Abstract
Present study aims at estimation and validation of net primary productivity (NPP) using production efficiency model (PEM), and its possible relationship with tree diversity. The PEM estimates NPP, based on light use efficiency (LUE) and intercepted photosynthetically active radiation (IPAR). Weighted average LUE varied between 0.02 gC/μmol/m2 of PAR (Mixed forest (miscellaneous)) to 0.08 gC/μmol/m2 of PAR (Acacia forest), in growing phase (GP), and 0.0008 gC/μmol/m2 of PAR (Boswellia mixed forest) to 0.023 gC/μmol/m2 of PAR (Acacia forest) during the senescent phase (SP). The average weighted LUE for tropical dry and Moist deciduous forest (MDF) in GP were 0.05 gC/μmol/m2 of PAR and 0.03 gC/μmol/m2 of PAR, respectively. The average IPAR for different forest types was 2079.58 μmol/m2/s during GP and 1510.58 μmol/m2/s during SP. The PEM based NPP varied between 0.58–275.78 gC/m2/year during GP and 0.43–74.34 gC/m2/year during SP. The PEM based NPP and conventional (ground based) NPP were related with R 2 of 0.55. The tree diversity and NPP relationship was observed with R 2 of 0.55 at the level of both plot and forest types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Net primary productivity (NPP) is an important indicator to understand the functioning, of the ecosystem and thus, provides a basis to understand the associated carbon sequestration patterns, ecosystem services and its response to climate change. Biodiversity on the other hand is believed to be important, for maintaining and increasing the ecosystem resilience in the event of climate change. Understanding the relationship between species diversity and ecosystem functioning (NPP) is of paramount importance to understand the impact of biodiversity loss on NPP patterns and consequently climate change.
Many studies have investigated positive relationship between NPP and biodiversity (species richness) in different ecosystems of the world (Naeem and Thompson 1994; Tilman et al. 1996; Tilman and Knops 1997; Lawton 1998, Woodward and Kelly 2008) mainly at local scales, while it is less understood at regional or landscape levels (Thompson et al. 2009). It has been believed that the functioning of the ecosystem is caused by a few species only (functional biodiversity) and remaining species are redundant, however, these redundant species may play dominant role in the event of climate change (Walker 1995; Paine 2002). It is not necessary that the species with high productivity in monoculture will also dominate production in mixtures (Hooper and Vitousek 1997; Troumbis et al. 2000; Engelhardt and Ritchie 2001; Spae`kova’I and Leps 2001; Hector et al. 2002; Hooper and Dukes 2004).
Many of the NPP models evolved in last two decades (Potter et al. 1993; Chong et al. 1993; Prince and Goward 1995; Marcon and Peterson 2002; Matsushita and Masayuki 2002; Awaya et al. 1993) provide estimates at global levels. On the other hand, many local level models require extensive data related to hydrology, carbon and nutrients for estimation of forest growth (Running and Coughlan 1988; Running and Gower 1991; Aber and Federer 1992; Weinstein et al. 1991; Wang et al. 1998) and posses the challenges of extrapolation at broader spatial scales (Jarvis and McNaughton 1986; Veroustraete et al. 1996). Production efficiency model (PEM), which uses light use efficiency (LUE) and intercepted photosynthetically active radiation (IPAR) is one of the efficient ways to estimate the NPP at different scales (Monteith 1972; Ahl Douglas et al. 2004; Lagergren and Eklundh 2005; Yuan et al. 2007) as (i) It follows the basic principals of the process of photosynthesis and (ii) is satellite driven.
It is the relationship of ground based IPAR with satellite derived indices mainly normalised difference vegetation index (NDVI), which makes PEM satellite driven. The PEM is based on the principle of functional convergence of LUE, which suggests that the LUE is more or less similar for same functional forest types. Many subsequent studies, however, have observed fivefold variations (Prince 1991; Running and Hunt 1993; Ruimy et al. 1994; Houborg et al. 2009). This needs to be validated for tropical dry deciduous forests. Data related to LUE is sparsely available in India, mainly because of complicacies involved in data collection, at the species level. Couple of studies for NPP modeling have been carried out previously (Nayak et al. 2010, Chabbra and Dadhwal 2004), however, at coarser spatial scales.
