonged to with Plateau and Oueme Valley composed of at least 90% of local species, respectively 90.59 and 97.69%. Therefore, in terms of the capacity to host In-PD plant species, gardens of Plateau and Oueme Valley were more efficient than those of Bassila and Zou, although the latter were more diversified with 134 and 89 local species respectively.
When analysing the capacity of HGs to host plant species belonging to other PDs (i.e. out-PD species), the analysis showed a positive and significant but weak correlation (r = 0.312, p-value <0.01) between the proportion of out-PD species and distance from the PD. The influence of the flora of a given PD on the flora from another PD, even if weak, depended on the distance between those PDs. Thus, neighbouring PDs were generally similar in terms of garden flora composition: Atacora Chain and Mekrou–Pendjari (r = 0.79, p-value <0.01), Oueme Valley and Plateau (r = 0.75, p-value <0.01), Northern Borgou and Southern Borgou (r = 0.75, p-value <0.05), Zou and Plateau (r = 0.63, p-value <0.05). This trend was confirmed by the non-metric multidimensional scaling of the garden flora of PDs (Fig. 4a), which separated groups of PDs based on the similarity of their garden flora: Atacora Chain and Mekrou–Pendjari; Northern Borgou and Southern Borgou; Zou; Plateau and Oueme Valley; Pobe; Bassila. At the AEZ level, garden flora of the semi-arid zone (Atacora Chain, Mekrou–Pendjari, Northern Borgou and Southern Borgou) was different to that of the humid zone (Plateau and Oueme Valley). Depending on their geographical proximity, the garden flora of sub-humid zones was similar to that of either the semi-arid zone or the humid zone. Then, the garden flora of Southern Borgou, geographically close to the semi-arid zone, was similar to that of this zone, whereas that of Zou, geographically close to humid zone, was similar to that of the humid zone. Gardens from Bassila and Pobe showed singularity in their composition and were not similar from each other or from the gardens of other PDs.
Garden occupancy and conservation status of HG plant species
Regardless of the native or exotic status, most of the 323 plant species recorded occurred in less than 10% of visited HGs (Fig. 5a). Indeed, 90.82% of plant species were found in less than 10% of visited HGs, with 48.73% occurring in less than 1% of visited HGs and 76.89% occurring in less than 5% of visited HGs (Fig. 5a). None of the plant species occurred in more than 75% of HGs (Fig. 5a).
There were significant differences of garden occupancy across PDs (χ 2, ψ2 = 170.02, DF = 32, p-value <0.001; Fig. 5b–j), with more than 50% of plant species at PD level found in less than 10% of HGs.
Significant differences were found in garden occupancy of native and exotic species at the country level (χ 2 test, ψ2 = 13.5, DF = 4, p-value = 0.009). All native plant species occurred in less than a quarter of HGs, with 95.61% of them found in less than 10% of HGs and 57.92% occurring in less than 1% of HGs (Fig. 5a). As regards to exotic plant species, all of them occurred in almost 75% of HGs, with 88.16% occurring in less than 10% of HGs and 41.42% in less than 1% of HGs. Therefore, native plant species were more restricted in their occupancy of gardens compared to exotic plant species.
The 360 HGs flora contained 76 singleton species and 42 doubleton species. Singleton species were found mostly in Bassila (29 singleton species), Atacora Chain (12 singleton species) and Zou (9 singleton species). 24 doubleton species in HGs within the same PD were found mainly in Bassila (12 doubleton species) and Plateau (4 doubleton species). 18 doubleton species occurring in two PDs were found mostly in Mekrou–Pendjari (7 doubleton species), Plateau (6 doubleton species) and Bassila (5 doubleton species).
Of the 76 singleton species, 40 were native and 36 exotic. 75% of native singleton species were found in Bassila (37.5%), Zou (20%) and Atacora Chain (17.5%). 75% of exotic singleton species were confined to Bassila (38.89%), Atacora Chain (13.89%), Northern Borgou (11.11%) and Southern Borgou (11.11%).
