Abstract
Salvage logging—the removal of dead trees in disturbed forest stands—has been controversially discussed. We investigated the impact of bark beetle attacks and subsequent salvage logging on insectivorous bats in a temperate mountain forest. We quantified bat activity (25,373 min counts; 32 plots) using batcorders during 221 all-night surveys in stands killed by bark beetles, with dead trees removed or not, and in vital, single- or multi-layered mature forest stands. We analysed the differences in activity of all bats in general and of bats of foraging guilds (open habitat, forest edge, closed habitat) in these habitats using a generalized linear Poisson mixed model, with plot and observation as random factors, and temperature and habitat as fixed factors. Only open-habitat foragers were slightly more active in salvage-logged stands than in bark-beetle-affected stands; they generally benefited from an open forest canopy, whereas closed-habitat foragers did not. Our results indicated that: (1) bats are less affected by salvage logging after a disturbance of a magnitude typical for European forests, probably because enough roosts are present in surrounding areas, (2) habitats for open foragers are improved by bark beetle infestation and (3) bats are poor bioindicators of negative impacts of salvage logging after natural disturbance in forests with a composition typical for Central Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural disturbances, such as windthrow, forest fires, or insect outbreaks, can lead to rapid changes in the structure of forests (Noss and Lindenmayer 2006). In commercial forests, economic constraints determine management decisions that lead to the prompt removal of dead trees to avoid any loss in wood value or an increase in pest species, such as some scolytids (Wermelinger 2004). Nevertheless, the impact of dead wood on forest health and the value of dead wood for biodiversity are intensely discussed (Shore et al. 2003; Ulbricht et al. 1999), and the necessity of salvage logging in naturally disturbed, protected forests is controversial (Connell 1978; Noss and Lindenmayer 2006; Pickett 1985). For example, the infestation of the Bavarian Forest National Park in south-eastern Germany (Fig. 1a, b) since the 1990s by bark beetles, mainly Ips typographus (Müller et al. 2008), has evoked an ongoing intense discussion about the necessity of salvage logging even in protected areas of Central European low-range mountains (Müller and Job 2009). Owing to an increasing stock of coniferous wood (Schelhaas et al. 2003), a warm period within the last 20 years (Ipcc 2007), and an increasing number of protected areas worldwide, a deeper understanding of the effects of the various management strategies, including salvage logging, will become increasingly important for forest and conservation managers (Schröder 2007).
Forest management strategies focus on a number of taxonomical groups. During the last decade, especially bats have been considered as valuable bioindicators because of their worldwide decline (Jennings and Pocock 2009; Jones et al. 2009; Wickramasinghe et al. 2003). Technological progress in the registration of these cryptic and nocturnal species provide better opportunities and better data sets for research (Parsons and Szewczak 2009). Investigations of the influence on bats of different logging practices in forest ecosystems indicate a lower bat diversity after logging because of the decrease in gleaning species (Clarke et al. 2005; Patriquin and Barclay 2003; Peters et al. 2006).
Studies on the effects of natural disturbances such as windstorms (Gannon and Willig 1994; Jones et al. 2001; Wolff et al. 2009) and forest fires (Carter et al. 2000; Fisher and Wilkinson 2005; Hayes 2009; Patriquin-Meldrum 1999) on bats indicate that the impact of natural disturbance is species specific and depends on the bat diet and foraging behaviour. Only two studies have evaluated the influence on bats of salvage logging after a natural disturbance: in central Oregon after post-fire salvage logging, the highest bat activity was found in more intensely logged sites (Hayes 2009), and in Canada, the activity of the little brown bat (Myotis lucifugus) was higher in forest stands affected by bark beetles than in salvage-logged areas (Randall et al. 2009).
Due to the differences in manoeuvrability and echolocation structure of bats, the use of habitats is highly species specific (Jones et al. 2001; Morris et al. 2010; Norberg and Rayner 1987; Patriquin and Barclay 2003). With respect to this adaption, central European bats have been classified into foraging guilds (Schnitzler and Kalko 2001; Fenton 1989): species foraging in open, or uncluttered habitats (open-habitat guild); species foraging at the edge of vegetation, or background-cluttered habitat (edge-habitat guild), and species foraging within the closed vegetation, or highly cluttered habitat (closed-habitat guild).