It is important to carry out biomass (NPP)-biodiversity studies in combination rather than in isolation (Miles and Kapos 2008). The less biodiverse systems having high carbon sequestration capability may not be preferable to, high biodiversity and less carbon sequestration systems, as they may actually become the source of carbon-di-oxide (CO2) in the event of climate change (UNEP-WCMC 2008; Harvey et al. 2009). The present research is aimed at estimation of NPP using PEM, validating the same with parallel ground based investigations and studying its possible relationship with tree diversity.
Study area
Shivpuri district (Madhya Pradesh, India) is located between 24°50′N and 25°53′N and from 76°59′E to 78°29′E (Fig. 1). Physiographically, the area is divided in plains, plateau and mountain ranges. The mountain ranges are relatively low in elevation (250–510 m) and are known as upper Vindhyan mountain ranges of central India. The climate is hot and dry and classified under semiarid to arid region. There are four evident seasons including, summer (from March to June); rainy season (from July to September); post rainy season (from October to November) and winter (from December to February). The annual mean maximum temperature in the region is highest during May and lowest during January. The temperature touches to 42°C during the month of June. The average rainfall in the district is 875 mm (http://shivpuri.mp.gov.in/aboutshivpuri.html. cited 08 August 2011). The rainfall is highest during the months of June–August (about 3,300 mm) (Mooley 1981; Parthasarathy et al. 1987; Pant and Rupa 1997). Forests cover 23.44% of the total area. The major forest types are tropical dry deciduous forest with some moist deciduous forests (MDF) in the western parts (Table 1).
Anogeissus forest is one of the dominant forest types and is found almost everywhere. In some areas, particularly in eastern parts, these forests have become stunted due to high anthropogenic pressure. Boswellia mixed forest grow well in the extreme conditions; i.e., less water, high temperature and shallow soil, and are predominant on hill slopes and plateau, however, with occasional presence in plains. Acacia forest is predominant in northern parts. Mixed forest (miscellaneous) is found in all parts of the districts, with dominance in western region. The Medium density forest with grasses at bottom (MDG) is savannah type of forest predominantly present in Madhav National Park. The MDF are found in western and central parts of the district.
The biotic pressure and low resource availability has made these forests critical from ecological point of view (Roy and Jain 1998). Regeneration is poor in almost all the forest types due to frequent fire and cattle grazing. Above forests are characterized by distinct phenological phases, i.e., Growing phase (GP) (mid-August to mid-December), Senescent phase (SP) (mid- December to mid-April) and Defoliated phase (DP) (mid-April till the commencement of rainy season). The GP is when the forests are covered with full canopy, SP represents sparse canopy and DP, when there is no canopy present.
Method
Total 29 permanent sampling plots (0.1 ha each) were laid down in different forest types of the study area (Kale et al. 2001) for taking observations in different phases. These observations were used for conventional and PEM based NPP estimation and comparing their results. For conventional NPP estimation, all the individual trees of all the species coming inside the plots were measured for their height and permanently marked for measuring yearly girth increment. For PEM based NPP estimation, IPAR and LUE observations were made during GP and SP (Table 2).
NPP estimation (conventional method)
The NPP was measured based on; [NPP = ∆ (Standing biomass) + Losses] (Waring et al. 1998). Plot-wise bole biomass for different species found in the study area was estimated using available species and area-specific biomass equations (Singh and Misra 1979; Tiwari 1992; Roy and Ravan 1996; Kale et al. 2004). In the event of non availability of species specific equation, ‘pooled biomass equation’ developed by Singh and Misra (1979) for similar forest types was used. These equations require tree girth and height information as inputs. Plot-wise bole biomass was estimated by aggregation of biomass of all the trees coming inside the plot. Forest type-wise average per hectare biomass was estimated by averaging plot-wise biomass for all the plots coming inside that particular forest type. Forest type-wise biomass was estimated by multiplying per hectare average biomass with total area of that particular forest. The procedure was repeated for next year to know the rate of change of biomass.
