Abstract
Timber plantation forestry is a major threat to indigenous grassland biodiversity, with ecological networks (ENs) currently being used to mitigate this threat. Being composed mostly of linear corridors, ENs create more edge than would occur naturally. To determine the minimum width of corridors for maximising biodiversity conservation, we need first to establish the extent of edge effects from plantation blocks into corridors. We compared arthropod diversity along transects that ran from within plantation blocks into grassland corridors. We also studied the edge effects of natural forest adjacent to natural grasslands within ENs. Sites in grasslands of neighbouring protected areas acted as natural reference sites against which the biodiversity of the EN transects were compared. Two types of exotic plantation trees and various tree age classes were studied. We found a 32 m edge zone from plantation blocks into grassland corridors. Few significant edge effects from plantation blocks occurred at greater distances than this, which suggested that grassland corridors with a width <64 m are essentially all edge. However, and importantly, this situation was complex, as different arthropod taxonomic groups responded differently to edges of plantation blocks and natural forest patches. Natural forest supported many additional species, not just within the forest, but also in associated grassland corridors. This means that maintaining natural forest imbedded within the ENs will protect both indigenous grassland and indigenous forest species as well as help maintain biodiversity across this timber production landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the global demand for timber increases, so more areas of the world will turn to plantation forestry as a wood source (Cubbage et al. 2010). Plantation forestry is a risk to global biodiversity as the plantations themselves contribute little to biodiversity, especially when non-native trees are used (Armstrong and van Hensbergen 1994; Pryke and Samways 2009; Bremer and Farley 2010). Ecological networks (ENs) are currently being used to mitigate the adverse effects of plantation forestry (Samways et al. 2010). They are large-scale interconnecting corridors and nodes that ensure connectivity between habitat patches for organism dispersal on evolutionary as well as on ecological time scales (Beier and Noss 1998).
ENs consists of both natural patches and corridors that connect them (Jongman 1995). Corridors are often simply defined as movement corridors for focal species (Hilty et al. 2006). As the aim of ENs is to conserve biodiversity, they also need to include the inherent biological complexity of the whole ecosystem (Jongman 1995). Conceptually much work has gone into the biodiversity value of ENs, although only a few areas of the world have actually implemented them (Yu et al. 2006; Jongman et al. 2011).
ENs have more edges than occur naturally due to the linear nature of their corridor components (Koh et al. 2010). Understanding these EN edge effects is important to conservation planning in that it determines minimal corridor width. The influence that transformed areas have on natural areas is often a two-zoned effect: (1) the edge zone which is influenced by the interface between a transformed area and a natural one, and (2) the interior zone where species richness, abundance and assemblage composition are no longer influenced by the distance to the edge (Cadenasso et al. 2003; Ries et al. 2004). Some biodiversity responds positively to the edge, and many species need the edge zone (van Halder et al. 2011). However, for conservation purposes it is the interior zone which is of most concern. The reason for this is that the interior is more difficult to conserve, as it requires enough space for edge zones to surround it. If corridors are too small they will consist entirely of edge zone and without the vital interior zone.
Edge effects are caused by structural changes along the edge boundary (Cadenasso et al. 2003; Harper et al. 2005), as well as through changes in soil moisture and nutrients (Li et al. 2007). Over time, secondary effects such as roads and invasion by alien species can further deteriorate the habitat along the edge. Disturbance on the edge zone allows generalist species to enter the corridor and disrupt natural systems (Pinheiro et al. 2010; Ivanov and Keiper 2010), although given enough space this edge effect will give way to more valuable core area (Slawski and Slawska 2000; Hochkirch et al. 2008).
Edge effects change in size for small mammals between natural forest patch and plantation block edges (Wilson et al. 2010), while there seems to be no general edge effects between natural Afromontane forest and its associated grassland (Kotze and Samways 2001). The type of transformed landscape also contributes to the extent of the edge and will determine those species found in it. This has been shown with changes in edge effects in rural versus urban contexts (Vallet et al. 2010) and even the age class of plantation blocks (Armstrong and van Hensbergen 1994).