Here we investigate the activity of all bat species in general and the activity of the three foraging guilds in areas of salvage logging and bark beetle infestation in the managed and unmanaged parts of the Bavarian Forest National Park. Based on the general knowledge about bat foraging behaviour in temperate and boreal forests, we expected the different foraging guilds to respond differently to salvage logging, namely that in areas of salvage logging, the activity of bats of the open-habitat guild would be higher, the activity of bats of the forest-edge guild would not change, and the activity of bats of the closed-habitat guild would be lower because of a reduction in the habitat complexity.
Materials and methods
Study area
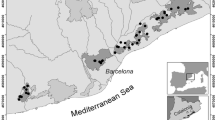
Our study was conducted in the Bavarian Forest National Park (48.54°N, 13.29°E) in south-eastern Germany along the border to the Czech Republic. This area (24,000 ha) contains an altitudinal gradient between 650 and 1,400 m a.s.l., with a high variety of structures distributed almost independently of elevation. The national park is dominated by mixed mountain forest consisting of spruce (Picea abies), beech (Fagus sylvatica), and fir (Abies alba). The area is part of the largest forested landscape in Central Europe, and represents a rather homogenous near-natural forested landscape, compared to the cultivated landscape of Europe. Owing to the no-take forestry policy, trees infested by bark beetles on an area of around 5,400 ha in the core zone of the national park remain untouched, whereas infested trees in the management zone have been salvage logged on about 1,200 ha to protect nearby private forests.
Within this study area, we randomly selected 32 plots along the whole elevation gradient. To quantify the effects of bark beetles and salvage logging on bat activity, we selected four types of forest stands (Fig. 1), (a) salvage logged, with removal of almost all vertical tree structures, i.e. snags and dead trees; (b) bark-beetle-affected stands without management, dominated by a rich vertical structure owing to snags; (c) vital single-layered stands with a dense canopy in the over-storey but open in the lower layers; and (d) vital multi-layered stands with a high density in the under-storey and mid-storey. Each type of forest stand was replicated by 8 plots in stands of at least 5 ha each. The minimum distance between two plots of the same habitat type was 800 m.
To date, 16 species of bats have been recorded in the national park. We assigned these species to the following guilds basically according to the arguments in (Fenton 1989): open-habitat guild: Nyctalus noctula, Nyctalus leisleri, Vespertilio murinus, Eptesicus serotinus, Eptesicus nilsonii and Pipistrellus nathusii; forest-edge guild: Barbastella barbastellus, Myotis myotis, Myotis daubentoni, Myotis brandtii, Myotis mystacinus, Pipistrellus pipistrellus, and Pipistrellus pygmaeus; and closed-habitat guild: Myotis nattereri, Myotis bechsteinii, Plecotus auritus, and Plecotus austriacus [for further information see Table in Online Resource 3 and (Müller et al. 2011)].
Bat activity
In the centre of each sampling plot, we installed a batcorder (batcorder 2.0; ecoobs, Runkel et al. 2009) 2.7 m above ground on a wooden pole for acoustic sampling of bats as an index of activity (Online Resource 1). The batcorder is an autonomous working system that records bat calls. We used the recording mode “Auto + Timer” and the same settings (quality: 20, threshold: −27 dB, post-trigger: 60 ms, critical frequency: 16 kHz) in all-night surveys. The microphone was directed 10° upwards from horizontal to avoid problems with condensation of water at the tip. We placed each batcorder in a forest gap and not facing a closed clutter of vegetation, such as a tree or shrub (Patriquin et al. 2003). Acoustic surveys ran from 30 min before sunset to 30 min after sunrise. Ten batcorders were available; therefore, we made efforts each night to cover the whole gradient of altitude and all four habitats. Each of the 32 plots was surveyed on seven different nights from May to September 2009. Three surveys failed because of technical problems; therefore, we performed altogether 221 all-night surveys.
