Abstract
Macrofungal species richness generally increases with forest continuity as does the richness of so-called ancient forest plant species (AFS). Based on this assumption, we examined the ability of AFS to indicate macrofungal diversity in six study areas covering a range of elevations and environments in the Czech Republic. In total, we used data from 106 sampling plots (2,500 m2 each) distributed over six types of forest stands reflecting different intensities and temporal stages of forest management. Species composition of vascular plants and macrofungi was recorded by a single inventory and regular 2-year monitoring, respectively. In total, we found 71 AFS and 1,413 macrofungal species, of which 150 were red-listed macrofungal species. We documented that AFS show potential for being used in the prediction of macrofungi species richness, including endangered species, at the local scale (α-diversity). Additionally, we found significant differences in macrofungal species richness depending on study area and type of forest management, which did not, however, derogate the effect of AFS. Spatial congruence between species composition of AFS and macrofungi communities (β-diversity) increased with forest age and decreased with intensity of forest management. If we consider the simplicity of monitoring AFS in comparison to regular monitoring of macrofungi, we found a widely usable tool for estimating macrofungal diversity in all dominant types of managed forest in central Europe. However, we should be aware of the limited ability of AFS to capture macrofungal diversity across a broader spatial context (γ-diversity), especially in areas with a low diversity of AFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although macrofungi represent the most visible part of the fungal kingdom, our knowledge of their diversity is far from complete, even in the best-explored temperate forests (Hawksworth 2001; Mueller and Schmit 2007; Halme et al. 2012). Apart from taxonomic obstacles and the paucity of skilled mycologists needed for correct identification of the fruiting bodies, there is also a lack of systematic macrofungal surveys (Cannon 1997; Hawksworth 2003; Halme and Kotiaho 2012). However, macrofungi rank among the taxa most seriously threatened by forest management in temperate forests (Lonsdale et al. 2008; Brunet et al. 2010).
In contrast to the macrofungi, monitoring of the vascular plant composition of forest herb layers is a basic and widespread technique traditionally included as a substantial part of temperate forest studies (e.g. Mueller-Dombois and Ellenberg 2002; Canullo et al. 2011). Plant communities are the primary autotrophic component of terrestrial ecosystems and are therefore one of the crucial (co)determinants of overall ecosystem composition, function and biodiversity, reflecting site environmental conditions. Consequently, vascular plants have been repeatedly tested as surrogate taxa for estimating the diversity of other poorly known and inaccessible taxa (e.g. Pharo et al. 1999; Sætersdal et al. 2003; Wolters et al. 2006). Taxon surrogacy is based on the assumption of cross-taxon congruence in spatial patterns of species richness and similarity of species composition, respectively (Prendergast et al. 1993; Rodrigues and Brooks 2007). It cannot be expected that congruence of species richness and composition between vascular plants and any target taxa will be so concise as to be able to provide us with accurate and error-free information comparable with the results of regular monitoring. However, the present extinction rate of threatened species of many taxa associated with temperate forests (including fungi) probably exceeds the progress made in monitoring, taxonomic descriptions, and the increase in knowledge of spatial distributions and ecology (Pimm et al. 1995; Blackwell 2011). Hence, finding an easily obtainable and reliable surrogate would help us to conserve the diversity of these poorly described taxa.
In the case of macrofungi species distribution in temperate forests, we have inconsistent evidence for congruence in species composition, and especially species richness, with vascular plants (Chiarucci et al. 2005; McMullan-Fisher et al. 2010; Blasi et al. 2010; Santi et al. 2010). The low coincidence between species richness of vascular plants and macrofungi is not surprising if we take into account the well-known differences in ecological requirements between these taxa. The species richness of forest herb layer vegetation is generally higher in sunny sites under open tree canopies (Peterken and Francis 1999; Hofmeister et al. 2009), while fruit-bodies of macrofungi mostly occur in more humid and shady habitats (Küffer et al. 2008; Bässler et al. 2010). Interpretation of the effectiveness of vascular plants as surrogate species, which can be extracted from previous studies, is further complicated by differences in spatial scale, which can explain some of the detected heterogeneity (Favreau et al. 2006; Wolters et al. 2006).
An essential factor in macrofungal diversity appears to be forest continuity and management (Ódor et al. 2006; Brunet et al. 2010; Halme et al. 2013). “Forest continuity” is herein defined as time since the last human-caused disturbance. The influence of forest continuity is rather ambiguous for vascular plants considered as a whole, because their diversity may be highest in heavily disturbed stands such as clearings, owing to the appearance of many light demanding as well as non-forest species (Peterken and Francis 1999; Skov and Lawesson 2000). However, the distribution of forest plant species classified as “ancient forest species” (AFS, sensu Hermy et al. 1999) coincides well with forest continuity (Lawesson et al. 1998; Honnay et al. 2002; Flinn and Vellend 2005), analogous to macrofungi.
Therefore, we hypothesized that species richness and composition of AFS and macrofungi at particular sites are correlated due to their shared relationship with forest continuity. If we document spatial proximity in species richness and composition of AFS and macrofungi, we will obtain a simple and widely applicable tool for preliminary detection and evaluation of habitats suitable for macrofungi. Since the highest number of total macrofungal species does not necessarily include the highest number of rare and endangered species (Heilmann-Clausen et al. 2005; Müller et al. 2007a), it would also be necessary to demonstrate the validity of this tool for the restricted group of red-listed macrofungi. Spatial resemblance of species composition is another important matter in cross-taxon congruence studies, even if there is a lack of congruence in species richness (Ferrier 2002; Su et al. 2004). A last question relevant to the item of surrogacy is to what extent plots (areas) selected as a surrogate (richness of AFS) capture the target taxa (macrofungi) (Rodrigues and Brooks 2007).