We chose to use arthropods as representatives of biodiversity as they are relatively small, hyperdiverse and can be sampled in large numbers, while some are sensitive to environmental variability at point localities (Weaver 1995; McGeoch 1998). They rely almost entirely upon the resources provided locally (Stork and Eggleton 1992), and are important for conservation as they have major functional roles (particularly as pollinators, herbivores, detritivores, and as keystone predators and parasitoids) (Rohr et al. 2007). We have also taken a multi-taxon approach as studies have shown that it is important to measure all ranges of arthropod responses (Tropek et al. 2008; Pryke and Samways 2011).
To design ENs that successfully conserve biodiversity in the plantation timber landscape, we need to determine the minimum width for a corridor and also determine the maximum distance from a plantation block edge where edge effects become dissipated. In response, we compare biodiversity of various arthropod groups along plantation/grassland edges to grasslands in protected areas (reference sites). We do this using plantation blocks comprising two different types of plantation tree species and plantation trees of different ages. We also investigate natural Afromontane forests to determine the natural edge effect in association with indigenous grassland and in doing so we provide a measure of the biological value of natural edge zones within the timber landscape.
Methods
Study area and design
We chose the ENs of the South African timber industry to study these edge effects as they have sharp edges between plantation blocks and the natural vegetation of the grassland corridors. Furthermore, many of the plantations border on protected areas, which can serve as reference sites. The South African timber industry occupies 1.8 million ha, or 1.5% of the surface area of the country. Of this, 1.3 million ha are planted to alien Eucalyptus, Acacia and Pinus species (Kirkman and Pott 2002). A further ca 500,000 ha are maintained mostly as conservation areas of protected grassland, wetland and natural forest, but also with some firebreaks, power lines and vehicle tracks. On average, one-third of a plantation remains unplanted to timber and it is these unplanted areas which constitute the ENs (Kirkman and Pott 2002).
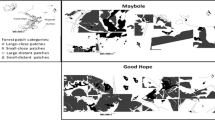
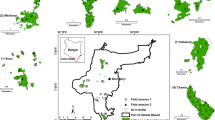
This study was extensive on three separate plantations across the KwaZulu-Natal Midlands in South Africa. This is an area dominated by threatened Midlands Mistbelt Grassland and Drakensberg Foothill Moist Grassland with patches of Southern Mistbelt Forest dispersed within the grassland matrix (Mucina and Rutherford 2006). Exotic timber plantations are the most abundant form of land transformation in the area (Kirkman and Pott 2002). The three plantations were Gilboa (29°16 S; 30°18 E), Good Hope (29° 40 S; 29° 58 E) and Maybole (29° 44 S; 30° 15 E). Each plantation had similar elevations ca 1000-1700 m asl, with Gilboa lying ±50 km from both Good Hope and Maybole, which were ±30 km from each other (Fig. 1).
Location map showing the three areas and the 40 transects used in this study in the KwaZulu-Natal Midlands, South Africa. Dark grey areas show the extent of the commercial plantation area, light grey show the extent of the natural area managed by the timber company. NF natural forest, MaP mature pine, Eu mature eucalypt, MeP medium-aged pine and YP young pine
Transects were laid out in five wooded areas, each with eight replicated sampling transects: old pine (>10 years old), medium-aged pine (4–6 years old), young pine (<3 years old), mature eucalypt plantation blocks, as well as in natural forest patches for a natural edge comparison. This gave a grand total of 40 transects and 320 sampling stations. A further 16 sites were set out in the grasslands of the neighbouring protected areas > 64 m from wooded areas, as reference sites for original species richness and community composition. All field work was between February and April 2009.
Transects were set-up from inside wooded areas, and into and across the standardized matrix of a grassland corridor within an EN. Eight stations were placed along each transect, and these were set out on a log2 scale. Three sampling stations were in the wooded area (either in plantation blocks or natural forest patches; 32, 16 and 8 m from the edge), one on the edge itself, and four stations were into the grassland corridor (8, 16, 32 and 64 m).
Arthropod sampling
At each station and each reference site, sampling used four techniques: two pitfall traps, 200 m diurnal searches, 100 m nocturnal searches and 100 sweeps of a sweep net. Target organisms were Formicidae, Araneae, Scorpiones, Opiliones, Odonata, Mantodea, Phasmatodea, Orthoptera, Lepidoptera, Neuroptera and the two beetle families Carabidae and Scarabaeidae (Scarabaeinae and Trogidae).