All digital records were saved on SDHC cards (SanDisk, SDHC 2, 8 GB). To measure the echolocation structure of all bat recordings, we used the software bcAdmin1.11 (ecoobs, Runkel et al. 2009). To determine the bat species of the recordings, we used the automated software bcDiscriminator1.14 statistical call discrimination for Central-European bat calls (ecoobs, Runkel et al. 2009). This program works by using a discrimination tree based on random forest (Breiman 2001) and provides probabilities for a species or groups of non-separated species for each record (Online Resource 2). The system is not able to distinguish between Myotis brandtii and Myotis mystacinus (M. brandtii/mystacinus) because their call structures are highly similar (Parsons and Jones 2000), and between Plecotus austriacus and Plecotus auritus (Plecotus auritus/austriacus) for the same reason. For species with longer time lag between calls, the batcorder tends to produce records for each call. To make the data comparable between species, we counted the number of 1-min intervals per night in which at least one record of one of our foraging guilds was recorded (for more details, see Müller et al. 2011).
Temperature
Temperature affects the activity of bats (Arbuthnott and Brigham 2007; Ciechanowski et al. 2007). Since it was necessary to survey on different nights, the differences in temperature among the nights and plots could influence the activity and thereby bias our analyses (Yates and Muzika 2006). To control our comparison of forest stands, we measured the mean temperature each night and each acoustic recording simultaneously using a Data Logger EL-USB-2 (LASCAR electronics, Salisbury) mounted at the batcorder, and used the measurement as a covariable.
Statistics
Results were analysed with R (R Development Core Team 2010). The count data consisted of the number of minute intervals per night for each bat guild. Therefore, to test the influence of habitat types, we fitted a generalized linear Poisson mixed model with multivariate normal random effects based on penalized likelihood (function glmm.PGQL of package MASS; Venables and Ripley 2002) with plot and observation as random factors to account for replicated measurements on each plot and with an observation-specific random intercept to account for possible overdispersion (Elston et al. 2001) as well as with temperature as covariable. Such an approach considers all critical points of our bat count data from an statistical point of view, the replicated samplings at our plots as well as the distribution of the count data with a few very large numbers, without a loss in information (for more details on mixed models see Quinn and Keough 2002). To identify significant differences between pairs of habitats, we computed Tukey’s all-pair comparisons and associated confidence intervals corrected for multiple comparisons (function glht of package multcomp, (Hothorn et al. 2008a).
Results
Our 221 all-night surveys revealed 28,971 bat records from 13 species and 2 groups of non-separated species. Using the discrimination functions, we were able to identify 11,198 bat records to the species level, remaining records could be identified only on higher taxonomical levels (Online Resource 2). The most common species were E. nilssonii, followed by M. brandtii/mystacinus, P. pipistrellus, and M. daubentonii. Temperature had a positive effect on the activity of all three guilds (Fig. 2d). The general bat activity did not significantly differ among the four habitat types, but the general bat activity in bark-beetle-affected stands was somewhat higher than in salvage-logged areas and single-layered stands, which in turn had somewhat higher activities than multi-layered stands (Fig. 3).
Boxplot of raw data minute counts of three bat foraging guilds (a–c) and their correlation to temperature (d). The colours in d correspond to those in a–c. A Salvage-logged area after bark-beetle infestation, B bark-beetle-affected forest stand, C single-layered forest stand, and D multi-layered forest stand
Difference in general bat activity (1-min counts) between pairs of different habitats. Tukey’s all-pair comparisons and associated confidence intervals, corrected for multiple comparisons (function glht of package multcomp; (Hothorn et al. 2008b) are given. Note that temperature was included as a confounding variable and random factor was given for observation and sampling plot. The general bat activity did not significantly differ among the four habitat types (confidence band includes the zero line), but in bark-beetle-affected stands it was somewhat higher than in salvage-logged areas and single-layered stands, which in turn had somewhat higher activities than multi-layered stands A Salvage-logged area after bark-beetle infestation, B bark-beetle-affected forest stand, C single-layered forest stand, and D multi-layered forest stand
The activity of the foraging guilds in the four habitat types, controlled for temperature, differed. The activity of open-habitat foragers was higher in salvage-logged areas than in bark-beetle-affected areas, and was significantly higher in salvage-logged areas than in the two vital forest stands (single-layered and multi-layered) (Figs. 2a–c, 4). The activity of the forest-edge foragers did not significantly differ between salvage-logged areas and bark-beetle-affected areas. Here, only the activity in single-layered stands was significantly higher than in salvage-logged areas and multi-layered stands.