Of the 42 doubleton species, 28 were native species, and 14 were exotic species. However, 17 native doubleton species occurred in the same PD, whereas 11 occurred in two PDs. 7 exotic doubleton species occurred in the same PD, whereas 7 other exotic doubleton species occurred in two PDs. Native doubleton species were mostly found in Bassila, comprising 13 doubleton species among which 10 doubleton species were from the same PD. With regards to exotic doubleton species, they occurred mainly in Plateau, with 5 doubleton species (3 within the same PD), and in Bassila, with 4 doubleton species (2 within the same PD).
From the pool of 323 plant species recorded in HGs, only 32 species, representing 9.91% of the total, have already been evaluated for their conservation status and red listed by IUCN, Benin or both IUCN and Benin.
Of the 23 species red listed by IUCN and recorded in HGs, three were threatened by extinction (vulnerable): Khaya senegalensis, Rhodognaphalon brevicuspe and Vitellaria paradoxa, whereas two were near threatened: Irvingia gabonensis, Milicia excelsa; and the other 18 were of least concern or data deficient.
Of the 12 species red listed in Benin and recorded in HGs, 11 were threatened, among which 7 were vulnerable species: Borassus aethiopum, Chrysophyllum albidum, Kigelia africana, Pentadesma butyracea, Terminalia superba, V. paradoxa and Zanthoxylum zanthoxyloides, 2 were endangered species (K. senegalensis, Christiana africana); and 2 were extinct in the wild (Acanthus montanus, Caesalpinia bonduc).
Some of the abovementioned threatened species were widely distributed across PDs. For instance, K. senegalensis was recorded in 6 out of 9 PDs, C. bonduc was found in 5 out 9 PDs and V. paradoxa was found in 4 out of 9 PDs. Other threatened species were confined to two PDs (i.e. M. excelsa in Bassila and Zou) and some to one PD (P. butyracea in Bassila).
Regardless of their conservation status assigned by IUCN or Benin red lists of threatened species, the analysis of rarity index of the 323 plant species recorded in HGs revealed that 91.40% of HG plant species were not frequent, with 36.53% of the flora being singleton or doubleton species. Based on the number of locations—the number of PDs where the species occurred—four categories of conservation status of species in HGs were obtained: CR (41.02%), EN (33.68%), VU (18.95%) and LC (6.62%). When considering three of the threatened species categories (CR + EN + VU), more than 90% of uncommon HG plant species were threatened. Although exotic plant species dominated HG flora, they were more threatened than native species (Fig. 6).
Conservation status of uncommon plant species based on number of locations (a) and zones of occurrence of rare plant species (b). Threatened status: LC least concern, VU vulnerable, EN endangered, CR critically endangered. PDs: AC Atacora Chain, MP Mekrou-Pendjari, NB Northern Borgou, SB Southern Borgou, BA Bassila, ZO Zou, PO Pobe, OV Oueme Valley, PL Plateau.
Considering the proportion of threatened species hosted at PD level, more than 50% of the garden flora at all PDs levels (Table 6) were threatened, with BA having more than 80% of its species threatened.
Discussion
This study examined the conservation benefits of HGs in Benin. It investigated their contribution to the maintenance of In-PD plant species, assessed the conservation status of plant species at the HG ecosystem level and discussed the implications of the observed results for the conservation of plant species, either crops or wild.
The findings showed that the HG flora in Benin is composed of diversified plant species that represent the PD’s surrounding vegetation. Unfortunately, most of these species were found to be threatened at the HG level.