Each station and reference site had two pitfall traps, one metre apart. Each trap was 70 mm in diameter, which effectively captures many rare species of ants (Abensperg-Traun and Steven 1995) and spiders (Brennan et al. 2005). Traps were half-filled with a 50% ethylene glycol solution (Woodcock 2005), and were left open for 3 days. Diurnal searches targeted flying arthropods and were carried out between 10 h and 15 h on sunny-windless days. Nocturnal searches were conducted with search lights at night after 20 h. Both diurnal and nocturnal searches were done by one observer (J.S.P) to reduce observer bias. The observer walked 200 m for diurnal and 100 m for nocturnal searches parallel to the wooded edge (i.e. either the edge of the plantation block or the edge of the natural forest), while recording all focal arthropods listed above. If a specimen was not familiar, it was captured and preserved for later identification. Insects on vegetation were sampled using 100 sweeps of a 40 cm diameter sweep net. These were also conducted parallel to the wooded edge. Due to the high number of transects and the difficulties in accessing some transects, they had to be sampled on random rotation, with the type of wooded area randomised. All transects were only visited once to avoid seasonal effects. All individuals sampled were identified to species or morphospecies.
Data analyses
Species richness data were rarefied for abundance with Mao Tao output from EstimateS (version 8; Colwell 2006) to standardize these data. As with most arthropod studies, the accumulation curves here were just short of the asymptote (Gotelli and Colwell 2001). This rarefication was done for the stations per wooded area (either plantation blocks or natural forest patches). Further analyses were calculated for all arthropods together, and also for ants, spiders, grasshoppers, butterflies, dragonflies, as well as for the dung and ground beetles separately. Generalized Linear Models (GLMs) with Poisson distribution (log-link functions) were used to compare the zones of the various wooded types for species richness only (O’Hara 2009; Zuur et al. 2010) in SAS 9.1. The zones analysed were the wooded area (either plantation or natural forest), the edge zone (<32 m from the wooded edge) and those in the corridor interior zone (≥32 m from the wooded edge into the grassland corridor). The fixed effects in the model were the type of wooded area in which the transect began, the transect zone and which of the three plantation holdings hosted the transect. These analyses were repeated per arthropod group. These GLMs were calculated with Poisson distribution for all data, as means were >5, and the minimum number of successes and failures were <5 (Bolker et al. 2009). As these analyses showed no overdispersion of the variances compared to the models, Wald χ2 (Z) statistics were calculated using the penalised quasilikelihood technique (Bolker et al. 2009). Abundance data had highly localized responses, and so abundance alone was not used as a biodiversity measure in this study (see supplementary Table 1, 2 for abundance results).
Multivariate analyses using Permutational multivariate analysis of variance (PERMANOVA) (Anderson 2001) and PRIMER 6 (PRIMER-E 2008) were used to calculate t and P-values for the similarity of the arthropod assemblage along the all the transects to the grassland reference sites. Similar PERMANOVAs were also calculated for each wooded area separately. Further PERMANOVAs calculated changes in assemblage composition for each of the three transects zones (see above for categorization) for each of the five wooded areas. This was done for all arthropods sampled, and for ants, spiders, grasshoppers, butterflies, beetles and dragonflies independently. These analyses were calculated with type of wooded area in which the transect began and transect zone as fixed factors. Analyses were performed using Bray-Curtis similarity measures with these data fourth-root transformed to reduce the weight of common species (PRIMER-E 2008).
Results
Overall edge effect for the whole assemblage and for various wooded areas
Overall, 7,689 individuals from 251 species (46 families and 12 orders) were sampled. When all wooded areas (both plantation blocks and natural forest patches) and all arthropod groups were combined, stations in the wooded area of the transect were significantly lower in species richness compared to all the other stations. In the edge zone arthropods increased and peaked at 8 m from the wooded edge but then showed similar species richness to the reference sites by the 16 m station (Fig. 2). Species assemblage composition of all transects combined was significantly different for all the stations except the last two (32 and 64 m from the edge) (Table 1). This meant that we could define: wooded area (plantation block or natural forest), edge zone (<32 m from the wooded edge) and interior zone (≥32 m from the wooded edge into the grassland corridor).