The losses (due to death and decay) were estimated by taking the litter sample in 1 m × 1 m quadrates throughout the GP and SP (except rainy season) in four corners and center of the plot. Average value was extrapolated at the level of plot and forest type to estimate plot and forest-type-wise litter value (Kale 2006). The rate of change of biomass was added to losses to know the plot and forest type-wise NPP. The species-wise biomass increment for all the sampled trees in all the forest types was investigated in order to understand the role of different species in NPP variations.
NPP estimation (PEM based)
The PEM based NPP was estimated through, \( NPP = \varepsilon * \left( {\sum IPAR} \right) \) where, ε is the weighted (relative density and relative basal area of tree species were used as weight) LUE and IPAR is the Intercepted Photosynthetically Active Radiation (μmol/m2/s). Green plants in the presence of sunlight convert CO2 to energy. The fraction of the radiation that is used for this purpose is called photosynthetically active radiation (PAR). The IPAR is that part of electromagnetic radiation, which is utilized by the plants for the process of photosynthesis. The measurements of radiation within the canopy were made based on the method described by Norman and Jarvis (1975); IPAR = I o − T c, where, I o is the total incident PAR and T c is the PAR transmitted through the canopy. If all the radiation is intercepted by the canopy, then IPAR = 1. If the entire radiation pass through the canopy gaps without being intercepted, then IPAR = 0 (Roy and Jain 1998). Plot-wise IPAR was estimated for all the plots for the representative GP and SP months using calibrated ceptometer. First I o was measured in open area, where direct sunlight was available and then, average T c was measured by moving diagonally inside the sample plot starting from the central tree and taking measurements (total 16) at regular intervals in east, west, north and south directions. Plot-wise average IPAR was estimated using the above equation. Monthly IPAR values were integrated for GP (October–November and December, 2001) and SP (January and February, 2002) and tested for their relationship with different indices, mainly NDVI using different linear and curvilinear regression models. The NDVI data was generated based on atmospherically corrected Indian Remote Sensing Satellite (IRS)—Wide field sensor (WiFS) sensor data having spatial resolution of 188 m (Kale 2006). This data correspond to the same month and year during which field investigations were carried out. The most significant relation in respective phases was regressed to estimate per pixel IPAR for GP and SP.
The LUE (or ‘energy conversion efficiency’) is the efficiency of green plants to convert the radiation incident on them to energy (Prince 1991). Although, it is technically dimensionless, it usually has associated units such as grams dry matter produced per megajoule of absorbed radiation (gMJ−1) (Landsberg et al. 1996; Ahl Douglas et al. 2004).
Total 12 g of carbon (molar mass of carbon) sequestered per mole of CO2 exchanged was considered for estimation of LUE. Unit of LUE was gC/μmol/m2/s of PAR incident. The species-wise LUE were estimated for all the plots in both GP and SP, using ultra compact leaf chamber analyzer (LCA), which were averaged at the level of plot and forest types (Kale 2006). The forest type-wise weighted average LUE for GP and SP were multiplied by per pixel IPAR for GP and SP, respectively, to know the per pixel NPP for GP and SP. The NPP estimates of conventional and PEM based methods were compared to investigate the accuracy of PEM model. While investigating these relationships, the tolerable threshold distance for selecting a pixel was maintained below 0.5 (pixel). This was because the tolerable image registration error in the present research was up to 0.5 pixels.
Tree diversity estimation
The tree diversity was estimated based on ‘Shannon–Weiner’ index. Higher is the value of the index, greater is the tree diversity.
\( \overline{H} = - \sum {[(n_{i} } /N)\ln (n_{i} /N)] \), where, \( \overline{H} \) is the Shannon–Weiner index, n i is importance value of species and N is total importance value of all the species available in that forest type. The importance value of each species was estimated based on its relative frequency, relative density and relative abundance in that forest type and was named as importance value index (IVI). The forest type wise \( \overline{H} \) was investigated for its relationship with forest type-wise average NPP, whereas, plot-wise tree diversity (species count) was tested for its relationship with plot-wise NPP.