We noticed a remarkable relationship between richness of AFS and macrofungi when analyzing data from a broad-based monitoring study conducted in forest stands covering a range of gradients in both environmental conditions and forest management intensity in the Czech Republic. Accordingly, we tested this relationship in order to answer the following questions:
-
a)
Can species richness of all, and of red-listed macrofungi, on a local scale (α-diversity) be derived from AFS richness?
-
b)
Is the relationship between the species richness of AFS and macrofungi affected by the type of forest management (including characteristics of forest stands such as prevalent management practices, tree species composition and forest age)? If so, can we easily characterize the changes in a statistical way?
-
c)
How congruent is spatial heterogeneity of species composition (β-diversity) between plant and macrofungal assemblages in the forest herb layer?
-
d)
How effective is selecting plots according to the number of AFS in capturing the regional species pool (γ-diversity) of macrofungi?
Methods
Study areas
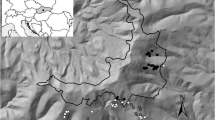
This study was carried out in six study areas (SA1–SA6) distributed over the Czech Republic to capture as much spatial, elevational and other relevant environmental gradients (e.g. geology, continentality) as possible (Fig. 1; Table 1). Each study area comprised part of a large continuous forest patch with an area of 1.4–10.0 km2. The size and boundary of the study areas were designed to include sufficient areas of forest stands of predefined types of forest management. We distinguished six types of forest management, corresponding to prevalent management practices, age structure, and tree species composition of forests within the Czech Republic. Firstly, we distinguished three categories of mature forest stands (>70 years old) growing in a total area of more than 2 ha: (a) nature reserves without regular forest management (hereinafter referred to as unmanaged forest), (b) managed stands of deciduous or mixed tree species (moderate management) and (c) even-aged monocultures of coniferous species (intensive management). Secondly, we divided regularly managed stands younger than 70 years into two habitat types: (d) regularly managed stands of deciduous, mixed and coniferous species 11–69 years old (young forests) and (e) clear-cut stands which originated 2–10 years ago (clearings). Finally, we established a category (f), which included all remaining forest stands unclassifiable as one of the previous habitat types due to insufficient area or habitat heterogeneity (forest mosaics). The largest part of every study area was occupied by stands under intensive management, followed by moderately managed stands. The size of nature reserves ranged from 10 to 100 ha, respectively. There was one nature reserve inside each study area with the exception of SA4 and SA5, where there were two and none, respectively. Therefore, the aim of having at least one example of all six forest management types inside each study area was met in four study areas (SA1–SA4) while the two remaining study areas at the lowest elevations (SA5, SA6) met this aim only if they were coupled (Table 1).
The unmanaged forests were predominantly composed of beech (Fagus sylvatica) with admixtures of spruce (Picea abies) and fir (Abies alba), which both increased with elevation. Ash (Fraxinus excelsior), maples (Acer pseudoplatanus, A. platanoides) and lime (Tilia cordata) were other common native tree species. An exception was unmanaged forests at the lowest elevation, which were dominated by oak (Quercus petraea) instead of beech. Stands under moderate management were generally composed of beech and oak at higher and lower elevations, respectively, whereas monocultures of spruce and pine (Pinus sylvestris) comprised the stands under intensive management. The mature managed forests were around 100 years old and, in the case of forests with moderate management regime, rarely reached 180 years. The age of presently unmanaged forests ranged between 150 and 400 years. They have been mostly protected since the first half of 20th century, the oldest and youngest stands since 1838 and 1964, respectively.
Forest continuity in the study areas was established according to maps of the first and second military mapping efforts of the Austrian Empire from 1764 to 1768 and 1836 to 1852, respectively (archived in Czech Office for Surveying, Mapping and Cadastre, Prague).
Sampling of vegetation and fungi
We located one to four square sampling plots, each 2,500 m2, proportionally in each type of forest management of all study areas to cover the internal heterogeneity of forest stands present in the study areas. We established 20 sampling plots in each of SA1–SA4 and 13 sampling plots in each of SA5–SA6, respectively, i.e. a total of 106 sampling plots (Table 1; for details, see Appendix 1 of supplementary material).
In June and July 2009 or 2010, we conducted a field survey from which we compiled lists of all vascular plant species present in the sampling plots. Three vertical layers were distinguished: a herb layer (plants up to 1.3 m tall), a shrub layer (from 1.3 m to the lower edge of the canopy layer, but not higher than 4 m), and a tree layer (>4 m). Nomenclature of plant species follows Kubát et al. (2002). The number of AFS was determined according to the list of AFS compiled by Hermy et al. (1999).
Two-year inventories of fungi were conducted from early spring 2009 to late autumn 2010 or from early spring 2010 to late autumn 2011. The presence of macrofungal fruiting bodies was regularly recorded in five or six surveys each year in all sampling plots, especially in periods with weather conditions favourable for the fructification of macrofungi. Red-listed species were determined according to the list of red-listed macrofungi of the Czech Republic (Holec and Beran 2006). The samples not readily identified in the field, were collected, dried and determined using either microscopic analysis and/or with the help of specialists in the respective groups of fungi. However, we were not able to determine every sample. In Appendix 5 (supplementary material) the names of records with doubtful determination are indicated by the abbreviation “cf.” (meaning that the record is probably the given species) or “aff.” (meaning that the record is similar to the given species, but not that species). We also had some records identified to genus only (indicated by “sp.” and serial number in Appendix 5 of supplementary material). In the final dataset, we included only those records identified to genus, which clearly represented other species than those already mentioned in the list. Hence, <6 % records remained out of list. Fungal nomenclature is united according to the Index Fungorum database (Landcare Research and Royal Botanic Gardens Kew: Mycology 2013), except in a very few cases, where we have different taxonomic opinion.