Summary results of rarefied species richness data (with 95% confidence intervals) on means (1 ± SD) for all transects in this study starting in natural forest and various plantation blocks, as well as for the six focal taxa. Different letters above bars represent significantly different means (5% level)
Transects for all plantation types had similar species richness in overall assemblage (all arthropod groups combined), with the exception of the young pine (Fig. 2). Natural forest transects followed the same general pattern as the plantations, but with greater variability in species richness, both within the natural forest patch and in the corridor (Fig. 2). Young pine showed high species richness within the pine block, although the transect trend followed that of the other plantations outside the plantation block (Fig. 2). Species assemblage composition only showed significant similarity to the reference sites at distances ≥16 m from edges for the natural forests, mature eucalypt and medium-aged pine and ≥32 m from the edges mature and young pine blocks (Table 1). The wooded areas had highly significant differences between both edge and interior zones, while the edge and interior zones were similar to each other. The young pines were an exception as they showed a significant difference between the plantation block and the grassland interior zone (Table 2).
When all stations along the transect were combined and the various wooded areas were compared for species richness, only mature pines were significantly higher than either the natural forest or mature eucalypts (Table 3). When only wooded areas were combined and compared, young pines were significantly higher in species richness than all other woodland types, while medium-aged pines were significantly more species rich compared to mature pine and eucalypt blocks (Table 3). Combined stations from the edge zone showed mature pines had significantly higher species richness for all wooded areas, while mature eucalypts were significantly higher in species richness than natural forest or young pines. There was no significant difference in species richness between the various interior zones (Table 3).
When all stations along a transect were combined, each of the five types of wooded area showed significant differences to each other in assemblage composition. There were also significant differences between each of the wooded areas in assemblage composition when only wooded stations were included in the analyses (Table 3). There were also highly significant compositional differences for the natural forest edge zone and significant composition differences for mature pine and young pine edge zones compared to all other edges zones (Table 3). The only corridor interior zone that showed significant differences were those of the natural forest transects, which were significantly different from all other corridor interior zones (Table 3).
Wooded edge effects on various taxa
Different taxa showed a variety of responses in species richness to woodland edges. Ants were the most numerous group (64 species, 5 288 individuals) and showed a trend similar to that of the overall curve, being low in species richness in wooded areas and then increasing until 8 m from the wooded edge, after which the graph flattened out (Fig. 2). Spiders (50 species, 768 individuals) showed little difference in species richness along the transects (Fig. 2). Grasshoppers (46 species, 599 individuals) had low diversity in wooded areas, with two peaks at 8 and 64 m (Fig. 2). Butterflies (30 species, 384 individuals) and dragonflies (15 species, 217 individuals) showed similar patterns, with very low species richness in the wooded areas, but increased steeply at the wooded edge, after which the graph flattened out (Fig. 2). Beetles (29 species, 246 individuals) showed a different trend to the other taxa as they had low species richness in the wooded areas, which then steeply increased and peaked 8 m from the wooded edge, after which the values in the graph slowly declined. At 64 m, beetle species richness was equivalent to that in the wooded areas (Fig. 2).
As ground beetles and dung beetles have very different ecological requirements, they were further analysed separately. Ground beetle species richness was significantly lower in the interior zone compared to the either the wooded area (Walt-Z = 5.82; P = 0.016) or the edge zone (Walt-Z = 14.63; P < 0.001), while the edge and wooded areas showed no significant difference (Walt-Z = 2.75; P = 0.097). Dung beetle species richness was significantly higher in the edge zone compared to the wooded area (Walt-Z = 23.30; P < 0.001) and interior zone (Walt-Z = 10.70; P = 0.001), although there were no significant differences between the wooded areas and interior zones (Walt-Z = 1.07; P = 0.301).
Ants, grasshoppers, butterflies and dragonflies showed similar patterns in their assemblage compositional changes along the transect. Wooded areas were highly significantly different from the edge and interior zones, with no significant difference between edge and interior zones (Table 2). Spiders also showed highly significant differences between wooded areas and edge and interior zones. They also showed a significant difference between edge and interior zones (Table 3). The only significant change in the beetle assemblage composition was between wooded and interior zones (Table 3).
Edge effects of different wooded areas on individual taxa
There was very little change in species richness or assemblage composition between edge and interior zones for the different wooded areas per taxon (Tables 1, 3). Natural forest edge or interior zones had a significantly higher species richness of ants, butterflies and dragonflies compared to the wooded areas (Table 4). There were also significant compositional differences in ants, grasshoppers and butterflies of wooded areas versus edge and interior zones of natural forests (Table 2).