Results and discussion
NPP estimation (conventional method)
Higher biomass increment at the level of plot is attributed to favourable growth conditions due to availability of moisture. Biomass reduction from 2000 to 2001 in Boswellia mixed forest plot 4 owes to death of some trees during 2001 (Table 3). Forest type-wise percent biomass increment was comparatively higher in mixed formations like Mixed forest (miscellaneous) and MDF rather than in the forests dominated by single species, i.e., Anogeissus forest and Acacia forest. The above forests are located in favorable micro-climatic conditions induced due to the topographic variations and availability of moisture. Minimum growth rate was obtained for Boswellia mixed forest; hence, they were comparatively more stable as far as biomass dynamics is concerned. Species-wise girth increment varied between 0.18 cm/year (Boswellia serrata) and 1.58 cm/year (Miliusa tomentosa) (Table 4). These values compared well with the girth increment values observed by Murphy and Lugo (1986) and Singh and Singh (1991) for tropical dry forests.
Plot-wise Litter values varied between 85.4 g/m2 for Mixed forest (miscellaneous) plot 12 to 688.2 g/m2 for Boswellia mixed forest plot 2 (Table 3). This difference is attributed to delay in senescence of some of the species in Mixed forest (miscellaneous) as compared to early senescence of Boswellia mixed forest resulting in higher litter accumulation. The high plot-wise NPP is generally attributed to availability of moisture and consequently less frequent fires and higher biomass increment (Table 3).
NPP estimation (PEM based)
Plot-wise IPAR followed the decreasing trend from GP to SP. Average IPAR for GP and SP for tropical dry deciduous forest were 2055.19 and 1486.80 μmol/m2/s, respectively, whereas, for MDF these were 2291 and 1766.16 μmol/m2/s, respectively (Table 3).
Among different vegetation indices, i.e., maximum, sum, minimum, amplitude (difference of maximum and minimum NDVI for GP/SP, image stack) and mean NDVIs; best relationship of IPAR was obtained with maximum-NDVI using ‘linear model’, with R 2 of 0.73 and 0.34 for GP and SP, respectively (Fig. 2). Lower R 2 in SP was due to decreased leaf vigor and increased background reflectance. Amplitude NDVI also depicted equal correlation with IPAR in GP with ‘log model’, but the ‘linear model’ was preferred over the log model for regression analysis.
Higher IPAR was obtained for the forests that maintained intact canopies, which is generally linked to availability of moisture.
Species-wise LUE (without weight) varied between 0.08 (Albizia procera) to 5.02 (Syzygium cumini) gC/μmol/m2 of PAR. Higher values were obtained for the species present in moist zones, i.e., Syzygium cumini, Nerium indicum, Tectona grandis, Terminalia arjuna, Aegle marmelos and Mitragyna parviflora, whereas, generally lower values were obtained for the species which were located in drier regions. The LUE varied significantly between forest types, however, lower values were observed in SP as compared to GP (Fig. 3). Different biophysical and climatic parameters and the amount of nitrogen present in the leaf are responsible for heterogeneity of LUE (Hirose and Bazzaz 1998). Forest type-wise weighted average LUE in GP varied between 0.02 gC/μmol/m2 of PAR (Mixed forest (miscellaneous)) to 0.08 gC/μmol/m2 of PAR (Acacia forest), whereas, during the SP, it varied between 0.0008 gC/μmol/m2 of PAR for Boswellia mixed forest to 0.023 gC/μmol/m2 of PAR for Acacia forest (Table 5). Lower LUE of Boswellia mixed forest is attributed to sparse and semi-dried canopy cover, which resulted in low CO2 exchange from the leaves as compared to the radiation incident on it.
The theoretical range of annual LUE is between 0.5 and 4.0 g/C/MJ (Collatz et al. 1991; Prince and Goward 1995). Empirically derived LUE varies from about 0.2 to 1.8 g/C/MJ among a range of biome, crop verities and forest stands. Simulated values derived from ecophysiological models of plant growth, driven by meteorological data have a similar range of values (0.2–1.95 g/C/MJ) for a variety of ecosystem in North America (Running and Hunt 1993). Results from global primary production models occupy somewhat narrower range (0.1–1.0 g/C/MJ) probably as a result of spatial averaging (Potter et al. 1993; Ruimy et al. 1994; Prince and Goward 1995). In the present research, LUE has been estimated considering the morning 2.5 h (9:30–1200 h) during which the rate of photosynthesis is optimum.