When considering the pool of flora either observed (323 plant species) or predicted (398.79 ± 11.81), HGs in Benin hosted between 11.51 and 14.21% of plant species and 44.32% of plant families of the national flora. The species accumulation curves did not reach the asymptote after the 360 visited gardens, suggesting that additional effort is required for sampling and garden inventories. Therefore, the true proportion of national flora hosted in HGs could be higher. These figures confirm HGs are important hotspots of agro-biodiversity as already reported in the past (Galluzzi et al. 2010; Srithi et al. 2012; Clarke et al. 2014; Salako et al. 2014) and commonly accepted by scientists and conservation organizations around the world. HGs in Benin are also proven to host important wild plant species (57.27% of garden flora). However, looking at the membership of garden flora, the findings showed the predominance of exotic plant species (52.63%), as also observed by Smith et al. (2006) in HGs in the UK. With regards to the distribution of plant diversity and its membership across PDs and AEZs, gardens from Bassila and Zou (sub-humid zone) were the richest (ranging 128–166 species), whereas the less rich gardens (ranging 79–87 species) were from Plateau, Oueme valley and Pobe (humid zone). For gardens in the sub-humid zone, their greater plant diversity was also reported by Salako et al. (2014). This could be explained by the geographical position and natural attributes of the sub-humid zone, which, acting as a crossroad, concentrates most ethnic groups of the country (Judex et al. 2009; Etnologue 2015) and offers large farmlands where people from the two other AEZs migrate from temporarily or indefinitely for agricultural purposes. Regarding the relatively low plant diversity in the humid zone, among other reasons, the small size of gardens of humid zone (Salako et al. 2014) is probably the driver of its low species richness (Smith et al. 2006; Gbedomon et al. 2015).
For all PDs in the AEZs, exotic plant species dominated the garden flora, suggesting that HGs are better conservation ecosystem at regional and global level rather than local level. The high prevalence of exotic plant species in gardens can be related to the attributes of gardens where important tradeoffs (Taylor et al. 2016) occur to maintain different functions (Idohou et al. 2014) including food, medicine, ornamentation, etc. Because food production is the primary function of HGs (Kumar and Nair 2004), garden flora is consistently influenced by this function (Skarbø 2014) and is largely composed of food species, as for instance in Benin (Idohou et al. 2014; Salako et al. 2014). These food species, mainly the domestic crops, are well known to be mostly exotic in most of the places they are grown (Marris 2014). The presence of exotic plant species in HGs is then an intrinsic attribute of HGs and is observed elsewhere in the world (Smith et al. 2006; Woldeyes et al. 2016). In addition to food species, HGs also harbor numerous medicinal and nutraceutical plant species worldwide (Albuquerque et al. 2005; Bajpai et al. 2013; Clarke et al. 2014; Furlan et al. 2016) and, in particular, in Benin (Idohou et al. 2014; Salako et al. 2014). Some studies have reported the predominance of exotic species in the pool of medicinal herbs. (Albuquerque 2006) explained this predominance of exotic species by the hypothesis of diversification. This hypothesis considers that the presence of exotic species in local traditional medicine takes the role of increasing the spectrum of alternatives for treating specific illnesses. Therefore, exotic species shall ameliorate the local medicinal plant formulary with species containing highly bioactive secondary metabolic compounds. This is likely to be one reason supporting the predominance of exotic species in HGs and corroborates the view that the primary driver of HGs is their composition and that conservation outcomes should be viewed as a positive added value. Although exotic plant species are not yet an issue in Benin, its prevalence in HGs could indicate an increasing presence of these plant species in the different ecosystems in Benin, and then an environmental issue in coming years. However, at HG level, this pattern should not be counted as a disadvantage. In opposition to the global thinking that all non-native species are invasive and “not good”, all exotic plant species planted or intentionally maintained in gardens are useful and under control of gardeners. In any case, “nativeness” is just an environmental value, and maintaining more or fewer exotic/native plant species in HGs does not prevent plant species from extinction.