Data analysis
Data were analysed in several ways in R (R Development Core Team 2013), using ‘nlme’ (Pinheiro et al. 2013) and ‘vegan’ packages (Oksanen et al. 2012). We first examined the relationships between the species richness of all vascular and ancient forest plant species in the herb layer and macrofungi in each sampling plot (α-diversity) using the Pearson correlation coefficient. Admittedly, this is a transparent but simplistic approach that can be criticized from several points of view. Nevertheless, the encouraging results motivated us to study the relationship of macrofungi to AFS more directly, using appropriate statistical models accounting for various explanatory variables and spatial autocorrelation. To this end, we used the linear mixed model (LME) framework (Laird and Ware 1982; Pinheiro and Bates 2000). We modelled spatial autocorrelation with the exponential covariogram, whose parameters were estimated via restricted maximum likelihood as a part of model identification. The response variable consisted of number of macrofungi and number of red-listed macrofungi, respectively. Apart from number of AFS that was treated as a regression, i.e. first degree of freedom variable, we analysed the effect of spatial context and types of forest management by adding study area and type of forest stands (defined above) as additional as fixed effect explanatory variables.
Species accumulation indices (SAI) were computed from optimal, surrogate and random species accumulation curves to assess the efficiency of macrofungal diversity inferences from AFS richness (Ferrier 2002; Rodrigues and Brooks 2007). Species accumulation curves depict the relationship between the cumulative number of plots selected and the cumulative number of species present. The optimal curve indicates the maximum possible saturation of macrofungal diversity, as the surrogate curve, which is based on AFS richness. The random curve and its standard deviation were computed with 9,999 permutations of the data. We created a separate set of species accumulation curves for each study area and a set for all study areas together. This set consisted of species accumulation curves for (i) all macrofungal species, (ii) saprotrophic species, (iii) mycorrhizal species and (iv) red-listed species.
Spatial turnover in community composition as an example of β-diversity (sensu Anderson et al. 2011; Vellend et al. 2007) was described with two indices suitable for presence-absence data: the Jaccard dissimilarity index (βJ) and the Raup and Crick index (βRC). While βJ is traditionally used, but may be largely biased by differences in species richness between the compared pairs of plots, βRC offers probabilistic dissimilarity independent of species richness. We assessed this probability using 999 simulations, in which the observed number of shared species between pairs of sampling plots was evaluated against a community null model. Dissimilarity indices (βJ and βRC) were calculated for all possible pairwise plot comparisons of both vascular and macrofungi assemblages (n = 5,565 for the set of all plots). A partial Mantel test was carried out to recognize congruence between (dis)similarities of herb layer plant and macrofungal communities, taking into account the geographic distances of respective plots. Significance of the Mantel statistic was assessed with the Monte Carlo procedure with 9,999 permutations.
In order to assess the affinity of plant species to red-listed macrofungi we set up a sequence of all recorded vascular plant species according to frequency in plots occupied by more than five red-listed macrofungi species (relative to the total frequency in all plots). Based on this we established common properties of plant species that preferentially occupied plots both rich and poor in red-listed macrofungi species.
Results
Species richness of herb layer plants and macrofungi
In all 106 plots, we recorded a total of 372 plant species in the herb layer (including tree and shrub seedlings), 71 of which were AFS (20.5 %). At the plot scale, plant species richness in the herb layer averaged 37 and ranged from 4 to 98 species; AFS represented an average of 28 % (ranging from 5 to 56 %) of the total plant species richness found in each plot. The number of AFS correlated well with total plant species richness (r = 0.655; p < 0.001).
As expected, species richness of macrofungi was generally higher than plant species richness, representing 1,413 species in total and ranging from 18 to 284 species per plot (average 134 species). In terms of substrate, the majority of species belonged to the saprotrophic fungi (69 %), with mycorrhizal fungi contributing 29 % and otherwise related fungi (parasitic, lichenicolous etc.) at 2 %. The ratio of saprotrophic to mycorrhizal species per plot averaged out to 3.0, but with substantial differences between plots (min. = 0.8; max. = 9.8).
In total, 150 red-listed species of macrofungi were recorded in 94 plots (89 % of all plots). The number of red-listed species per plot was closely correlated with total species richness of macrofungi (r = 0.848; p < 0.001), although the number of red-listed species was, on average, <4 % of the total species number (0–12 %) in the plots. As for substrate, the proportion of saprotrophic, mycorrhizal and otherwise related macrofungi in red-listed species corresponded well to their proportion in the total macrofungus richness, representing 63, 35 and 3 %, respectively.
Relationship between local species richness of AFS and macrofungi
Species richness of macrofungi per plot did not correlate with total vascular plant species richness in the herb layer (r = 0.028; p = 0.77), but was closely correlated with the number of AFS (r = 0.871; p < 0.001). In contrast to total plant species richness, the number of AFS coincided with the number of red-listed macrofungal species, although less strongly (r = 0.496; p < 0.001). According to LME, species richness of macrofungi can be statistically derived from the number of AFS. This relationship was found to be linear with AFS as the most important variable followed by the other significant effects of study area and type of forest management (Table 2). Observed and fitted species richness of macrofungi in the sampling plots differed on average by 19 species (16 % of species richness). Variances of fitted values ranged from up to 10 and 25 % of observed species richness of macrofungi in 42 and 87 % of sampling plots, respectively (Fig. 2). The effect of the number of AFS interacted significantly with neither study area nor forest management type (Table 2). Consequently, the relationship between AFS and macrofungi richness can be considered generally consistent across both regions and forest management. Based on the number of AFS, we were able to predict not only total species richness of macrofungi, but also the number of red-listed macrofungi species in particular plots, albeit with lower accuracy (Table 2).