Differences in bat activity (1-min counts) for each of the three foraging guilds. Tukey’s all-pair comparisons are shown in the same way as in Fig. 3. The activity of open-habitat foragers was significantly higher in salvage-logged areas than in the two vital stand types. The activity of the forest-edge foragers did not significantly differ. The activity of the closed-habitat foragers was significantly higher in the two vital stand types. A Salvage-logged area after bark-beetle infestation, B bark-beetle-affected forest stand, C single-layered forest stand, and D multi-layered forest stand
The activity of the closed-habitat foragers also did not significantly differ between salvage-logged areas and bark-beetle-affected areas, but the activity in both these areas was significantly lower than in the two vital stand types (Figs. 2a–c, 4).
Discussion
Our study assessed the influence of alteration of forest structures by bark beetles and salvage logging on bat activity. The most important observation was the clear difference in the activity of bats of different foraging guilds. Therefore, measurements of general bat activity, as conducted by Hayes (2009), could mask the specific responses depending on functional traits. In our study area and in Germany in general, owing to the occurrence of several guilds with a specific, distinct response to forest structure and an unequal distribution of bat activity across the foraging guilds (in our study most activity was of edge foragers), the results of all bat activity could be strongly biased.
A second important finding was that the activity of open-habitat guild species was higher in salvage-logged areas, which does not support a negative effect of salvage logging for this group. For edge-habitat and closed-habitat guilds, salvage logging seemed to be irrelevant in our study area. In contrast, their activity seemed to be triggered more by the general process of forest opening by bark beetles, independent of subsequent salvage logging. Only for edge-habitat species did the habitat suitability seem to decrease because of salvage logging; the activity was higher in single-layered stands than in salvage-logged stands, but not higher than in bark-beetle-affected stands.
We are aware that the effects or lack of effects observed in our study could be affected by the methodology used to detect bat activity. One problem is that habitat differences could influence the ability to detect echolocation calls produced by bats, e.g. by masking bat activity in more cluttered habitats (Patriquin et al. 2003). To reduce or eliminate the differences in the detection of bat calls, we followed the suggestion of Patriquin et al. (2003) and placed batcorders in gaps within multi-layered dense forest stands. Furthermore, we are also aware that bat species with quiet calls, particularly those of the closed-habitat guild, are more difficult to record than bat species with louder calls, such as those of the open-habitat guild, which weakens our database for the analysis of the closed-habitat guild. But the detection ability was conservative within the three foraging guilds (Online Resource 3) and was analysed here separately. Thus, we are convinced that the variation in detectability had no influence on our results. Furthermore, we followed the suggestion of Yates and Muzika (2006) and included temperature as a covariable to compare bat activity between habitats on different nights.
A second critique may arise from our sampling near the ground. It is well documented that bat activity differs among strata (Adams et al. 2009; Hayes and Gruver 2000). In our study, the batcorders were installed 2.7 m above ground and detected bat activity within a spherical radius of approximately 20 m. In mature forest stands, this measure is below the uppermost part of the canopy, which reaches 35–45 m in height, depending on the tree species. However, only a few of the bat species in our study (Nyctalus sp., Pipistrellus sp.) regularly occur above the canopy in similar forest stands, but they also forage in lower strata (Aschoff et al. 2006; Runkel 2008). Therefore, our method seems to be appropriate to estimate bat activity within our forest stands.
Three major factors may trigger the occurrence of bats in our forest area: food, flight space and day roosts. In a previous analysis, we have shown that insect abundance is important for the occurrence of bats only in the open-habitat forager guild. Similarly, only for this guild is flight space due to open forest stands an important determinant for their occurrence (Müller et al. 2011). These analyses also show that the abundances of moths and flies, the most important bat food, decrease with an increase in forest opening but are of lower importance for bat-foraging activity. Therefore, one may expect that salvage logging reduces the critical number of roosts—a limiting factor for the occurrence of bats in many commercial forests (Barclay and Kurta 2007). This may be the reason for a negative impact of salvage logging on the forest-edge forager Myotis lucifugus after bark beetle attack in Canada (Randall et al. 2009). Our data do not allow us to confirm this finding because the higher activity in bark-beetle-affected areas compared to salvage-logged areas was far from significant (Fig. 4). Our study area thus differs from those larger areas of bark beetle infestation, as in the Northwest Territories of North America (Raffa et al. 2008), where large-scale logging could reduce roost availability below critical values on the landscape scale, with negative effects on bat activity. All of our intensive logging areas are still surrounded by stands rich in snags and tree cavities (Kanold et al. 2009) at distance of less than 1 km, and some uninfected trees still remain in the logged areas. The flight distances of most of the local bat species are between 5 and 10 km (Dietz et al. 2007); therefore, distances of less than 1 km can be easily bridged. A similar weaker effect of selective salvage logging after beetle infestation on several cavity breeders has also been reported (Kroll 2010).