Regardless of the “nativeness” or not of plant species, HGs are part of the larger ecosystem—in this study, at PDs and AEZ levels—which offers a pool of plant species to gardeners. The findings showed that garden flora is composed of a large part (more than 60% and up to 97%) of the flora of the PDs they belong to. Therefore, HGs are not isolated ecosystems but rather connected to and representative of the vegetation of their surrounding landscape. The influence of out-PDs vegetation on the garden flora of another PD was found to be remarkable mainly in some HGs of Sub-humid and semi-arid zones, determining 16.66 to 84.62% of the flora of some HGs. Even if not presented in this manuscript, people migration was found to drive the influence of out-PDs vegetation of garden flora, with owners of HGs which have experienced migration at least once having high proportion of Out-PD plant species in their gardens. Such an interaction between plant distribution and ethnic migration was also evidenced in Benin by Assogbadjo et al. (2012). In another way, the influence of out-PDs vegetation on the garden flora of another PD was found to be fairly dependent on the physical distance between PDs. Overall, neighbouring PDs were very similar regarding their gardens’ flora. For instance, the garden flora of the following assemblages (AC and MP), (NB and SB), (ZO, PL and OV) was very similar, whereas that of BA and PO was very distinctive. Even if not investigated in this paper, beyond environmental and socio-cultural factors, the existence and extent of the seed and seedling exchange network could explain the similarity and dissimilarity among PDs with regards to their garden flora (Calvet-Mir et al. 2012a, b). Indeed, gardeners within the same PD or neighbouring PDs are probably members of the same seed and seedling exchange network. With increasing distance, the influence of the network gets weaker. As a consequence, for sites selection for future in situ conservation purposes, the assemblage defined by similar PDs could be selected rather than each PD member of the assemblage being selected separately. Because the distinctive PDs (BA and PO) act as places of particular interest, site selection should compulsorily account for each of these PDs.
Beyond the richness and quality of membership (native/exotic) of garden flora, the distribution of individual species as well as their conservation status at the HG level is another issue of interest. Indeed the relevance of HGs for biodiversity conservation should be analysed with their capacity to represent agro-biodiversity at multiple levels over small spaces (Hodgkin et al. 2002). The garden occupancy analysis indicated that most plant species (90.82%) occurred in less than 10% of gardens, with a high proportion of singleton and doubleton (36.53%). The conservation status based on garden occupancy and number of locations of presence indicated that most HG plant species are rare (threatened) including plant species already red listed by IUCN and Benin. These alarming observations could indicate a critical situation whereby some plant species, mainly singleton and doubleton, will no longer exist within HG ecosystems. However, again, this situation does not necessarily suggest that HGs are not good systems of conservation. The situation should rather be analysed taking into account the intrinsic pattern of HGs. Indeed HGs are composed of different vegetation strata (Fernandes and Nair 1986; Abdoellah et al. 2002; Abebe et al. 2013) but are predominantly of the herbaceous strata (Abebe et al. 2013; Gbedomon et al. 2015). With such a structure, HG flora composition is highly dynamic (Coomes and Ban 2004; Abebe et al. 2006) even across the seasons and years (Bernholt et al. 2009; Cruz-Garcia and Struik 2015). Plant species may disappear and reappear later in the same garden or elsewhere, depending on the choice of gardeners. As long as the conservation function will be a by-product of the production function in home gardening rather than an objective, the efficacy of these ecosystems in sustainably maintaining plant species diversity will be questionable.
In this paper, the findings are based on one year of data collection and capture only variation across the seasons. Time series data over a longer period are required to show a stable and robust trend in spatial occupancy and conservation status of plant species at the HG level. Furthermore, the rarity cut-off point (rarity index >90%), defined as the threshold of occurrence below which plant species are considered as being widespread, was based on empirical case studies and does not stand as an absolute value. Therefore, this value may be flexible (Leroy et al. 2012) and, consequently, may affect the conclusions with regards to the conservation status of HG species in Benin.