Relation between species richness of macrofungi observed in 106 sampling plots associated with six forest management stand types in six study areas within the Czech Republic and predicted by linear mixed models from richness of ancient forest plant species (AFS) with resolution of a study areas, b type of forest management (A unmanaged forest, B moderately managed, C intensively managed, D young forest, E clearing, F forest mosaic)
Relationship between regional species richness of AFS and macrofungi
As strong as predictions of macrofungal richness via AFS richness are on the local scale, this relationship is weak on the regional scale. We documented only moderate surrogate efficiency across all study areas evaluated as a whole (Fig. 3a) or in each separately (Appendix 3 of supplementary material), because of the relatively small differences between surrogate and random curves. The main reason for this is that the number of AFS correlated with species diversity of macrofungi according to their trophic status unevenly. The surrogate curve counted the regional diversity of saprotrophic macrofungi (both coarse and fine woody debris) better than the random curve, while it failed (with the exception of the first four plots) for mycorrhizal macrofungi (Fig. 3b, c). Similarly, the plots richest in AFS accumulated an above-average portion of regional diversity for red-listed fungi species, however the number of non-detected species was quite high in these plots (Fig. 3d). For example, the first ten plots ranked according to the richness of AFS were occupied by a total of 58 red-listed fungi species, which is significantly more than number of species accumulated randomly (43 ± 12), but less than the maximum possible value of 97 species resulting from the optimal accumulation curve (Fig. 3d).
Macrofungal species accumulation curves obtained in 106 sampling plots associated with six forest management stand types in six study areas within the Czech Republic: a Optimal (according to the fungi), surrogate (according to the ancient forest plant species (AFS) richness) and random species accumulation curves of all macrofungi species, b saprotrophic macrofungi species, c mycorrhizal macrofungi species and d red-listed macrofungi species. The random curve is appended by the standard deviation derived from random permutations of the data (gray area). Species area indices (SAI) are provided in the graphs
The local richness of AFS effectively captured regional diversity of macrofungi only in those areas and types of forest management with higher AFS richness and a broad gradient of environmental conditions (Appendices 3, 4 of supplementary material). In particular, the sampling plots in forest mosaics were able to represent a regional richness of macrofungi appropriately, because that type of forest management included the forest stands with the most diverse range of site condition regarding tree species composition, stand structure and age (Appendix 4d of supplementary material).
Relationship between species composition of AFS and macrofungi
The minimum number of plots (59 of the 106 plots) for a full representation of the herb layer plant taxa contained 87 and 83 % of total macrofungal and red-listed macrofungal taxa, respectively. Results of the partial Mantel tests documented that variances in βJ and βRC of vascular plant and macrofungal communities corresponded significantly, with the exception of those of types of forest management representing the earliest stages of forest management cycle (clearings and forests up to 70 years old) (Table 3). Compositional patterns of AFS and macrofungi revealed similar pairwise plot resemblance, reflected in a consistent ratio of macrofungi species to AFS at various spatial scales: 20.6, 17.8, and 20.1 in sampling plots, study areas, and the pooled data of all study areas, respectively.
We found that some AFS and other plant species strongly preferred plots exceptionally rich in both red-listed and total macrofungi species (Appendix 2 of supplementary material). Incidences of many AFS (Actaea spicata, Circaea intermedia, Daphne mezereum, Dentaria bulbifera, Mercurialis perennis, Paris quadrifolia, Pulmonaria obscura, Sanicula europaea, Stellaria nemorum, Veronica montana, etc.) almost exclusively associated with the plots distinguished by the highest number of records of red-listed and total macrofungal species. On the other hand, a few acid-tolerant AFS (Carex pallescens, Convallaria majalis, Luzula sylvatica) occurred in plots inhabited by species-poor macrofungal communities.
Discussion
Surrogate efficiency of ancient forest species
Based on the hypothesis of a similar importance of forest continuity for both AFS and macrofungi, we tested the applicability of AFS for the evaluation of macrofungal diversity in study areas covering broad environmental gradients in central Europe. The data supported the efficiency of AFS richness in predicting macrofungal diversity, especially at the local scale, where it provided a highly accurate estimate of macrofungal richness (α-diversity). Species richness of AFS also enabled us to find hot spots of red-listed macrofungal diversity, in spite of the lower accuracy of predicted richness of red-listed species for particular plots.
The advantages of substituting regular monitoring of macrofungi with an assessment of AFS are apparent. Although the time required for an AFS assessment may differ according to herb layer cover and richness in various types of forests, it remains only a fraction of the time required for regular surveys of macrofungi. In addition, fungal species can be identified by a fraction of the number of experts in comparison with AFS (Mueller et al. 2007; Halme and Kotiaho 2012). Consequently, there are plenty of data regarding herb layer composition of the various forests in central Europe resulting in a good understanding of patterns in plant species diversity (Chytrý and Rafajová 2003; Dengler et al. 2011). Our understanding of macrofungi diversity does not cover all remnants of ‘virgin’ forests in central Europe nor does it encompass an extensive area of managed forests (Senn-Irlet et al. 2007).
In the present study, we showed that stands suitable for macrofungi can be successfully located through AFS richness, particularly if we take into consideration forest management and presence or absence of the best AFS indicator species. By using this information, AFS approximates both total and red-listed macrofungal richness on the local scale and can be used for preliminary searches for fungi-rich stands in large areas of managed forests in central Europe. A recognition of macrofungal diversity in managed forests in central Europe is necessary, but impossible using mycological approaches only, so AFS can be considered a useful substitute, particularly if we can extract this information from previously collected herb layer data. On the other hand, the weakness of species area indices that we obtained from our data (Fig. 3), revealed limitations of AFS in capturing regional diversity of macrofungi. Hence, AFS cannot completely replace regular macrofungal monitoring.