Ants, grasshoppers, butterflies and dragonflies all showed significantly higher species richness in the edge and grassland interior zones along mature pine and eucalypt transects compared to those in the wooded area of the transect (Table 4). Wooded mature pine was compositionally similar to the equivalent edge and interior zones for ants, grasshoppers, butterflies and dragonflies (Table 2). Only grasshoppers and butterflies showed compositional differences between wooded and edge or interior zones (Table 2).
Edge and interior zones along the medium-aged pine transect were significantly higher than the wooded areas in species richness for the ants, spiders, butterflies and dragonflies (Table 4). Compositionally, there were significant differences in ant and spider assemblages between stations in medium-aged pine blocks and edge or interior zones (Table 2).
Only species richness of grasshoppers showed significant differences between the wooded sites of young pine plantations compared to associated corridor edge and interior zones (Table 4). The only significant compositional differences for young pine transects were for the butterflies in wooded stations compared to those on the edge zone and in the interior zone, as well as beetles between wooded areas and interior zones (Table 2).
Discussion
Edge effects of various plantations and natural forests on grassland corridors
Within the plantation blocks there was low species richness that increased across the edge and then peaked at around 8 m into the grassland corridor. At 16–32 m into the corridor, species richness stabilised. Our results showed that some edge effects from the plantation could still be seen at least 16 m from the plantation edge in terms of both species richness and assemblage composition. This suggests that overall a 32 m edge zone needs to be observed when planning ecological networks in this transformed landscape, with a grassland corridor interior zone being ≥32 m from the edge of the plantation.
The high species richness along the wooded edge (whether plantation or natural forest) could have been caused by the mixing of species from the interiors of the wooded areas and the grassland corridors. However, the assemblage composition in the wooded area was not the same as that at the edge stations. Another possibility is that the wooded edge acted as a barrier for the corridor interior species, which increased the catch along the wooded edge. The most likely reason is that the edge often has anthropogenic disturbances such as roads, or aliens (Holway 2005), which allow generalist opportunistic species to enter the system (Didham et al. 1996). This appeared to be the case here, but not to the demise of the specialist species.
The assemblage composition of for all the wooded areas (including the natural forests) was significantly different from those at either the edge or in the grassland corridor interior zones. Yet edge and interior zones were always similar to each other, no matter which wooded type was compared. Furthermore, when the the various wooded areas were compared to each other, they tended to host significantly different species. Although, there were also some significant differences in assemblage composition of the various edge zones, this was not the case with the interior zones associated with plantation blocks, which were similar.
Young pines showed different arthropod species richness and species assemblage composition compared to mature pines. Moreover, there was greater species richness within young pines compared to other plantation blocks, although when edge zones were compared, mature pines had significantly more species. Corridor interior zones showed no difference between young and mature pine. Species composition along the young pine transects showed no differences between stations in the edge zone compared to those within the pine block itself or those in the corridor interior zone. This suggested that the high level of disturbance from harvesting and replanting plantation blocks created a situation where the young plantations consisted of a high number of species which normally occurred at the edge. Yet this effect was not seen in the interior of the corridor.
Natural forest patch edges, unlike those of the plantation blocks, made it more difficult to define edge zones from corridor interior zones. These results echo those of Kotze and Samways (2001) where there is much variation in response to natural Afromontane forest and grassland ecotones. Although species richness in natural forest was not higher than in the plantations, the natural forests had many unique species. Furthermore, the grassland corridors associated with natural forests were significantly different to all corridors associated with plantations. This suggests that some species were interacting with both the natural forest and grassland, giving these ecotones conservation value.
Response of various taxa to edge effects
Ants are known to respond to patch boundary contrast and abiotic conditions (Dauber and Wolters 2004). Here we found that ant species richness was significantly higher in the edge zone and in the corridor interior compared to the wooded areas, except in the case of young pines. Ant assemblage composition also differed significantly between the edge and corridor interior zones compared to all the wooded areas, except for the young pine and eucalypt blocks. Edge effects for ants disappeared within 8 m of the wooded edges, which was considerably shorter than the >25 m response that ants showed to road boundaries in the same geographical area (Samways et al. 1997) and to mesophytic forest urban boundaries (Ivanov and Keiper 2010).