References
Abdoellah OS, Parikesit BG, Hadikusumah HY (2002) Home gardens in the upper citarum watershed, West Java: a challenge for in situ conservation of plant genetic resources. Home gardens and in situ conservation of plant genetic resources in farming systems:140
Abebe T, Wiersum KF, Bongers FJJM, Sterck F (2006) Diversity and dynamics in homegardens of southern Ethiopia. Tropical Homegardens. Springer Netherlands, pp. 123–142
Abebe T, Sterck F, Wiersum K, Bongers F (2013) Diversity, composition and density of trees and shrubs in agroforestry homegardens in Southern Ethiopia. Agrofor Syst 87:1283–1293
Adomou AC (2005) Vegetation patterns and environmental gradients in Benin: implications for biogeography and conservation. Wageningen Universiteit, Wageningen
Akoègninou A, Van der Burg W, Van der Maesen L (2006) Flore analytique du Bénin. Backhuys Publishers, Leiden
Albuquerque UP (2006) Re-examining hypotheses concerning the use and knowledge of medicinal plants: a study in the Caatinga vegetation of NE Brazil. J Ethnobiol Ethnomed 2:1
Albuquerque UD, Andrade L, Caballero J (2005) Structure and floristics of homegardens in Northeastern Brazil. J Arid Environ 62:491–506
Amberber M, Argaw M, Asfaw Z (2014) The role of homegardens for in situ conservation of plant biodiversity in Holeta Town, Oromia National Regional State, Ethiopia. Int J Biodivers Conserv 6:8–16
Assogbadjo AE, Fandohan B, Kakaï RG, Kyndt T, Hardy OJ, Gheysen G, Sinsin B (2012) Genetic evidence of the contribution of ethnic migrations to the propagation and persistence of the rare and declining scrambling shrub Caesalpinia bonduc L. Hum Ecol 40:117–128
Ausems E (1980) Convention on the conservation of European wildlife and natural habitats. Environ Conserv 7:143–144
Baillie J, Hilton-Taylor C, Stuart SN (2004) 2004 IUCN red list of threatened species: a global species assessment. IUCN, Gland
Bajpai S, Sharma A, Kanungo V (2013) Traditional home gardens: a preserve of medicinal plants. Int J Herb Med 1:152–161
Bernholt H, Kehlenbeck K, Gebauer J, Buerkert A (2009) Plant species richness and diversity in urban and peri-urban gardens of Niamey, Niger. Agrofor Syst 77:159–179
Calvet-Mir L, Calvet-Mir M, Molina JL, Reves-Garcia V (2012a) Seed exchange as an agrobiodiversity conservation mechanism. A case study in Vail Fosca, Catalan Pyrenees, Iberian Peninsula. Ecol Soc 17:29
Calvet-Mir L, Gómez-Baggethun E, Reyes-García V (2012b) Beyond food production: ecosystem services provided by home gardens. A case study in Vall Fosca, Catalan Pyrenees, Northeastern Spain. Ecol Econ 74:153–160
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67
Clarke LW, Li L, Jenerette GD, Yu Z (2014) Drivers of plant biodiversity and ecosystem service production in home gardens across the Beijing Municipality of China. Urban Ecosyst 17:741–760
Coomes OT, Ban N (2004) Cultivated plant species diversity in home gardens of an Amazonian peasant village in northeastern Peru. Econ Bot 58:420–434
Cribari-Neto F, Zeileis A (2009) Beta Regression in R. Research Report Series/Department of Statistics and Mathematics, 98. Department of Statistics and Mathematics x, WU Vienna University of Economics and Business, Vienna
Cruz-Garcia GS, Struik PC (2015) Spatial and seasonal diversity of wild food plants in home gardens of Northeast Thailand. Econ Bot 69:99–113
Dagnelie P (1998) Inférence statistique à une et à deux dimensions. De Boeck Supérieur, Bruxelles
Dawson IK, Guariguata MR, Loo J, Weber JC, Lengkeek A, Bush D, Cornelius J, Guarino L, Kindt R, Orwa C (2013) What is the relevance of smallholders’ agroforestry systems for conserving tropical tree species and genetic diversity in circa situm, in situ and ex situ settings? A review. Biodivers Conserv 22:301–324
Díaz S, Fargione J, Chapin FS III, Tilman D (2006) Biodiversity loss threatens human well-being. PLoS Biol 4:e277
Etnologue (2015) Language of the world:Benin
Fernandes EC, Nair PR (1986) An evaluation of the structure and function of tropical homegardens. Agric Syst 21:279–310