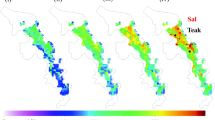
NPP for GP varied between 0.58 and 275.78 gC/m2/year (Fig. 4a), whereas, for SP it varied from 0.43 to 74.34 gC/m2/year (Fig. 4b). Plot-wise NPP estimated based on conventional methods were compared well with NPP (GP) estimated using PEM (R 2 = 0.55) (Fig. 5). The NPP estimation using PEM is a complicated task mainly because it requires species-wise LUE information, thus an average weighted LUE value of 0.05 for tropical dry deciduous forest and 0.03 for MDF can be used for straightforward NPP estimation, however, overall there is 24% overestimation of NPP. Since MDF constitutes only 0.57% of the total forest area, it is required to test the observations in other areas as well.
The weight of relative density and relative basal area given to LUE, affects the NPP patterns. Based on the conventional ground based investigations it has been observed that higher NPP was obtained for MDF and Mixed forest (miscellaneous), which is mainly due to dominance of species having higher incremental growth rate, and comparatively higher litter production. Hence, it is obvious to consider the weighted LUE in order to have representation of species dominance. However, in forests dominated by one particular species, like Anogeissus forest, Boswellia mixed forest and Acacia forest, comparatively higher weighted LUE were obtained. In contrast to this, mixed forest like MDF and Mixed forest (miscellaneous) have dominance of higher number of species, which resulted in lower weighted LUE due to averaging. Nevertheless, LUE is important parameters for NPP estimation and faithfully represent the incremental growth. This has been validated by testing relationship between plot-wise biomass increments and plot-wise average LUE (without weight) for GP (October–November). A significant correlation (R 2 = 0.76) was resulted (Fig. 6).
Tree diversity NPP relationship
The forest type wise \( \overline{H} \) is listed in Table 5. The maximum and minimum values were obtained for Mixed forest (miscellaneous), and MDG, respectively. A fair NPP-diversity relationship (R 2 = 0.55) was obtained at the level of plot (Fig. 7) and forest types (R 2 = 0.55), i.e., Shannon–Weiner Index.
It has been observed that few species like Acacia catechu, Anogeissus latipholia, Butea monosperma, Diospyros melanoxylon are dominating the forests of the Shivpuri districts and play a key role in NPP variations. Even when their incremental growth rates are lower than other species like Lannea coromandelica, Bauhinia racemosa, Miliusa tomentosa and Tectona grandis (Table 4) their percent contribution in overall biomass increment is comparatively higher because of their dominance. However, the Boswellia serrata species which constituted 12.20% of biomass of all the sampled trees in all the forest types contributes only 1.42% towards the total biomass increment. This is because of its lower increment rate and comparatively lower dominance. On the contrary, the Anogeissus latipholia constitutes 9.38% of biomass of all the sampled trees of all the forest types; however, its contribution in overall biomass increment is 14.98%, which is attributed to its higher increment rate and higher dominance. The NPP is governed by biomass increment and litter accumulation. The two most species rich forests, i.e., MDF and Mixed forest (miscellaneous) have high biomass increment; high litter fall and consequently high NPP (Table 5).
Presence of species, i.e., Miliusa tomentosa, Bauhinia racemosa, Lannea coromandelica and Elaeodendron glaucam having high incremental growth in combination with above dominant species further enhances NPP of these forests. The highest NPP of MDF is mainly attributed to presence of Tectona grandis, which in addition to higher incremental growth and dominance (highest IVI in MDF) also contributes significantly in litter accumulation. The forests dominated by single species like Anogeissus forest, Boswellia mixed forest and Acacia forest had lower NPP as compared to mixed formations.
Discussion and conclusions
It has been observed that Acacia catechu, Anogeissus latifolia, Anogeissus pendula and Diospyros melanoxylon are responsible for 65% of overall biomass increment by all the forest types of Shivpuri district. Presence of other species having high incremental growth in association with above species further enhances the NPP. Therefore, species rich forests like Mixed forest (miscellaneous) and MDF also have comparatively higher NPP. Such species associations are important for enhancing the carbon sequestration and consequently governing the climate change. In order to maintain/improve the resiliency of such areas, there is a strong need for conservation and protection.