For closed-habitat foragers, all types of forest opening—whether by bark beetles or logging—reduces activity. This underlines the assumption that these species are typical inhabitants of more-closed stands. Many of them are able to collect prey effectively by gleaning and are thereby rather independent of prey density (Safi and Kerth 2007; Müller et al. 2011), but the resources for gleaning are clearly reduced in both open-habitat types.
One major impact of salvage logging on ecosystems is the change in physical structure (Noss and Lindenmayer 2006). Our results and results of previous studies (Grindal and Brigham 1998; Adams et al. 2009) showed that the opening of the canopy by the bark beetle outbreak, independent of salvage logging, improved the habitat conditions for members of the open-habitat guild. An additional benefit of salvage logging, as has been shown for the Sharp-tailed Grouse (Tympanuchus phasianellus) in Canada, which thrives on salvage cuts (Niemuth and Boyce 2004), and for bird species richness after salvage logging of windthrows in Poland (Zmihorski and Durska 2011), was observed for the open-habitat guild also in our study, but was just below the significance level (Fig. 4).
Both negative and positive impacts of salvage logging on various animals have been reported. Those negatively affected by salvage logging include saproxylic beetles, which rely on dead-wood structures (Müller et al. 2010; Paillet et al. 2010), rare fungi that require high amounts of dead wood (Bässler and Müller 2010). One could conclude that the higher impact of salvage logging on these species is due to their low mobility. Yet birds, which are of similar mobility as bats, are also affected by salvage logging, particularly because cavity trees are removed, e.g. after a fire (Cahall and Hayes 2009; Greenberg et al. 1995; Morissette et al. 2002; Castro et al. 2010; Dickson et al. 1983; Hutto 2006). However, bat and bird behaviours differ greatly in some respects. Most bird species are strongly territorial with foraging activities in a few hectares (see discussion in Moning and Müller 2008), whereas bats in temperate forests as well as in tropical forests show little interspecific competition (Heller and Helversen 1989; Kingston et al. 2000), which allows them to respond more flexibly to forest structure alteration and to use patches rich in food. Here foraging distances from roost per night of around 5 km is common in Central European bats (Dietz et al. 2007). Furthermore, a lack of negative salvage logging effects on bats is not singular. It has been shown that salvage logging has neutral or positive effects on microbial assemblages (Khetmalas et al. 2002) and plants (Ne’eman et al. 1997; Elliott 2002). For plants, the opening of the soil by heavy logging machines enhances the possibility for many plant species to colonize the forests after disturbance (Paillet et al. 2010). However, the consequences of salvage logging can be more complex, affecting also trophic relationships, such as that of wolves and ungulates in Canada, in which ungulates, in the face of increasing forage plant biomass, avoid salvage-logged areas because of higher predation risk (Hebblewhite et al. 2009).
Conclusions
In the conservation of protected areas, salvage logging has been criticized because tree removal after natural disturbances decreases the habitats of many organisms. However, bats, which are attractive species in conservation, barely responded to salvage logging in the Bavarian Forest National Park. This unexpected result can be explained by the relatively small logging stands in Central European forests, which conserves enough roosts on the landscape scale. Furthermore, we found very distinct responses to forest opening by bark beetles and post-beetle salvage logging by the three foraging guilds. This underlines the importance of conducting studies of the activity of separate bat foraging guilds and not of general bat activity. Species of open-habitat foragers preferred both types of openings over vital forests. The positive effect of both canopy openings demonstrates that natural disturbance is the force that creates important gaps for open-habitat species. We conclude that the enigmatic bats, as compared to other taxonomic groups, are not the best choice for quantifying negative impacts of different forest managements on wildlife in Central European forests after a disturbance.