Overall, spider species richness showed little variation in species richness along the transects, although they were significantly more abundant in wooded areas than in some edge zones. Only the medium-aged pine, mature pine and eucalypt blocks showed significantly higher spider species richness in the edge zones compared to within the plantation blocks. Spider assemblage composition showed a similar pattern. Natural forest and mature pine blocks had similar spider species composition as corridor interiors, which suggested that some spider species could occupy natural forest and mature pines as well as corridor interiors. It appeared that they did not favour the constant disturbance (either natural or anthropogenic) in the edge zone.
All wooded areas were significantly lower in grasshopper species richness than were the edge or interior zones. This pattern was particularly strong in the mature pines and eucalypts. Grasshopper assemblage composition was also very different between mature wooded areas and those of edge and interior zones, with significant added differences between the natural forest and medium-aged pine. Despite grasshoppers responding strongly to management (Bazelet and Samways 2011), we found that the edge effect was highly significant, suggesting that design features involving boundary contrast are still influential for this group.
Butterflies had lower species richness in wooded areas compared to corridor edges and interior zones. There were also changes in species composition between wooded areas and either edge or interior zones. This lack of edge effect was probably because the butterflies have high mobility compared to the other groups, and do not linger in narrow corridors, as noted by Pryke and Samways (2001). Edge zones can be important for some butterflies, although most butterfly species use both edge and interior zones (van Halder et al. 2011). Quality of the vegetation is as important for butterfly conservation as is the size of the corridor or habitat (Pryke and Samways 2001), with connectivity being important for maintaining populations (Haddad 1999).
Beetle species richness was higher in the edge zone compared to wooded areas. However, at 64 m this declined to the same level as in the wooded area. Species richness also did not differ between the various wooded areas and the edge or the corridor interior zones. Ground beetles here preferred both wooded areas (natural and planted) and edge zones. In turn, dung beetles were edge specialists in this system. Yet at forest-cerrado edges in Brazil, habitat type was more important than the edge (Durães et al. 2005). The edge preference here in South Africa is probably because many small mammals are abundant on the edges of both natural forest patches and plantation blocks (Wilson et al. 2010), and large animals rest in the shade of these areas during the heat of the day, so providing more dung at the edges.
Dragonflies responded in a similar way to butterflies, with an increase in species richness between the wooded areas and the open grassland corridors, although no edge affect was noticeable for either the plantation blocks or natural forest patches.
Conclusions
We found a distinct grassland edge zone adjacent to plantation blocks. This edge zone was about 32 m wide. Beyond 32 m the effects of the plantation blocks were negligible. However, this figure of 32 m is liberal as some groups showed an edge effect of only 8 or 16 m. In general terms, this means that corridors <64 m wide are mainly edge. The minimum 250 m corridor width suggested by Pryke and Samways (2001) for maintaining biodiversity in this area is still appropriate, as this incorporates a great deal of interior space for more sensitive species. Following on from this, establishment of wider habitat corridors as suggested by Samways et al. (2010) would certainly reduce the total amount of edge across the entire network.
The multi-taxon approach we have taken here has highlighted some monitoring advantages and pitfalls that would need to be taken into account in future research on conservation in agroforestry. The most notable issue is that different taxonomic groups responded differentially to the wooded edges, whether exotic or native trees. Grasshoppers, butterflies, dragonflies and to a lesser extent ants all responded similarly to the edge, but the beetles we studied here (ground and dung beetles) included edge specialists. In contrast, the edges were relatively insignificant for spiders. When using a single taxon for these kinds of edge studies, care needs to be taken in selecting the right taxon/a for the right conservation question. This is probably more difficult than is generally appreciated. So we suggest that at least a few taxa should be included, at least initially to fully assess how arthropods in general respond to various environmental changes associated with edges.
Monitoring of young plantation blocks for edge effects is not really necessary, as there is little difference between these young plantation blocks and the edge or interior zones. Furthermore, the rotation of the plantation blocks for harvesting to planting new seedlings has little effect on the interior of the corridors, with the corridors associated with young plantation blocks showing no difference from those associated with mature plantation blocks. Yet we still caution that for biodiversity conservation, all harvesting and planting activities should be restricted to the plantation blocks, and the use of corridors for vehicle access or other harvesting activities should be confined to the edge zones, leaving the corridor interiors intact.