The inclusion of LUE in PEM provides the connection between energy harvested by the vegetation and the efficiency of its use in assimilation of carbon. The LUE is a heterogeneous parameter, which varies significantly with species; and generally remains lower for stressed forest types. For some species like Syzygium cumini and Terminalia arjuna, which are present in moist areas, high LUE (nearly six and four times the mean LUE values, respectively) were obtained. It has been believed that this heterogeneity is attributed to biophysical and climatic parameters and the amount of nitrogen present in the leaf (Hirose and Bazzaz 1998). More research in the direction will help in further calibrating the PEM.
The weight of relative density and relative basal area provides a basis to consider the relative dominance of constituent species of different forest types, however, in case of the forests dominated by single species, the LUE are affected by high species dominance and results in higher LUE, which ultimately enhances the NPP of such forest types, whereas, in mixed formations it is lower due to averaging effect. Therefore, it may be appropriate to consider only the physiological basis of LUE rather than physiological as well as structural basis for estimation of NPP.
Worldwide there is a lot of emphasis on reducing CO2 emissions by avoiding deforestation and forest degradation (REDD). Indian government has set targets to carry out afforestation/eco restoration of 20 million ha. land in the next 10 years and increase in greenhouse gas removals by India’s forest to 6.35% of India’s total greenhouse gas emissions by the year 2020. Studies like this forms a strong basis, both for identification of appropriate areas for afforestation/eco-restoration and conservation prioritization of existing areas to ensure high CO2 sequestration while maintaining the biodiversity in the imminent climate change scenario.
Abbreviations
- NPP:
-
Net primary productivity
- PEM:
-
Production efficiency model
- LUE:
-
Light use efficiency
- IPAR:
-
Intercepted photosynthetically active radiation
- PAR:
-
Photosynthetically active radiation
- NDVI:
-
Normalized difference vegetation index
- LCA:
-
Leaf chamber analyzer
- CO2 :
-
Carbon di-oxide
- MDF:
-
Moist deciduous forest
- MDG:
-
Medium density forest with grasses at bottom
References
Aber JD, Federer CA (1992) A generalized, lumped-parameter model of photosynthesis, evapotranspiration and net primary production in temperate and boreal forest ecosystems. Oecologia 92:463–474
Ahl Douglas E, Gower ST, Scott MD, Burrows SN, Norman JM, Diak GR (2004) Heterogeneity of light use efficiency in a northern Wisconsin forest: implications of modeling net primary productivity with remote sensing. Remote Sens Env 93:168–178
Awaya Y, Kodani E, Tanaka K, Lius J, Zhuang DM, Yonking J (2004) Estimation of global net primary productivity using NOAA images and meteorological data: changes between 1988 and 1993. Int J Remote Sens 25(9):1597–1613
Chabbra A, Dadhwal VK (2004) Estimating terrestrial net primary productivity over India using satellite data. Curr Sci 86(2):269–271
Collatz GJ, Ball JT, Cirivet C, Berry JA (1991) Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agri for meteo 54:107–136
Engelhardt KAM, Ritchie ME (2001) Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 411:687–689
Harvey A, Barney D, Cyril K (2009) Opportunities for achieving biodiversity conservation through REDD. Cons Lett: 1–9
Hector A, Bazeley-White E, Loreau M, Otway S, Schmid B (2002) Over yielding in plant communities: testing the sampling effect hypothesis with replicated biodiversity experiments. Ecol Lett 5:502–511
Hirose T, Bazzaz FA (1998) Trade-off between light-and Nitrogen-use efficiency in canopy photosynthesis. Ann of Bot 82:195–202
Hooper DU, Dukes JS (2004) Over yielding among plant functional groups in a long-term experiment. Ecol Lett 7:95–105
Hooper DU, Vitousek PM (1997) The effects of plant composition and diversity on ecosystem processes. Science 277:1302–1305