References
Adams MD, Law BS, French KO (2009) Vegetation structure influences the vertical stratification of open- and edge-space aerial-foraging bats in harvested forests. Forest Ecol Manag 258:2090–2100
Arbuthnott D, Brigham RM (2007) The influence of a local temperature inversion on the foraging behaviour of big brown bats, Eptesicus fuscus. Acta Chiropterologica 9(1):193–201
Aschoff T, Holderied MW, Marckmann U, Runkel V (2006) Forstliche Maßnahmen zur Verbesserung von Jagdlebensräumen von Fledermäusen. Abschlussbericht für die Vorlage bei der Deutschen Bundesstiftung Umwelt, Germany
Barclay RMR, Kurta A (2007) Ecology and behavior of bats roosting in tree cavities and under bark. In: Lacki MJ, Hayes JP, Kurta A (eds) Bats in forests. The John Hopkins University Press, Baltimore, pp 17–60
Bässler C, Müller J (2010) Importance of natural disturbance for recovery of the rare polypore Antrodiella citrinella Niemelä & Ryvarden. Fungal Biol 114:129–133
Breiman L (2001) Random forest. Mach Learn 45:5–32
Cahall RE, Hayes JP (2009) Influences of postfire salvage logging on forest birds in the Eastern Cascades, Oregon, USA. Forest Ecol Manag 257:1119–1128
Carter TC, Ford WM, Menzel MA (2000) fire and bats in the southeast and mid-atlantic: more questions than answers? In: Ford WM, Russel KR, Moorman CE (eds) The role of fire in nongame wildlife management and community restoration. Traditional Uses and New Directions, Nashville
Castro J, Moreno-Rueda G, Hodar JA (2010) Experimental test of postfire management in pine forests: impact of salvage logging versus partial cutting and nonintervention on bird-species assemblages. Conserv Biol 24:810–819
Ciechanowski M, Zając T, Biłas A, Dunajski R (2007) Spatiotemporal variation in activity of bat species differing in hunting tactics: effects of weather, moonlight, food abundance, and structural clutter. Can J Zool 85(12):1249–1263
Clarke FM, Pio DV, Racey PA (2005) A comparison of logging systems and bat diversity in the neotropics. Conserv Biol 19:1194–1204
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Dickson JG, Conner RN, Williamson JH (1983) Snag retention increases bird use of a clear-cut. J Wildlife Manage 47(3):799–804
Dietz C, von Helversen O, Nill D (2007) Handbuch der Fledermäuse Europas und Nordwestafrikas. Kosmos, Stuttgart
Elliott KJ, Hitchcock SL, Krueger L (2002) Vegetation response to large scale disturbance in a southern Appalachian forest: hurricane Opal and salvage logging. J Torrey Bot Soc 129:48–69
Elston DA, Moss R, Boulinier T, Arrowsmith C, Lambin X (2001) Analysis of aggregation, a worked example: numbers of ticks on red grouse chicks. Parasitology 122:563–569
Fenton MB (1989) The foraging behaviour and ecology of animal-eating bats. Can J Zool 68:411–421
Fisher JT, Wilkinson L (2005) The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Rev 35:51–81
Gannon MR, Willig MR (1994) The effects of hurricane Hugo on bats of the luquillo experimental forest of puerto rico. Biotropica 26:320–331
Greenberg CH, Harris LD, Neary DG (1995) A comparison of bird communities in burned and salvage-logged, clearcut, and forested Florida sand pine scrub. Wilson Bull 107:40–54
Grindal SD, Brigham RM (1998) Short-term effects of small scale habitat disturbance on activity by insectivorous bats. J Wildlife Manage 62:996–1003
Hayes JP (2009) Post-fire salvage logging in central oregon: short-term response in bats, birds and small mammals. Fire Sci Brief 1–6
Hayes JP, Gruver JC (2000) Vertical stratification of bat activity in an old-growth forest in western Washington. Northwest Sci 74:102–108
Hebblewhite M, Munro RH, Merrill EH (2009) Trophic consequences of postfire logging in a wolf-ungulate system. Forest Ecol Manag 257(3):1053–1062
Heller KG, Helversen O (1989) Resource partitioning of sonar frequency bands in rhinolophid bats. Oecologia 80:178–186