As the natural forest and adjacent grassland had unique biodiversity value, we encourage the conservation of these natural forests in the corridors. Maintaining the natural forest imbedded within the ENs would help protect both natural forest and grassland species, and would also help maintain total biodiversity across this production landscape.
References
Abensperg-Traun M, Steven D (1995) The effects of pitfall trap diameter on ant species richness (Hymenoptera: Formicidae) and species composition of the catch in a semi-arid eucalypt woodland. Aust J Ecol 20:282–287
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Armstrong AJ, van Hensbergen HJ (1994) Comparison of avifaunas in Pinus radiata habitats and indigenous riparian habitat at Jonkershoek, Stellenbosch. S Afr J Wildlife Res 24:48–55
Bazelet CS, Samways MJ (2011) Relative importance of management vs. design for implementation of large-scale ecological networks. Landscape Ecol 26:341–353
Beier P, Noss RF (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Brennan KEC, Majer JD, Moir ML (2005) Refining sampling protocols for inventorying invertebrate biodiversity: influence of drift-fence length and pitfall trap diameter on spiders. J Arachnol 33:681–702
Cadenasso ML, Pickett STA, Weathers KC, Bell SS, Benning TL, Carreiro MM, Dawson TE (2003) An interdisciplinary and synthetic approach to ecological boundaries. Bioscience 53:717–722
Colwell RK (2006) http://viceroy.eeb.uconn.edu/EstimateS. Accessed March 2008
Cubbage F, Koesbandana S, Mac Donagh P, Rubilar R, Balmelli G, Olmos VM, De La Torre R, Murara M, Hoeflich VA, Kotze H, Gonzalez R, Carrero O, Frey G, Adams T, Turner J, Lord R, Huang J, MacIntyre C, McGinley K, Abt R, Phillips R (2010) Global timber investments, wood costs, regulation, and risk. Biomass Bioenergy 34:1667–1678
Dauber J, Wolters V (2004) Edge effects on ant community structure and species richness in an agricultural landscape. Biodivers Conserv 13:901–915
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260
Durães R, Martins WP, Vaz-de-Mello FZ (2005) Dung beetle (Coleoptera: Scarabaeidae) assemblages across a natural forest-cerrado ecotone in Minas Gerais, Brazil. Neotrop Entomol 34:721–731
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Haddad NM (1999) Corridor and distance effects on interpatch movements: a landscape experiment with butterflies. Ecol Appl 9:612–622
Harper KA, Macdonald SE, Burton PJ, Chen J, Brosofske KD, Saunders SC, Euskirchen ES, Roberts D, Jaiteh MS, Esseen P-A (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19:768–782
Hilty JA, Lidicker WZ, Merenlender AM (2006) Corridor ecology: the science and practice of linking landscapes for biodiversity conservation. Island Press, Washington
Hochkirch A, Gartner AC, Brandt T (2008) Effects of forest-dune ecotone management on the endangered heath grasshopper, Chorthippus vagans (Orthoptera: Acrididae). B Entomol Res 98:449–456
Holway DA (2005) Edge effects of an invasive species across a natural ecological boundary. Biol Conserv 121:561–567
Ivanov K, Keiper J (2010) Ant (Hymenoptera: Formicidae) diversity and community composition along sharp urban forest edges. Biodivers Conserv 19:3917–3933
Jongman RHG (1995) Nature conservation planning in Europe—developing ecological networks. Landsc Urban Plan 32:169–183
Jongman RHG, Bouwma IM, Griffioen A, Jones-Walters L, Doorn AM (2011) The Pan European Ecological Network: PEEN. Landscape Ecol 26:311–326
Kirkman KE, Pott RM (2002) Biodiversity conservation in plantation forestry. In: Pierce SM, Cowling RM, Sandwith T, MacKinnon K (eds) Mainstreaming biodiversity in development—case studies from South Africa. The World Bank Environmental Department, Washington, DC, pp 33–42
Koh LP, Lee TM, Sodhi NS, Ghazoul J (2010) An overhaul of the species-area approach for predicting biodiversity loss: incorporating matrix and edge effects. J Appl Ecol 47:1063–1070
Kotze DJ, Samways MJ (2001) No general edge effects for invertebrates at Afromontane forest/grassland ecotones. Biodivers Conserv 10:443–466