Ancient forest species
Recently, many authors in central and western Europe have compiled lists of vascular plant species according to their affinity to old (ancient) forest in comparison with secondary (recent) ones (e.g. Honnay et al. 1994; Wulf 1997; Hermy et al. 1999; Wulf 2003). Generally, there is a substantial overlap in these AFS lists. Therefore, we used the broadest list of AFS elaborated by Hermy et al. (1999) which includes 127 plant species, 71 of which occurred in our study areas. Although most AFS occurred more frequently on plots rich in macrofungal species, the indicator ability of particular AFS species varied (see “Results” section and Appendix 2 of supplementary material). A few rare AFS were strongly connected to the plots with the highest species richness of macrofungi (e.g. Adoxa moschatellina, Asarum europaeum, Chrysosplenium alternifolium). Some other AFS were quite common, but simultaneously occurred mostly in plots with more than five red-listed species of macrofungi (e.g. Dentaria bulbifera, Mercurialis perennis, Paris quadrifolia). The spatial distribution of these AFS was emulated by some other herb layer species not included in the AFS list (Acer platanoides, Galeobdolon montanum, G. luteum, Galium odoratum, Polygonatum verticillatum, Prenanthes purpurea, etc.). However, many of these species were included in other AFS lists (e.g. Wulf 2003) or represent diagnostic species of near-natural beech-dominated forests (Kučera and Chytrý 2001). We did not include these species in the analysis to obtain higher surrogate efficiency, because we would like to present the potential AFS as assembled by Hermy et al. (1999) as a well-defined group for most of Europe. When AFS are used to indicate macrofungal diversity in practice we, however, recommend enriching the list of subjected species with other species with a high affinity to stands rich in macrofungal species in a particular region (such as G. montanum, G. luteum, G. odoratum etc. in the case of our study; see Appendix 2 of supplementary material). On the other hand, exclusion of a few AFS with a negative affinity to macrofungal species-rich stands may be considered.
Forest continuity and forest management
We documented that forest continuity could be responsible for harmonizing the α-diversity pattern of macrofungi and those of vascular plant species with positive relationships with this factor. This is not impaired by the fact that the diversity of these two taxa may be encouraged by different aspects of forest continuity. The term forest continuity itself does not have one widely accepted definition and it is still somewhat questionable which features of forest stands and what temporal and spatial contexts are relevant (Willis 1993; Jonsson et al. 2005; McCarthy et al. 2011). In spite of these uncertainties, there are two main possible effects of forest continuity benefiting biodiversity: (a) sufficient time enabling species with strong dispersal limitations to colonize forest stands, (b) establishment of favourable microhabitats such as vertical structure and tree species composition, old-growth and decaying trees, variability of dead wood, etc., which are lacking in young forests (Nordén and Applequist 2001; Winter and Möller 2008). Whereas affinity of AFS to old forests results predominantly from dispersal limitations rather than from environmental conditions (Honnay et al. 2005; Hermy and Verheyen 2007), the presence of microhabitats is decisive for the maintenance of macrofungal diversity, even though dispersal constraints cannot be excluded (Christensen et al. 2004; Jonsson et al. 2005; Nordén et al. 2013).
Communities of AFS and macrofungal species were generally congruent, but strongly affected by forest management. We identified a relatively high congruence in unmanaged forest stands but a lower congruence in young forests, clearings and intensive managed stands. Forest management represents another aspect of forest continuity that differentially affects AFS and macrofungi. In the past centuries, most AFS subsisted in coppiced and/or pastured forests throughout Europe, but these forests are presently considered as ancient, i.e. possessing forest continuity (Peterken 1996; Decocq et al. 2005; Flinn and Vellend 2005). Regarding macrofungi, the definition should be more restrictive since continuity is only achieved by forest stands that have not been affected by human impacts for at least several decades or even centuries (Müller et al. 2007a, b; Nordén et al. 2008). In spite of this inconsistency, our data convincingly documented that AFS and macrofungi are similarly sensitive to prevalent silvicultural practices (clearing or shelterwood cutting followed by artificial replanting). Thus, communities poor in both AFS and macrofungi were generally found in forest stands disturbed by clear-cut logging and artificial replanting, although some other vascular plant species may emerge or colonise as a result of this disturbance (Skov and Lawesson 2000; Moora et al. 2007).
Similar to AFS within the group of vascular plants, not all macrofungal species respond equally to present silvicultural practices. There is enough evidence that diversity of saprotrophic fungi is associated with the volume and diversity of both coarse and fine woody debris (Nordén et al. 2004; Küffer et al. 2008; Blaser et al. 2013). Presence and diversity of dead wood have been reduced by forest management in general, but conspicuously in the case of large-sized logs and snags which are practically absent in most managed forests (Stokland et al. 2004; Lonsdale et al. 2008; Pouska et al. 2010, but see Christensen et al. 2004). However, the sensitivity of mycorrhizal macrofungi to forest management may not be so explicit and may be exhibited by species composition rather than species richness (Jones et al. 2003; Brunet et al. 2010). Consistent with these incomplete findings, AFS richness indicated saprotrophic diversity more successfully than that of mycorrhizal macrofungi.
Other relevant aspect of the present study
Although we carried out 2 years of detailed monitoring at each plot, we cannot argue that we recorded all macrofungal species (Straatsma et al. 2001; Halme and Kotiaho 2012). Extension of the survey to 3 or more years would undoubtedly extend the list of species observed in particular plots. However, on the basis of species increments established during particular visits (and also during 2 years of monitoring) we assume that the differences among plots would probably increase if we prolonged monitoring to more than 2 years.
As mentioned above, the final macrofungi dataset was less than occurs at each site. However, a statistical analysis cannot be biased by those missing species, because the proportion of excluded records was generally related to fungal richness of the respective plots. Therefore, if we identified species of all records completely, differences among plots would probably have been even larger.