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Hutto RL (2006) Toward meaningful snag-management guidelines for postfire salvage logging in North America conifer forests. Conserv Biol 20:984–993
IPCC (ed) (2007) Climate change 2007: impacts, adaption and vulnerability, contribution of working group II to the fourth assessment report of the IPCC. Cambridge University Press, Cambridge
Jennings N, Pocock MJO (2009) Relationship between sensitivity to agricultural intensification and ecological traits of insectivorous mammals and arthropods. Conserv Biol 23:1195–1203
Jones KE, Barlow KE, Vaugan N, Rodrígues-Durán A, Gannon MR (2001) Short-term impacts of extreme environmental disturbance on the bats of Puerto Rico. Anim Conserv 4:59–66
Jones G, Jacobs DS, Kunz TH, Willig MR, Racey PA (2009) Carpe noctem: the importance of bats as bioindicators. Endang Species Res 8:93–115
Kanold A, Rohrmann N, Müller J (2009) Einflussfaktoren auf das Baumhöhlenangebot und dessen Auswirkungen auf die Arten und Dichten von Höhlenbrütern in Bergwäldern. Ornithologischer Anzeiger 47:116–129
Khetmalas MB, Egger KN, Massicote HB, Tackaberry LE, Clapperton MJ (2002) Bacterial diversity associated with Subalpine Fir (Abiea lasiocarpa) ectomycorrhizae following wildfire and salvage-logging in central British Columbia. Can J Microbiol 48:611–625
Kingston T, Jones G, Zubaid A, Kunz TH (2000) Resource partitioning in rhinolophoid bats revisited. Oecologia 124:3332–3342
Kroll AJ, Edward BA, Altman B (2010) Effects of salvage logging on avian nest survival in beetle-killed forests. Forest Ecol Manag 269(9):1599–1606
Moning C, Müller J (2008) Envoronmental keyfactors and their trashholds for the avifauna of temperate mountain Forests. Forest Ecol Manag 256:1198–1208
Morissette JL, Cobb TP, Brigham RM, James PC (2002) The response of boreal forest songbird communities to fire and post-fire harvesting. Can J Forest Res 32:2169–2183
Morris AD, Miller DA, Kalcounis-Rueppell MCK (2010) Use of forest edges by bats in a managed pine forest landscape. J Wildl Manage 74:26–34
Müller M, Job H (2009) Managing natural disturbance in protected areas: tourists’ attitude towards the bark beetle in a German national park. Biol Conserv 142:375–383
Müller J, Bußler H, Goßner M, Rettelbach T, Duelli P (2008) The European spruce bark beetle Ips typographus (L.) in a national park: from pest to keystone species. Biodivers Conserv 17:2979–3001
Müller J, Reed N, Bussler H, Brandl R (2010) Learning from a “benign neglect strategy” in a national park: response of saproxylic beetles to dead wood accumulation. Biol Conserv 143:2559–2569
Müller J, Mehr M, Bässler C, Fenton MB, Hothorn T, Pretzsch H, Klemmt H-J, Brandl R (2011) Aggregative response in bats: prey abundance versus habitat. Oecologia 169(3):673–684
Ne’eman G, Perevolotsky A, Schiller G (1997) The management implications of the Mt. Carmel research project. Int J Wildland Fire 7:343–350
Niemuth ND, Boyce MS (2004) Influence of landscape composition on sharp-tailed grouse lek location and attendance in Wisconsin pine barrens. Ecoscience 11:209–217
Norberg UM, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia, Chiroptera): wing adaptions, flight performance, foraging strategy and echolocation. Philos Trans R Soc Lond B Biol Sci 316:335–427
Noss RF, Lindenmayer DB (2006) Special section: the ecological effects of salvage logging after natural disturbance. Conserv Biol 20:946–948
Paillet Y, Berges L, Hjälten J, Odor P, Avon C, Bernhardt-Römermann M, Bijlsma R-J, de Bruyn L, Fuhr M, Grandin U, Kanka R, Lundin L, Luque S, Magura T, Matesanz S, Meszaro I, Sebastia M-T, Schmidt W, Standovar T, Tothmeresz B, Uotila A, Valladares V, Vellak K, Virtanen R (2010) Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv Biol 24(1):101–112
Parsons S, Jones G (2000) Acoustic identification of twelve species of echolocating bat by discriminant function analysis and artificial neuronal networks. J Exp Biol 203:2641–2656