Ferrari C, Pezzi G, Portanova A (2000) The distribution of rare plant species on Mount Prado, a northern Apennine diversity hot spot. Acta Phytogeogr Suec 85:23–30
Furlan V, Kujawska M, Hilgert NI, Pochettino ML (2016). To what extent are medicinal plants shared between country home gardens and urban ones? A case study from Misiones, Argentina. Pharmaceutical biology, 54(9), 1628–1640
Galluzzi G, Eyzaguirre P, Negri V (2010) Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers Conserv 19:3635–3654
Gbedomon RC, Fandohan AB, Salako VK, Idohou AFR, Kakaï RG, Assogbadjo AE (2015) Factors affecting home gardens ownership, diversity and structure: a case study from Benin. J Ethnobiol Ethnomed 11:1
Gerard Grubben WK, Nono-Womdim Rémi, Everaarts Arij, Fondio Lassina, Nugteren Jan Arie, Corrado AM (2014) Vegetables to combat the hidden hunger in Africa. Chron Hortic 54:32
Goodman LA (1961) Snowball sampling. Ann Math Stat 32(1):148–170
Green RE, Cornell SJ, Scharlemann JP, Balmford A (2005) Farming and the fate of wild nature. Science 307:550–555
Heraty JM, Ellstrand NC (2016) Maize germplasm conservation in Southern California’s urban gardens: introduced diversity beyond ex situ. Econ Bot 70(1):37–48
Hodgkin T, Watson J, Eyzaguirre P (2002) Home gardens and the maintenance of genetic diversity. In: Home gardens and in situ conservation of plant genetic resources in farming systems. Proceedings of the Second International Home Gardens Workshop, Witzenhausen, Federal Republic of Germany, 17–19 July, 2001. International Plant Genetic Resources Institute (IPGRI), pp. 14–18
Hooper DU, Adair EC, Cardinale BJ, Byrnes JE, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108
Idohou R, Fandohan B, Salako VK, Kassa B, Gbèdomon RC, Yédomonhan H, Glèlè Kakaï RL, Assogbadjo AE (2014) Biodiversity conservation in home gardens: traditional knowledge, use patterns and implications for management. Int J Biodivers Sci Ecosyst Serv Manag 10(2):89–100
Igue AM, Floquet A, Stahr K (2000) Land use and farming systems in Benin. In: Adapted farming in West Africa: issues, potentials and perspectives, pp. 227–238
INSAE (2013) Resultats provisoires du RGPH4. Rapport
IUCN (2016) IUCN red list of threatened species. Version 2014.2. Downloaded on 6 November 2014
Jahnke HE, Jahnke HE (1982) Livestock production systems and livestock development in tropical Africa, vol 35. Kieler Wissenschaftsverlag Vauk, Kiel
Judex M, Röhrig J, Schulz O, Thamm H (2009) IMPETUS Atlas du Bénin. Résultats de recherche 2000–2007. Département de Géographie, Université de Boon, Boon
Junqueira A, Souza N, Stomph T, Almekinders C, Clement C, Struik P (2016) Soil fertility gradients shape the agrobiodiversity of Amazonian homegardens. Agric Ecosyst Environ 221:270–281
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27
Kumar BM, Nair PR (2004) The enigma of tropical homegardens. Agrofor Syst 61:135–152
Lebrun J, Stork A (1991) AL 1991-1997. Enumération des plantes à fleurs d’Afrique tropicale. Conservatoire et Jardin botanique de la ville de Genève 1:249
Leroy B, Petillon J, Gallon R, Canard A, Ysnel F (2012) Improving occurrence-based rarity metrics in conservation studies by including multiple rarity cut-off points. Insect Conserv and Divers 5:159–168
MAEP (2011) Agricultural database 1998–2010. Ministry of Agriculture, Livestock and Fishery, Department of Statistics, Cotonou, Benin
Marris E (2014) How can we best come to terms with the exotic species that surround us? Natl Geogr. http://news.nationalgeographic.com/news/2014/07/140724-invasive-species-conservation-biology-extinction-climate-science/
Nature IUCN Resources and ISS Commission (2001) IUCN Red List categories and criteria. IUCN, Gland
Negri V (2005) Agro-biodiversity conservation in Europe: ethical issues. J Agric Environ Eth 18:3–25
Neuenschwander P, Sinsin B, Goergen G (2011) Protection de la Nature en Afrique de l’Ouest: Une Liste Rouge pour le Bénin Nature Conservation in West Africa: Red List for Benin. IITA, Ibadan
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2016) vegan: community ecology package. R package version 2.3-3
Palmer MW (1990) The estimation of species richness by extrapolation. Ecology 71:1195–1198