Looking at the remarkable macrofungal diversity of red-listed macrofungi in unmanaged, but also some of the managed forests in three of the six study areas, we should mention that these three areas are presumably not representative of Central-European managed forest “standards”. The negative effect of forest management on macrofungal diversity should be apparent in these species-rich areas, which are therefore convenient to test surrogate effectiveness (Ferrier 2002; Favreau et al. 2006).
Conclusions and implications
Our results show that species richness of macrofungal species including endangered species at the local scale (α-diversity) can be appropriately predicted from AFS richness. Although we found significant effects of study area and type of forest management on macrofungal species richness, they did not detract from the effect of AFS. Spatial congruence between species composition of AFS and macrofungal communities (ß-diversity) increased with forest age and decreased with intensity of forest management. The ability of AFS to surrogate macrofungal diversity seems to be more limited in the regional context (γ-diversity), especially in regions rich in mycorrhizal fungal species and poor in AFS.
These conclusions are of interest from the purely theoretical/ecological viewpoint, but also from the methodological and practical point of view. While single sampling at an optimal time is sufficient to evaluate AFS diversity, multiple surveys arranged in more seasons are a norm for macrofungi (Straatsma et al. 2001; Halme and Kotiaho 2012). If we consider the simple monitoring of AFS to regular monitoring of macrofungi, we have found a widely usable tool to evaluate macrofungal diversity in the dominant types of managed forests in central Europe. Obviously, the tool is not perfect, as it cannot be expected to predict macrofungal diversity without errors, but it is practically usable in a statistical way. As we demonstrated for a wide range of environmental conditions, the macrofungi to AFS relationship is tight on medium spatial scales and can hence be used for relatively simple and cheap pre-screening of macrofungi diversity in rather large areas (a task that would be unthinkable by direct macrofungal assessment). However, even local and small-scale macrofungal studies are still needed for detailed assessments in the future.
References
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of ß diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28
Bässler C, Müller J, Dziock F, Brandl R (2010) Effects of resource availability and climate on the diversity of wood-decaying fungi. J Ecol 98:822–832
Blackwell M (2011) The fungi: 1, 2, 3…5.1 million species? Am J Bot 98:426–438
Blaser S, Prati D, Senn-Irlet B, Fischer M (2013) Effects of forest management on the diversity of deadwood-inhabiting fungi in Central European forests. Forest Ecol Manag 304:42–48
Blasi C, Marchetti M, Chiavetta U, Aleffi M, Audisio P, Azzella MM, Brunialti G, Capotorti G, del Vico E, Lattanzi E, Persiani AM, Ravera S, Tilia A, Burrascano S (2010) Multi-taxon and forest structure sampling for identification of indicators and monitoring of old-growth forest. Plant Biosyst 144:160–170
Brunet J, Fritz Ö, Richnau G (2010) Biodiversity in European beech forests: a review with recommendations for sustainable forest management. Ecol Bull 53:77–94
Cannon PF (1997) Strategies for rapid assessment of fungal diversity. Biodivers Conserv 6:669–680
Canullo R, Starlinger F, Granke O, Fischer R, Aamlid D, Neville P (2011) Assessment of ground vegetation. Manual part VII.1, 19 pp. In: ICP Forest (2010) Manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forests. UNECE ICP Forests Programme Co-ordinating Centre, Hamburg
Chiarucci A, D’Auria F, de Dominicis V, Laganà A, Perini C, Salerni E (2005) Using vascular plants as a surrogate taxon to maximize fungal species richness in reserve design. Conserv Biol 19:1644–1652
Christensen M, Heilmann-Clausen J, Walleyn R, Adamcik S (2004) Wood-inhabiting fungi as indicators of nature value in European beech forests. In: Marchetti M (ed) Monitoring and indicators of forest biodiversity in Europe—from ideas to operationality. EFI Proceedings No. 51, pp 229–237
Chytrý M, Rafajová M (2003) Czech National Phytosociological Database: basic statistics of the available vegetation-plot data. Preslia 75:1–15
Decocq G, Aubert M, Dupont F, Bardat J, Waltez-Franger A, Saguez R, de Foucault B, Alard D, Delelis-Dusollier A (2005) Silviculture-driven vegetation change in a European temperate deciduous forest. Ann For Sci 62:313–323
Dengler J, Jansen F, Glöckler F, Peet RK, De Cáceres M, Chytrý M, Ewald J, Oldeland J, Lopez-Gonzalez G, Finckh M, Mucina L, Rodwell JS, Schaminée JHJ, Spencer N (2011) The Global Index of Vegetation-Plot Databases (GIVD): a new resource for vegetation science. J Veg Sci 22:582–597
Favreau JM, Drew CA, Hess GR, Rubino MJ, Koch FH, Eschelbach KA (2006) Recommendations for assessing the effectiveness of surrogate species approaches. Biodivers Conserv 15:3949–3969
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Syst Biol 51:331–363
Flinn KM, Vellend M (2005) Recovery of forest plant communities in post-agricultural landscapes. Front Ecol Environ 3:243–250
Halme P, Kotiaho JS (2012) The importance of timing and number of surveys in fungal biodiversity research. Biodivers Conserv 21:205–219