Li L, He X, Li X, Wen Q, He HS (2007) Depth of edge influence of the agricultural-forest landscape boundary, southwestern China. Ecol Res 22:774–783
McGeoch MA (1998) The selection, testing and application of terrestrial insects as bioindicators. Biol Rev 73:181–201
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria
O’Hara RB (2009) How to make models add up—a primer on GLMMs. Ann Zool Fenn 46:124–137
Pinheiro ERS, Duarte LD, Diehl E, Hartz SM (2010) Edge effects on epigaeic ant assemblages in a grassland-forest mosaic in southern Brazil. Int J Ecol 36:365–371
PRIMER-E (2008) PERMANOVA and PRIMER 6. PRIMER-E, Lutton
Pryke JS, Samways MJ (2009) Recovery of invertebrate diversity in a rehabilitated city landscape mosaic in the heart of a biodiversity hotspot. Landsc Urban Plan 93:54–62
Pryke JS, Samways MJ (2011) Importance of using many taxa and having adequate controls for monitoring impacts of fire for arthropod conservation. J Insect Conserv. doi:10.1007/s10841-011-9404-9
Pryke SR, Samways MJ (2001) Width of grassland linkages for the conservation of butterflies in South African afforested areas. Biol Conserv 101:85–96
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Ann Rev Ecol Evol Syst 35:491–522
Rohr JR, Mahan CG, Kim KC (2007) Developing a monitoring program for invertebrates: guidelines and a case study. Conserv Biol 21:422–433
Samways MJ, Osborn R, Carliel F (1997) Effect of a highway on ant (Hymenoptera: Formicidae) species composition and abundance, with a recommendation for roadside verge width. Biodivers Conserv 6:903–913
Samways MJ, Bazelet CS, Pryke JS (2010) Provision of ecosystem services by large scale corridors and ecological networks. Biodivers Conserv 19:2949–2962
Slawski M, Slawska M (2000) The forest edge as a border between forest and meadow: vegetation and Collembola communities. Pedobiologia 44:442–450
Stork NE, Eggleton P (1992) Invertebrates as determinants and indicators of soil quality. Am J Alternative Agric 7:38–47
Tropek R, Spitzer L, Konvicka M (2008) Two groups of epigaeic arthropods differ in colonising of piedmont quarries: the necessity of multi-taxa and life-history traits approaches in the monitoring studies. Community Ecol 9:177–184
Vallet J, Beaujouan V, Pithon J, Rozé F, Daniel H (2010) The effects of urban or rural landscape context and distance from the edge on native woodland plant communities. Biodivers Conserv 19:3375–3392
van Halder I, Barbaro L, Jactel H (2011) Conserving butterflies in fragmented plantation forests: Are edge and interior habitats equally important? J Insect Conserv 15:591–601
Weaver JC (1995) Indicator species and scale of observation. Conserv Biol 9:939–942
Wilson JW, Stirnemann RL, Shaikh ZS, Scantlebury M (2010) The response of small mammals to natural and human-altered edges associated with Afromontane forests of South Africa. Forest Ecol Manag 259:926–931
Woodcock BA (2005) Pitfall trapping in ecological studies. In: Leather S (ed) Insect sampling in forest ecosystems. Blackwell Science, Oxford, pp 37–57
Yu K, Li D, Li N (2006) The evolution of greenways in China. Landsc Urban Plan 76:223–239
Zuur AF, Elena NI, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Method Ecol Evol 1:1–14
Acknowledgments
The authors thank R. Gaigher and G. Schreiner for field assistance. C. Burchmore, D. Burden, P. Gardiner, and D. van Zyl of Mondi for providing maps, accommodation at field sites, technical assistance and local knowledge. C. Bazelet, A. Dippenaar-Schoeman, C. Haddad, and F. Roets kindly provided species identifications. The authors also thank Ezemvelo KZN Wildlife, Mondi Business Paper and Mondi Shanduka Newsprint for permitting sampling on their holdings. Funding was from the Mondi Ecological Network Programme (MENP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pryke, J.S., Samways, M.J. Conservation management of complex natural forest and plantation edge effects. Landscape Ecol 27, 73–85 (2012). https://doi.org/10.1007/s10980-011-9668-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-011-9668-1