Parsons S, Szewczak JM (2009) Detecting, recording, and analyzing the vocalizations of bats. In: Kunz TH, Parsons S (eds) Ecological and behavioral methods for the study of bats. The Johns Hopkins University Press, Baltimore, pp 91–111
Patriquin KJ, Barclay RMR (2003) Foraging by bats in cleared, thinned and unharvested boreal forest. J Appl Ecol 40:646–657
Patriquin KJ, Hogberg LK, Chruszcz BJ, Barclay RMR (2003) The influence of habitat structure on the ability to detect ultrasound using bat detectors. Wildlife Soc B 31(2):475–481
Patriquin-Meldrum K (1999) The effect of fire and logging on forest dwelling bats. Bat Research News 40:185
Peters SL, Malcolm JR, Zimmerman PI (2006) Effects of selective logging on bat communities in the southeastern amazon. Conserv Biol 20:1410–1421
Pickett STA, White PS (1985) Patch dynamics: a synthesis. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic Press, Orlando, pp 371–384
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Raffa K, Aukema B, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58(6):501–517
Randall LA, Barclay RMR, Jung TS (2009) The effect of forest disturbance on bats of the southwestern Yukon. University of Calgary, Calgary
Runkel V (2008) Mikrohabitatnutzung syntoper Waldfledermäuse: Ein Vergleich der genutzten Strukturen in anthropogen geformten Waldbiotopen Mitteleuropas. Friedrich-Alexander-Universität Erlangen-Nürnberg
Runkel V, Marckmann U, Schuster P (2009) Manual. ecoobs GbR Nürnberg
Safi K, Kerth G (2007) Comparative analyses suggest that information transfer promoted sociality in male bats in temperate zones. Am Nat 170(3):465–471
Schelhaas MJ, Nabuurs GJ, Schuck A (2003) Natural disturbances in the European forests in the 19th and 20th centuries. Global Change Biol 9(11):1620–1633
Schnitzler HU, Kalko EKV (2001) Echolocation by insect-eating bats. Bioscience 51(7):557–569
Schröder LM (2007) Retention or salvage logging of standing trees killed by the spruce bark beetle Ips typographus: consequences for dead wood dynamics and biodiversity. Scand J Forest Res 22:524–530
Shore TL, Brooks JE, Stone JE (2003) Mountain pine beetle symposium: challenges and solutions. Victoria, British Columbia
Ulbricht R, Hinrichs A, Ruslim Y (1999) Technical guideline for salvage felling in rehabilitation areas after forest fires. Samarinda, Indonesia
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus: a review of recent research. Forest Ecol Manag 202:67–82
Wickramasinghe LP, Harris S, Jones G, Vaugan N (2003) Bat activity and species richness on organic and conventional farms: impact of agricultural intensification. J Appl Ecol 40:984–993
Wolff JM, Bottaglia L, Carter TC, Rodman LB, Britzke ER, Feldhammer GA (2009) Effects of tornado disturbance on bat communities in southern Illinois. Northeast Natural 16:553–562
Yates MD, Muzika RM (2006) Effect of forest structure and fragmentation on site occupancy of bat species in Missouri Ozark forests. J Wildl Manage 70:1238–1248
Zmihorski M, Durska E (2011) The effect of contrasting management types on two distinct taxonomic groups in a large-scaled windthrow. Eur J For Res 130:589–600
Acknowledgments
The study was financed by the INTERREG IV program (project Nr. 68). We thank Volker Runkel and Ulrich Marckman (Ecoobs GbR) and Christian Strätz for helpful discussions and comments, and Kaisa Kurvinen, Verena Riedinger, Robin Reiter, Sebastian Seibold, and Till Severon for assistance in the field. We thank Karen A. Brune for linguistic revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehr, M., Brandl, R., Kneib, T. et al. The effect of bark beetle infestation and salvage logging on bat activity in a national park. Biodivers Conserv 21, 2775–2786 (2012). https://doi.org/10.1007/s10531-012-0334-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0334-y