Halme P, Heilmann-Clausen J, Räma T, Kosonen T, Kunttu P (2012) Monitoring fungal biodiversity—towards an integrated approach. Fungal Ecol 5:750–758
Halme P, Ódor P, Christensen M, Plitaver A, Veerkamp M, Walleyn R, Siller I, Heilmann-Clausen J (2013) The effects of habitat degradation on metacommunity structure of wood-inhabiting fungi in European beech forests. Biol Conserv 168:24–30
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432
Hawksworth DL (2003) Monitoring and safeguarding fungal resources worldwide: the need for an international collaborative MycoAction Plan. Fungal Divers 13:29–45
Heilmann-Clausen J, Aude E, Christensen M (2005) Cryptogam communities on decaying deciduous wood—does tree species diversity matter? Biodivers Conserv 14:2061–2078
Hermy M, Verheyen K (2007) Legacies of the past in the present-day forest biodiversity: a review of past land-use effects on forest plant species composition and diversity. Ecol Res 22:361–371
Hermy M, Honnay O, Firbank L, Grashof-Bokdam C, Lawesson JE (1999) An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol Conserv 91:9–22
Hofmeister J, Hošek J, Modrý M, Roleček J (2009) The influence of light and nutrient availability on herb layer species richness in oak-dominated forests in central Bohemia. Plant Ecol 205:57–75
Holec J, Beran M (eds) (2006) Červený seznam hub (makromycetů) České republiky [Red list of fungi (macromycetes) of the Czech Republic]. Příroda, Praha 24:1–282 (in Czech with English summary)
Honnay O, Degroote B, Hermy M (1994) Ancient-forest plant species in Western Belgium: a species list and possible ecological mechanisms. Belg J Bot 130:139–154
Honnay O, Bossuyt B, Verheyen K, Butaye J, Jacquemyn H, Hermy M (2002) Ecological perspectives for the restoration of plant communities in European temperate forests. Biodivers Conserv 11:213–242
Honnay O, Jacquemyn H, Bossuyt B, Hermy M (2005) Forest fragmentation effects on patch occupancy and population viability of herbaceous plant species. New Phytol 166:723–736
Jones MD, Durall DM, Gairney JWG (2003) Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol 157:399–422
Jonsson BG, Kruys N, Ranius T (2005) Ecology of species living on dead wood—lessons for dead wood management. Silva Fenn 39:289–309
Kubát K, Hrouda L, Chrtek J jun, Kaplan Z, Kirchner J, Štěpánek J (eds) (2002) Klíč ke květeně České republiky [Key to the flora of the Czech Republic]. Academia, Praha
Kučera T, Chytrý M (2001) Bučiny [beech forests]. In: Chytrý M, Kučera T, Kočí M (eds) Katalog biotopů České republiky. Nature Conservation Agency of the Czech Republic, Prague, pp 190–198 (in Czech)
Küffer N, Gillet F, Senn-Irlet B, Aragno M, Job D (2008) Ecological determinants of fungal diversity on dead wood in European forests. Fungal Divers 30:83–95
Laird NM, Ware JH (1982) Random-effects models for longitudinal data. Biometrics 38:963–974
Landcare Research and Royal Botanic Gardens Kew: Mycology (2013) Index Fungorum. www.indexfungorum.org. Accessed Jan 2013
Lawesson JE, de Blust G, Grashof C, Firbank L, Honnay O, Hermy M, Hobitz P, Jensen LM (1998) Species diversity and area-relationships in Danish beech forests. Forest Ecol Manag 106:235–245
Lonsdale D, Pautasso M, Holdenrieder O (2008) Wood-decaying fungi in the forest: conservation needs and management options. Eur J For Res 127:1–22
McCarthy MA, Thompson CJ, Moore AL, Possingham HP (2011) Designing nature reserves in the face of uncertainty. Ecol Lett 14:470–475
McMullan-Fisher SJM, Kirkpatrick JB, May TW, Pharo EJ (2010) Surrogates for macrofungi and mosses in reservation planning. Conserv Biol 24:730–736
Moora M, Daniell T, Kalle H, Liira J, Pussa K, Roosaluste E, Opik M, Wheatley R, Zobel M (2007) Spatial pattern and species richness of boreonemoral forest understory and its determinants: a comparison of differently managed forests. Forest Ecol Manag 250:64–70
Mueller GM, Schmit JP (2007) Fungal biodiversity: what do we know? What can we predict? Biodivers Conserv 16:1–5
Mueller GM, Schmit JP, Lealock PR, Buyck B, Cifuentes J, Desjardin DE, Halling RE, Hjortstam K, Iturriaga T, Larsson KH, Lodge DJ, May TW, Minter D, Rajchenberg M, Redhead SA, Ryvarden L, Trappe JM, Watling R, Wu Q (2007) Global diversity and distribution of macrofungi. Biodivers Conserv 16:37–48
Mueller-Dombois D, Ellenberg H (2002) Aims and methods of vegetation ecology, 2nd edn. Blackburn Press, New Jersey
Müller J, Hothorn T, Pretzsch H (2007a) Long-term effects of logging intensity on structures, birds, saproxylic beetles and wood-inhabiting fungi in stands of European beech Fagus sylvatica L. Forest Ecol Manag 242:297–305
Müller J, Engel H, Blaschke M (2007b) Assemblages of wood-inhabiting fungi related to silvicultural management intensity in beech forests in southern Germany. Eur J For Res 126:513–527
Nordén B, Applequist T (2001) Conceptual problems of ecological continuity and its biodindicators. Biodivers Conserv 10:779–791
Nordén B, Ryberg M, Götmark F, Olausson B (2004) Relative importance of coarse and fine woody debris for the diversity of wood-inhabiting fungi in temperate broadleaf forests. Biol Conserv 117:1–10
Nordén B, Götmark F, Ryberg M, Paltto H, Allmer J (2008) Partial cutting reduces species richness of fungi on woody debris in oak-rich forests. Can J For Res 38:1807–1816
Nordén J, Penttila R, Siitonen J, Tomppo E, Ovaskainen O (2013) Specialist species of wood-inhabiting fungi struggle while generalist thrive in fragmented boreal forests. J Ecol 101:701–712