Houborg R, Anderson CM, Norman JM, Wilson T, Meyers T (2009) Intercomparison of a ‘bottom-up’ and ‘top-down’ modeling paradigm for estimating carbon and energy fluxes over a variety of vegetative regimes across the U.S. Agri For Meteo 149:2162–2182
Jarvis PG, Mcnaughton KG (1986) Stomatal control of transpiration: scaling up from leaf to region. Adv Ecol Res 15:1–49
Kale M (2006) Terrestrial biomass and productivity estimation in tropical dry deciduous forests of central India using satellite remote sensing. Dissertation, University of Pune, Ph. D
Kale MP, Singh S, Roy PS (2001) Satellite remote sensing (IRS -WiFS) for estimating intercepted photosynthetically active radiation in Tropical forest ecosystem. Asian J Geoin 2:59–71
Kale M, Singh S, Roy PS (2004) Biomass equations of dominant species of dry deciduous forest in Shivpuri district (M.P.). Curr Sci 87(5):683–687
Lagergren F, Eklundh L (2005) Net primary production and light use efficiency in a mixed coniferous forest in Sweden. Plant, Cell Environ 28:412–423
Landsberg JJ, Prince SD, Jarvis PG, McMurtrie RE, Luxmoore R, Medlyn BE (1996) Energy conversion and use in forests: the analysis of forest production in terms of radiation utilization efficiency. In: Gholz HL, Nakane K, Shimoda H (eds) The use of remote sensing in the modeling of forest productivity. Kluwer Acad Publ, Dodrecht, pp 273–298
Lawton JH (1998) Pigeons, peregrines and people. Oikos 83(2):209–211
Lo Seen CD, Mougin E, Etchegorry G (1993) Relating the global vegetation index to net primary productivity and actual evapotranspiration over Africa. Int J Remote Sens 14(8):1517–1546
Marcon CJ, Peterson KM (2002) The utility of estimating net primary productivity over Alaska using baseline AVHRR data. Int J Remote Sens 23(21):4571–4596
Matsushita B, Masayuki T (2002) Integrating remotely sensed data with an ecosystem model to estimate net primary productivity in East Asia. Remote Sens Environ 81:58–66
Miles L, Kapos V (2008) Reducing greenhouse gas emissions from deforestation and forest degradation: global land-use implications. Science 320:1454–1455
Monteith JL (1972) Solar radiation and productivity in tropical ecosystems. J Appl Ecol 9:747–767
Mooley DA (1981) Annual rain-water over India, its variability and impact on the economy. J Climatol 1:167–186
Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Anal Rev Ecol Syst 17:67–88
Naeem S, Thompson LJ (1994) Declining biodiversity can alter the performance of ecosystems. Nature 368(6473):734–737
Nayak KR, Patel NR, Dadhwal VK (2010) Estimation and analysis of terrestrial net primary productivity over India by remote-sensing-driven terrestrial biosphere model. Environ Monit Assess 170:195–213
Norman JM, Jarvis PG (1975) Photosynthesis in Sitka Spruce (Picea sitchensis Bong. Carr.) V. Radiation Penetration theory and a test case. J Appl Ecol 12:839–878
Paine RT (2002) Trophic control of production in a rocky intertidal community. Science 296(5568):736–739
Pant GB, Rupa KK (1997) Climates of South Asia. Wiley, Chichester 320
Parthasarathy B, Sontakke NA, Monot AA, Kothawale DR (1987) Droughts/floods in the summer monsoon rainfall season over different meteorological subdivisions of India for the period 1871–1984. J Climatol 7:57–70
Potter CS, Randerson JT, Field CB, Matson PA, Vitousek PM, Mooney HA, Klooster SA (1993) Terrestrial ecosystem production: a process model based on global satellite and surface data. Global Biogeochem Cycl 7:811–841
Prince SD (1991) A model for regional primary production for use with coarse-resolution satellite data. Int J Remote Sens 12:1313–1330
Prince SD, Goward SJ (1995) Global Primary Production: A remote sensing approach. J Biogeog 22:815–835
Roy PS, Jain A (1998) A satellite remote sensing based approach to estimate regional net primary productivity of forest. In: global change studies scientific results from ISRO geosphere biosphere program
Roy PS, Ravan SA (1996) Biomass estimation using satellite remote sensing data-An investigation on possible approaches for natural forest. J Biosci 21:535–561
Ruimy A, Dedieu G, Saugier B (1994) Methodology for the estimation of terrestrial net primary production from remotely sensed data. J Geophys Res 99:5263–5284