Perrin A, Basset-Mens C, Huat J, Yehouessi W (2015) High environmental risk and low yield of urban tomato gardens in Benin. Agron Sustain Dev 35:305–315
Pretty J, Sutherland WJ, Ashby J, Auburn J, Baulcombe D, Bell M, Bentley J, Bickersteth S, Brown K, Burke J (2010) The top 100 questions of importance to the future of global agriculture. Int J Agric Sustain 8:219–236
Prévot-Julliard A-C, Clavel J, Teillac-Deschamps P, Julliard R (2011) The need for flexibility in conservation practices: exotic species as an example. Environ Manag 47:315–321
Pushpakumara D, Marambe B, Silva G, Weerahewa J, Punyawardena B (2012) A review of research on homegardens in Sri Lanka: the status, importance and future perspective. Trop Agric 160:55–125
Quiroz D, Towns A, Legba SI, Swier J, Brière S, Sosef M, van Andel T (2014) Quantifying the domestic market in herbal medicine in Benin, West Africa. J Ethnopharmacol 151:1100–1108
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Salako V, Adebanji A, Kakai RG (2013) On the empirical performance of nonmetric multidimensional scaling in vegetation studies. Int J Appl Math Stat 36:54–67
Salako VK, Fandohan B, Kassa B, Assogbadjo AE, Idohou AFR, Gbedomon RC, Chakeredza S, Dulloo ME, Kakaï RG (2014) Home gardens: an assessment of their biodiversity and potential contribution to conservation of threatened species and crop wild relatives in Benin. Genet Resour Crop Evol 61:313–330
Sinsin B, Matig OE, Assogbadjo A, Gaoué O, Sinadouwirou T (2004) Dendrometric characteristics as indicators of pressure of Afzelia africana Sm. dynamic changes in trees found in different climatic zones of Benin. Biodivers Conserv 13:1555–1570
Skarbø K (2014) The cooked is the kept: factors shaping the maintenance of agro-biodiversity in the Andes. Hum Ecol 42:711–726
Smith RM, Thompson K, Hodgson JG, Warren PH, Gaston KJ (2006) Urban domestic gardens (IX): composition and richness of the vascular plant flora, and implications for native biodiversity. Biol Conserv 129:312–322
Srithi K, Trisonthi C, Wangpakapattanawong P, Srisanga P, Balslev H (2012) Plant diversity in Hmong and Mien homegardens in northern Thailand. Econ Bot 66:192–206
Sunwar S, Thornström C-G, Subedi A, Bystrom M (2006) Home gardens in western Nepal: opportunities and challenges for on-farm management of agrobiodiversity. Biodivers Conserv 15:4211–4238
Sutherland W, Adams W, Aronson R, Aveling R, Blackburn TM, Broad S, Ceballos G, Cote I, Cowling R, Da Fonseca G (2009) One hundred questions of importance to the conservation of global biological diversity. Conserv Biol 23:557–567
Taylor JR, Lovell ST, Wortman SE, Chan M (2016) Ecosystem services and tradeoffs in the home food gardens of African American, Chinese-origin and Mexican-origin households in Chicago, IL. Renew Agric Food Syst:1–18
Watson JW, Eyzaguirre PB (2002) Home gardens and in situ conservation of plant genetic resources in farming systems. Bioversity International, Rome
Woldeyes F, Asfaw Z, Demissew S, Roussel B (2016) Homegardens (Aal-oos-gad) of the Basket People of Southwestern Ethiopia: sustainable agro-ecosystems characterizing a traditional landscape. Ethnobot Res Appl 14:549–563
Acknowledgements
This work was funded by the International Foundation for Science (IFS), Research Grant D/5827-1. We are extremely grateful to the senior researchers for their helpful suggestions. We would like also to thank the visiting household for their collaboration. Special thanks go to the technician of the National Herbarium for his assistance in the identification of vouchers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Daniel Sanchez Mata.
This article belongs to the Topical Collection: Ex-situ conservation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gbedomon, R.C., Salako, V.K., Adomou, A.C. et al. Plants in traditional home gardens: richness, composition, conservation and implications for native biodiversity in Benin. Biodivers Conserv 26, 3307–3327 (2017). https://doi.org/10.1007/s10531-017-1407-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-017-1407-8