Ódor P, Heilmann-Clausen J, Christensen M, Aude E, van Dort KW, Piltaver A, Siller I, Veerkamp MT, Walleyn R, Standovár T, van Hees AFM, Kosec J, Matocec N, Kraigher H, Grebenc T (2006) Diversity of dead wood inhabiting fungi and bryophytes in semi-natural beech forests in Europe. Biol Conserv 131:58–71
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2012) Vegan: community ecology package. R—package, version 2.0-5
Peterken GF (1996) Natural woodlands. Cambridge University Press, Cambridge
Peterken GF, Francis JL (1999) Open spaces as habitats for vascular ground flora species in the woods of central Lincolnshire, UK. Biol Conserv 91:55–72
Pharo EJ, Beattie AJ, Binns D (1999) Vascular plant diversity as a surrogate for bryophyte and lichen diversity. Conserv Biol 13:282–292
Pimm SL, Russell GJ, Gittleman JL, Brooks TM (1995) The future of biodiversity. Science 269:347–350
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-plus. Springer, New York
Pinheiro J, Bates D, Debroy S, Sarkar D, R-core team (2013) Package ‘nlme’. R—package, version 3.1-108
Pouska V, Svoboda M, Lepšová A (2010) The diversity of wood-decaying fungi in relation to changing site condition in an old-growth mountain spruce forest, Central Europe. Eur J For Res 129:219–231
Prendergast JR, Quinn RM, Lawton JH, Eversham BC, Gibbons DW (1993) Rare species, the coincidence of diversity hotspots and conservation strategies. Nature 365:335–337
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org/. Accessed Feb 2013
Rodrigues ASL, Brooks TM (2007) Shortcuts for biodiversity conservation planning: the effectiveness of surrogates. Ann Rev Ecol Evol S 38:713–737
Sætersdal M, Gjerde I, Blom HH, Ihlen PG, Myrserth EW, Pommeresche R, Skartveit J, Solhøy T, Aas O (2003) Vascular plants as a surrogate species group in complementary site selection for bryophytes, macrolichens, spiders, carabids, staphylinids, snails, and wood polypore fungi in a northern forest. Biol Conserv 115:21–31
Santi E, Maccherini S, Rocchini D, Bonini I, Brunialti G, Favilli L, Perini C, Pezzo F, Piazzini S, Rota E, Salerni E, Chiarucci A (2010) Simple to sample: vascular plants as a surrogate group in a nature reserve. J Nat Conserv 18:2–11
Senn-Irlet B, Heilmann-Clausen J, Genney D, Dahlberg A (2007) Guidance for conservation of macrofungi in Europe. The Directorate of Culture and Cultural and Natural Heritage Council of Europe, Strasbourg, p 39
Skov F, Lawesson JE (2000) Estimation of plant species richness from systematically placed plots in a managed forest ecosystem. Nord J Bot 20:477–483
Stokland JN, Tomter SM, Söderberg U (2004) Development of dead wood indicators for biodiversity monitoring: experience from Scandinavia. In: Marchetti M (ed) Monitoring and indicators of forest biodiversity in Europe—from ideas to operationality. EFI Proceedings No. 51, pp 205–226
Straatsma G, Ayer F, Egli S (2001) Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol Res 105:515–523
Su JC, Debinski DM, Jakubauskas ME, Kindscher K (2004) Beyond species richness: community similarity as a measure of cross-taxon congruence for coarse-filter conservation. Conserv Biol 18:167–173
Tolasz R (ed) (2007) Climate atlas of Czechia. Czech Hydrometerorological Institute, Prague
Vellend M, Verheyen K, Flinn KM, Jacquemyn H, Kolb A, Van Calster H, Peterken G, Graae BJ, Bellemare J, Honnay O, Brunet J, Wulf M, Gerhardt F, Hermy M (2007) Homogenization of forest plant communities and weakening of species-environment relationships via agricultural land use. J Ecol 95:565–573
Willis KJ (1993) How old is ancient woodland? Trends Ecol Evol 8:427–428
Winter S, Möller GC (2008) Microhabitats in lowland beech forests as monitoring tool for nature conservation. Forest Ecol Manag 255:1251–1261
Wolters V, Bengtsson J, Zaitsev AS (2006) Relationship among the species richness of different taxa. Ecology 87:1886–1895
Wulf M (1997) Plant species as indicators of ancient woodland in northwestern Germany. J Veg Sci 8:635–642
Wulf M (2003) Preference of plant species for woodlands with differing habitat continuities. Flora 198:444–460
Acknowledgments
We thank M. Dančák, A. Jírová, L. Kratochvílová, M. Lepší, Z. Plesková and P. Veselý for surveying herb-layer vegetation and J. Novotný and J. Hlásek for participation in sampling of macrofungi. Some critical taxa were identified by Z. Pouzar (Corticiaceae s.l.), P. Vampola (Polyporales s.l.) and S. Komínková (Mycena). Technical support was provided by L. Antolík, K. Baltaziuk, L. Čížek, M. Flachs, J. Haščík, P. Hubený, H. Hunkařová, V. Iarema, M. Jakub, J. Jaroš, P. Koukal, J. Kšír, Z. Melín, A. Petrbok, K. Stupková, T. Svoboda, A. Tenčík, P. Zach, M. Zajíček and I. Zítková. We thank J.W. Jongepier and J. Titus for improving English and clarity of manuscript. Editor in Chief D. Hawksworth and three anonymous reviewers gave valuable comments on the manuscript. We are grateful to the respective forest administrations for cooperation in our research. This study was supported by grant VaV SP/2d1/146/08 from the Ministry of the Environment of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Francis Brearley.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hofmeister, J., Hošek, J., Brabec, M. et al. Richness of ancient forest plant species indicates suitable habitats for macrofungi. Biodivers Conserv 23, 2015–2031 (2014). https://doi.org/10.1007/s10531-014-0701-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0701-y