Running SW, Coughlan JC (1988) A general model of forest ecosystem processes for regional applications hydrologic balance, canopy gas exchange and primary production process. Ecol Mod 42:125–154
Running SW, Gower ST (1991) FOREST- BGC, a general model for forest ecosystem processes for regional applications II dynamic carbon allocation and nitrogen budgets. Tree Physiol 9:147–160
Running SW, Hunt ER (1993) Generalization of a forest ecosystem process model for other biomes, BIOME-BGC, and an application for global scale models. In: Ehleringer JR, Field CB (eds) Scaling Physiological Processes: Leaf to globe. San Diego, Academic, pp 141–158
Singh KP, Misra R (1979) Structure and functioning of natural, modified and silviculture ecosystems in eastern Uttar Pradesh, final technical report (1975–1978), MAB research project 1. Banaras Hindu University, Varanasi
Singh L, Singh JS (1991) Species structure, dry matter dynamics and carbon flux of a dry tropical forest in India. Ann of Bot 68:263–273
Spae`kova′I, Leps J (2001) Procedure for separating the selection effect from other effects in diversity–productivity relationships. Ecol Letts 4:585–594
Thompson I, Mackey B, McNulty S, Mosseler A (2009) Forest resilience, biodiversity, and climate change. A synthesis of the biodiversity/resilience/stability relationship in forest ecosystems. Secretariat of the Convention on Biological Diversity, Montreal. Technical Series, pp 3–67
Tilman D, Knops J (1997) The influence of functional diversity and composition on ecosystem processes. Science 277(5330):1300–1302
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379(6567):718–720
Tiwari AK (1992) Component-wise biomass models for trees: a non-harvest technique. Ind Fores 404–410
Troumbis AY, Dimitrakopoulos PG, Siamantziouras AS, Memtsas D (2000) Hidden diversity and productivity patterns in mixed Mediterranean grasslands. Oikos 90:549–559
UNEP-WCMC (2008) Carbon and biodiversity: a demonstration atlas. Eds. Kapos V., Ravilious C, Campbell A, Dickson B, Gibbs H, Hansen M, Lysenko I, Miles L, Price J, Scharlemann JPW, Trumper K. UNEP-WCMC, Cambridge
Veroustraete F, Patyn J, Myneni RB (1996) Estimating net ecosystem exchange of carbon using the normalised difference vegetation index and an ecosystem model. Remote Sens Environ 58:115–130
Walker B (1995) Conserving biological diversity through ecosystem resilience. Cons Biol 9:747–752
Wang YP, Rey A, Jarvis PG (1998) Carbon balance of young birch trees grown in ambient and elevated atmospheric CO2 concentrations. Global Change Biol 4(8):797–807
Waring RH, Landsberg JJ, Williams M (1998) Net primary production of forests: a constant fraction of gross primary production? Tree Physiol 18:129–134
Weinstein DA, Beolin RM, Yanni RD (1991) Modeling changes in red spruce carbon balance and allocation in response to interacting ozone and nutrient stresses. Tree Physiol 9:127–146
Woodward FI, Kelly CK (2008) Responses of global plant diversity capacity to changes in carbon dioxide concentration and climate. Ecol Lett 11:1229–1237
Yuan W, Liu S, Zhou G, Zhou Gu, Tieszen LL, Baldocchi D, Bernhofer C, Gholz H, Goldstein AH, Goulden ML, Hollinger DY, Hu Y, Law BE, Stoy PC, Vesala T, Wofs SC (2007) Deriving a light use efficiency model from eddy covariance flux data for predicting daily gross primary production across biomes. Agri For Meteo 143:189–207
Acknowledgments
Authors are thankful to Department of Space, Govt. of India for funding the present research under its ‘Indian Space Research Organisation-Geosphere Biosphere Program (ISRO-GBP)’. Thanks are due to Forest department Madhya Pradesh, for providing permission to carry field investigations in forested landscapes of Shivpuri district. We thank Dr. Arijit Roy of Indian Institute of Remote Sensing, Dehradun for his help in improving the contents of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kale, M.P., Roy, P.S. Net primary productivity estimation and its relationship with tree diversity for tropical dry deciduous forests of central India. Biodivers Conserv 21, 1199–1214 (2012). https://doi.org/10.1007/s10531-012-0226-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0226-1